Near-Infrared Spectroscopy (NIRS) in Traumatic Brain Injury (TBI)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Search Results
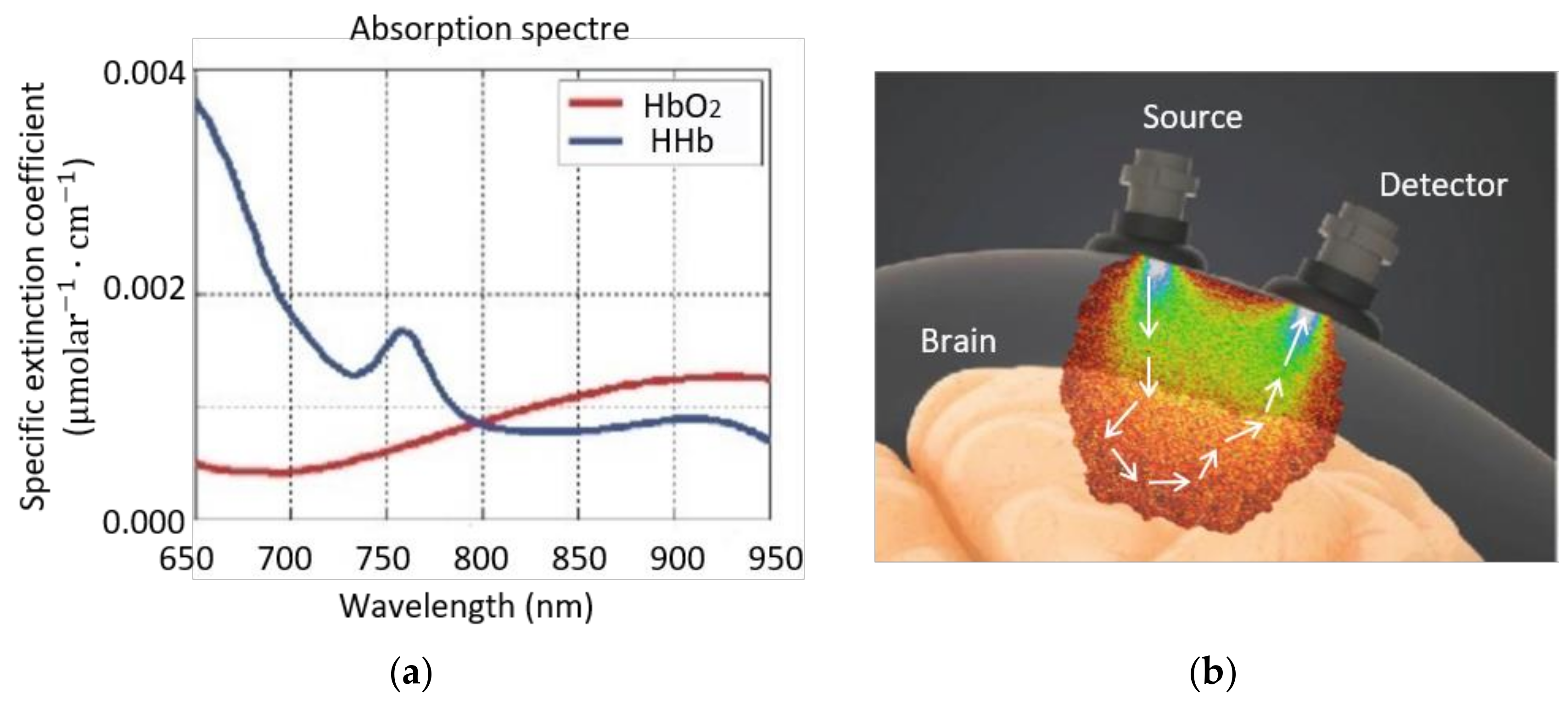
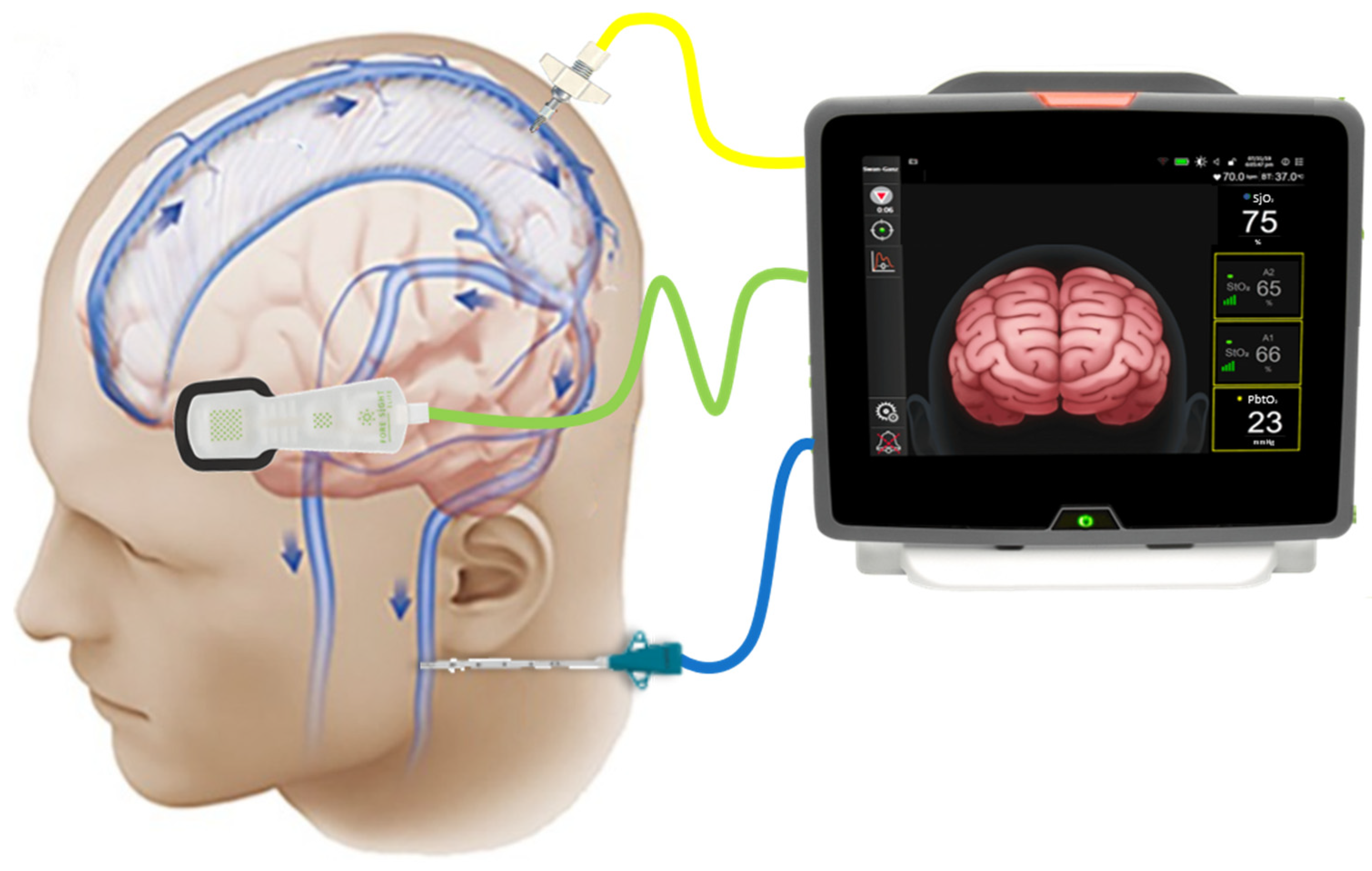
3.2. Physical and Technological Description of NIRS
3.3. NIRS Measurement Techniques
3.4. Subgroups
3.4.1. Oxygenation
Physiology of Oxygenation in TBI
Summary of the Evidence on NIRS-Derived Oxygenation in TBI
Sample Size and Patient Demographics
Distribution of NIRS Techniques
Appropriate Clinical Comparable Parameters
3.4.2. Autoregulation
Physiology of Autoregulation in TBI
Summary of the Evidence on NIRS-derived Autoregulation in TBI
Sample Size and Patient Demographics
Distribution of NIRS Techniques
Appropriate Clinical Comparable Parameter
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brazinova, A.; Rehorcikova, V.; Taylor, M.S.; Buckova, V.; Majdan, M.; Psota, M.; Peeters, W.; Feigin, V.; Theadom, A.; Holkovic, L. Epidemiology of Traumatic Brain Injury in Europe: A Living Systematic Review. J. Neurotrauma 2018. [Google Scholar] [CrossRef] [Green Version]
- Maas, A.I.R.; Menon, D.K.; Adelson, D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. The Lancet Neurology Commission Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research Executive summary The Lancet Neurology Commission. Lancet Neurol 2017, 16, 987–1048. [Google Scholar] [CrossRef] [Green Version]
- Van Dijck, J.T.J.M.; Dijkman, M.D.; Ophuis, R.H.; de Ruiter, G.C.W.; Peul, W.C.; Polinder, S. In-hospital costs after severe traumatic brain injury: A systematic review and quality assessment. PLoS ONE 2019, 14, e0216743. [Google Scholar] [CrossRef]
- Dismuke, C.E.; Walker, R.J.; Egede, L.E. Utilization and Cost of Health Services in Individuals with Traumatic Brain Injury. Glob. J. Health Sci. 2015, 7, 156–169. [Google Scholar] [CrossRef]
- Helmick, K.M.; Spells, C.A.; Malik, S.Z.; Davies, C.A.; Marion, D.W.; Hinds, S.R. Traumatic brain injury in the US military: Epidemiology and key clinical and research programs. Brain Imaging Behav. 2015, 9, 358–366. [Google Scholar] [CrossRef]
- Carney, N.; Ghajar, J.; Jagoda, A.; Bedrick, S.; Davis-O’Reilly, C.; Du Coudray, H.; Hack, D.; Helfand, N.; Huddleston, A.; Nettleton, T.; et al. Concussion guidelines step 1: Systematic review of prevalent indicators. Neurosurgery 2014, 75. [Google Scholar] [CrossRef] [Green Version]
- Corrigan, J.D.; Harrison-Felix, C.; Haarbauer-Krupa, J. Epidemiology of Traumatic Brain Injury. In Textbook of Traumatic Brain Injury; Silver, J.M., McAllister, T.W., Arciniegas, D.B., Eds.; American Psychiatric Association Publishing: Washington, DC, USA, 2018; pp. 3–24. ISBN 9781615371129. [Google Scholar]
- Rowson, B.; Rowson, S.; Duma, S.M. Biomechanical Forces Involved in Brain Injury. In Textbook of Traumatic Brain Injury; Silver, J.M., McAllister, T.W., Arciniegas, D.B., Eds.; American Psychiatric Association Publishing: Washington, DC, USA, 2018; pp. 25–39. ISBN 9781615371129. [Google Scholar]
- Williams, W.H.; Chitsabesan, P.; Fazel, S.; McMillan, T.; Hughes, N.; Parsonage, M.; Tonks, J. Traumatic brain injury: A potential cause of violent crime? Lancet Psychiatry 2018, 5, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, A.; Ayton, D.; Pritchard, E.; Tsindos, T.; O’brien, P.; Dr, M.K.; Braaf, S.; Berecki-Gisolf, J.; Hayman, J. Informing the Response to Recommendation 171 of the Victorian Royal Commission into Family Violence; Brain Injury Australia: Sydney, 2018; ISBN 978-0-6482640-1-9. [Google Scholar]
- Mian, M.; Shah, J.; Dalpiaz, A.; Schwamb, R.; Miao, Y.; Warren, K.; Khan, S. Shaken baby syndrome: A review. Fetal Pediatr. Pathol. 2015, 34, 169–175. [Google Scholar] [CrossRef]
- Stocker, R.A. Intensive Care in Traumatic Brain Injury Including Multi-Modal Monitoring and Neuroprotection. Med. Sci. 2019, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Herklots, M.W.; Moudrous, W.; Oldenbeuving, A.; Roks, G.; Mourtzoukos, S.; Schoonman, G.G.; Ganslandt, O. Prospective Evaluation of Noninvasive HeadSense Intracranial Pressure Monitor in Traumatic Brain Injury Patients Undergoing Invasive Intracranial Pressure Monitoring. World Neurosurg. 2017, 106, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-B. Multimodality Monitoring in the Neurointensive Care Unit: A Special Perspective for Patients with Stroke. J. Stroke 2013, 15, 99. [Google Scholar] [CrossRef] [Green Version]
- Mazzeo, A.T.; Gupta, D. Monitoring the injured brain. J. Neurosurg. Sci. 2018, 62, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, L.; Bösel, J. Noninvasive Neuromonitoring: Current Utility in Subarachnoid Hemorrhage, Traumatic Brain Injury, and Stroke. Neurocrit. Care 2017, 27, 122–140. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Lobo, D.; Bitot, V.; Couffin, S.; Escalard, S.; Mounier, R.; Cook, F. Prediction of Early Intracranial Hypertension After Severe Traumatic Brain Injury: A Prospective Study. World Neurosurg. 2019, 127, e1242–e1248. [Google Scholar] [CrossRef] [PubMed]
- Sokoloff, C.; Williamson, D.; Serri, K.; Albert, M.; Odier, C.; Charbonney, E.; Bernard, F. Clinical Usefulness of Transcranial Doppler as a Screening Tool for Early Cerebral Hypoxic Episodes in Patients with Moderate and Severe Traumatic Brain Injury. Neurocrit. Care 2019. [Google Scholar] [CrossRef]
- Wilde, E.A.; Little, D. Clinical Imaging. In Textbook of Traumatic Brain Injury; Silver, J.M., McAllister, T.W., Arciniegas, D.B., Eds.; American Psychiatric Association Publishing: Washington, DC, USA, 2018; ISBN 9781615371129. [Google Scholar]
- Roldan, M.; Abay, T.Y.; Kyriacou, P.A. Non-invasive techniques for multimodal monitoring in Traumatic Brain Injury (TBI): Systematic review and meta-analysis. J. Neurotrauma 2020, 37, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Jöbsis, F.F. Noninvasive, Infrared Monitoring of Cerebral and Myocardial Oxygen Sufficiency and Circulatory Parameters. Science 1977, 23, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Oxiplex, T.S. A non-invasive, real-time monitor of precise tissue oxygenation and hemoglobin concentration. Newt. Drive 2001, 6. Available online: http://www.iss.com/biomedical/instruments/oxiplexTS.html (accessed on 24 February 2020).
- Weigl, W.; Milej, D.; Janusek, D.; Wojtkiewicz, S.; Sawosz, P.; Kacprzak, M.; Gerega, A.; Maniewski, R.; Liebert, A. Application of optical methods in the monitoring of traumatic brain injury: A review. J. Cereb. Blood Flow Metab. 2016, 36, 1825–1843. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, F.; Khellaf, A.; Ku, J.C.; Donnelly, J.; Thelin, E.P.; Zeiler, F.A. Continuous Near-Infrared Spectroscopy Monitoring in Adult Traumatic Brain Injury. J. Neurosurg. Anesthesiol. 2019. [Google Scholar] [CrossRef]
- Karamzadeh, N.; Amyot, F.; Kenney, K.; Anderson, A.; Chowdhry, F.; Dashtestani, H.; Wassermann, E.M.; Chernomordik, V.; Boccara, C.; Wegman, E.; et al. A machine learning approach to identify functional biomarkers in human prefrontal cortex for individuals with traumatic brain injury using functional near-infrared spectroscopy. Brain Behav. 2016, 6. [Google Scholar] [CrossRef]
- Robertson, C.S.; Zager, E.L.; Narayan, R.K.; Handly, N.; Sharma, A.; Hanley, D.F.; Garza, H.; Maloney-Wilensky, E.; Plaum, J.M.; Koenig, C.H.; et al. Clinical evaluation of a portable near-infrared device for detection of traumatic intracranial hematomas. J. Neurotrauma 2010, 27, 1597–1604. [Google Scholar] [CrossRef]
- Robertson, C.S.; Gopinath, S.P.; Chance, B. A New Application for Near-Infrared Spectroscopy: Detection of Delayed Intracranial Hematomas after Head Injury. J. Neurotrauma 1995, 12, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.P.; Robertson, C.S.; Contant, C.F.; Narayan, R.K.; Grossman, R.G.; Chance, B. Early detection of delayed traumatic intracranial hematomas using near- infrared spectroscopy. J. Neurosurg. 1995, 83, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, S.P.; Robertson, C.S.; Grossman, R.G.; Chance, B. Near-infrared spectroscopic localization of intracranial hematomas. J. Neurosurg. 1993, 79, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.H.; Arabi, Y.M. Critical care management of severe traumatic brain injury in adults. Scand. J. Trauma. Resusc. Emerg. Med. 2012, 20, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitzan, M.; Nitzan, I.; Arieli, Y. The various oximetric techniques used for the evaluation of blood oxygenation. Sensors (Switzerland) 2020, 20, 1–28. [Google Scholar]
- Van de Graaff, K.M.; Rhees, R.W.; Palmer, S.L. Cardiovascular System: Blood. In Human Anatomy and Physiology; McGraw-Hill Education: New York, NY, USA, 2013; ISBN 9780071810791. [Google Scholar]
- Tortora, G.J.; Derrickson, B. The cardiovascular system: The blood. In Principles of Anatomy & Physiology; Wiley: Hoboken, NY, USA, 2014; pp. 693–719. [Google Scholar]
- Sen, A.N.; Gopinath, S.P.; Robertson, C.S. Clinical application of near-infrared spectroscopy in patients with traumatic brain injury: A review of the progress of the field. Neurophotonics 2016, 3, 031409. [Google Scholar] [CrossRef]
- Murkin, J.M.; Arango, M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br. J. Anaesth. 2009, 103, i3–i13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, Y.; Niwayama, M. Principles and Instrumentation. In Application of Near Infrared Spectroscopy in Biomedicine; Jue, T., Masuda, K., Eds.; Springer US: Boston, MA, USA, 2013; pp. 1–19. ISBN 978-1-4614-6252-1. [Google Scholar]
- Owen-Reece, H.; Smith, M.; Elwell, C.E.; Goldstone, J.C. Near infrared spectroscopy. Br. J. Anaesth. 1999, 82, 418–444. [Google Scholar] [CrossRef]
- Van der Zee, P.; Cope, M.; Arridge, S.R.; Essenpreis, M.; Potter, L.A.; Edwards, A.D.; Wyatt, J.S.; McCormick, D.C.; Roth, S.C.; Reynolds, E.O.R.; et al. Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. In Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1992; Volume 316, pp. 143–153. [Google Scholar]
- Choi, J.K.; Kim, J.M.; Hwang, G.; Yang, J.; Choi, M.G.; Bae, H.M. Time-Divided Spread-Spectrum Code-Based 400 fW-Detectable Multichannel fNIRS IC for Portable Functional Brain Imaging. IEEE J. Solid State Circuits 2016, 51, 484–495. [Google Scholar] [CrossRef]
- Abay, T.Y. Reflectance photoplethysmography for non-invasive monitoring of tissue perfusion. Ph.D. Thesis, University of London, London, UK, March 2016. [Google Scholar]
- Rolfe, P. In vivo near-infrared spectroscopy. Annu. Rev. Biomed. Eng. 2000, 2, 715–754. [Google Scholar] [CrossRef] [PubMed]
- Abay, T.Y.; Kyriacou, P.A. Reflectance Photoplethysmography as Non-Invasive Monitoring of Tissue Blood Perfusion. IEEE Trans. Biomed. Eng. 2015. [Google Scholar] [CrossRef] [PubMed]
- McNulty, J.; Born, M.; Pozos, R.S. Near-Infrared Spectroscopy (NIRS). In Springer Handbook of Medical Technology; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2011; pp. 423–438. [Google Scholar]
- Gagnon, R.E.; Macnab, A.J.; Gagnon, F.A.; Blackstock, D.; LeBlanc, J.G. Comparison of two spatially resolved NIRS oxygenation indices. J. Clin. Monit. Comput. 2002, 17, 385–391. [Google Scholar] [CrossRef]
- Ercole, A.; Gupta, A.K. Cerebral oxygenation. In Core Topics in Neuroanaesthesia and Neurointensive Care; Matta, B.F., Menon, D.K., Smith, M., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 72–84. ISBN 9780511977558. [Google Scholar]
- Ferrari, M.; Quaresima, V. Review: Near infrared brain and muscle oximetry: From the discovery to current applications. J. Near Infrared Spectrosc. 2012, 20, 1–14. [Google Scholar] [CrossRef]
- Van Essen, T.; Goos, T.G.; Van Ballegooijen, L.; Pichler, G.; Urlesberger, B.; Reiss, I.K.M.; De Jonge, R.C.J. Comparison of frequency-domain and continuous-wave near-infrared spectroscopy devices during the immediate transition. BMC Pediatr. 2020, 20, 94. [Google Scholar] [CrossRef] [PubMed]
- Veesa, J.D.; Dehghani, H. Functional near infrared spectroscopy using spatially resolved data to account for tissue scattering: A numerical study and arm-cuff experiment. J. Biophotonics 2019, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, F.; Tachtsidis, I. Clinical brain monitoring with time domain NIRS: A review and future perspectives. Appl. Sci. 2019, 9, 1612. [Google Scholar] [CrossRef] [Green Version]
- Okada, E. Photon migration in NIRS brain imaging. In Application of Near Infrared Spectroscopy in Biomedicine; Springer US: New York, NY, USA, 2013; pp. 37–58. ISBN 9781461462521. [Google Scholar]
- Watzman, H.M.; Kurth, C.D.; Montenegro, L.M.; Rome, J.; Steven, J.M. Arterial and Venous Contributions to Near-infrared Cerebral Oximetry | Anesthesiology | ASA Publications. Anesthesiology 2000, 93, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Vanderah, T.; Gould, D.; Nolte, J. Blood supply of the brain. In Nolte’s the human Brain: An Introduction to Its Functional Anatomy; Elsevier: Philadelphia, PA, USA, 2016; pp. 126–153. ISBN 978-1-4557-2859-6. [Google Scholar]
- Tortora, G.J.; Derrickson, B. The cardiovascular system: The heart. In Principles of Anatomy & Physiology; Wiley: Hoboken, NY, USA, 2014; pp. 720–760. [Google Scholar]
- Samraj, R.S.; Nicolas, L. Near infrared spectroscopy (NIRS) derived tissue oxygenation in critical illness. Clin. Investig. Med. 2015, 38, E285–E295. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, A.; Bhagat, H.; Grover, V. Jugular venous oximetry. J. Neuroanaesth. Crit. Care 2015, 02, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, G.; Hemphill, J.C.; Sorani, M.; Martin, C.; Morabito, D.; Obrist, W.D.; Manley, G.T. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit. Care Med. 2008, 36, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, N.; Patel, S.K.; Ojugbeli, A.; Nouri, A.; Shirani, P.; Grossman, A.W.; Cheng, J.; Zuccarello, M.; Prestigiacomo, C.J. Understanding the complex pathophysiology of idiopathic intracranial hypertension and the evolving role of venous sinus stenting: A comprehensive review of the literature. Neurosurg. Focus 2018, 45, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lifesciences, E. Edwards Lifesciences—the Leader in Heart Valves & Hemodynamic Monitoring. Available online: https://www.edwards.com/ (accessed on 12 August 2020).
- Lang, E.W.; Jaeger, M. Systematic and Comprehensive Literature Review of Publications on Direct Cerebral Oxygenation Monitoring. Open Crit. Care Med. J. 2013, 6, 1–24. [Google Scholar] [CrossRef]
- Ngwenya, L.B.; Burke, J.F.; Manley, G.T. Brain Tissue Oxygen Monitoring and the Intersection of Brain and Lung. Respiratory Care 2016, 61, 1232–1244. [Google Scholar] [CrossRef] [Green Version]
- Oddo, M.; Bösel, J.; Le Roux, P.; Menon, D.K.; Vespa, P.; Citerio, G.; Bader, M.K.; Brophy, G.M.; Diringer, M.N.; Stocchetti, N.; et al. Monitoring of Brain and Systemic Oxygenation in Neurocritical Care Patients. Neurocrit. Care 2014, 21, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, A.; Maekawa, T.; Soejima, Y.; Sadamitsu, D.; Yamamoto, M.; Matsushita, M.; Nakashima, K. Qualitative comparison of carbon dioxide-induced change in cerebral near-infrared spectroscopy versus jugular venous oxygen saturation in adults with acute brain disease | Ovid. Neurol. Crit. CARE 1995, 23, 1734–1738. [Google Scholar] [CrossRef]
- Kirkpatrick, P.J.; Smielewski, P.; Czosnyka, M.; Menon, D.K.; Pickard, J.D. Near-infrared spectroscopy use in patients with head injury. J. Neurosurg. 1995, 83, 963–970. [Google Scholar] [CrossRef]
- Lewis, S.B.; Myburgh, J.A.; Thornton, E.L.; Reilly, P.L. Cerebral oxygenation monitoring by near-infrared spectroscopy is not clinically useful in patients with severe closed-head injury: A comparison with jugular venous bulb oximetry | Ovid. Clin. Investig. 1996, 24, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Kampfl, A.; Pfausler, B.; Denchev, D.; Jaring, H.P.; Schmutzhard, E. Near Infrared Spectroscopy (NIRS) in Patients with Severe Brain Injury and Elevated Intracranial Pressure: A Pilot Study. Acta Neurochir. Suppl. 1997, 1997, 112–114. [Google Scholar] [CrossRef]
- Kerr, M.E.; Marion, D.; Orndoff, P.A.; Weber, B.B.; Sereika, S.M. Evaluation of near infrared spectroscopy in patients with traumatic brain injury. Adv. Exp. Med. Biol. 1998, 454, 131–137. [Google Scholar] [CrossRef]
- Ter Minassian, A.; Poirier, N.; Pierrot, M.; Menei, P.; Granry, J.C.; Ursino, M.; Beydon, L. Correlation between cerebral oxygen saturation measured by near-infrared spectroscopy and jugular oxygen saturation in patients with severe closed head injury. Anesthesiology 1999, 91, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, O.S.K.; Prowse, S.; Strong, A.J. Oscillations in the near-infrared signal in patients with severe head injury. Acta Neurochir. Suppl. 2002, 81, 135–137. [Google Scholar] [CrossRef]
- Brawanski, A.; Faltermeier, R.; Rothoerl, R.D.; Woertgen, C. Comparison of Near-Infrared Spectroscopy and Tissue Po 2 Time Series in Patients after Severe Head Injury and Aneurysmal Subarachnoid Hemorrhage. J. Cereb. Blood Flow Metab. 2002, 22, 605–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLeod, A.D.; Igielman, F.; Elwell, C.; Cope, M.; Smith, M. Measuring cerebral oxygenation during normobaric hyperoxia: A comparison of tissue microprobes, near-infrared spectroscopy, and jugular venous oximetry in head injury. Anesth. Analg. 2003, 97, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.N.; Durduran, T.; Frangos, S.; Edlow, B.L.; Buckley, E.M.; Moss, H.E.; Zhou, C.; Yu, G.; Choe, R.; Maloney-Wilensky, E.; et al. Noninvasive Measurement of Cerebral Blood Flow and Blood Oxygenation Using Near-Infrared and Diffuse Correlation Spectroscopies in Critically Brain-Injured Adults. Neurocrit. Care 2010, 12, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Leal-Noval, S.R.; Cayuela, A.; Arellano-Orden, V.; Marín-Caballos, A.; Padilla, V.; Ferrándiz-Millón, C.; Corcia, Y.; García-Alfaro, C.; Amaya-Villar, R.; Murillo-Cabezas, F.; et al. Invasive and noninvasive assessment of cerebral oxygenation in patients with severe traumatic brain injury. Intensive Care Med. 2010, 36, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Tachtsidis, I.; Tisdall, M.M.; Pritchard, C.; Leung, T.S.; Ghosh, A.; Elwell, C.E.; Smith, M. Analysis of the changes in the oxidation of brain tissue cytochrome-c-oxidase in traumatic brain injury patients during hypercapnoea: A broadband NIRS study. Adv. Exp. Med. Biol. 2011, 701, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Tachtsidis, I.; Kolyva, C.; Highton, D.; Elwell, C.; Smith, M. Normobaric Hyperoxia Does Not Change Optical Scattering or Pathlength but Does Increase Oxidised Cytochrome c Oxidase Concentration in Patients with Brain Injury. Adv. Exp. Med. Biol. 2013, 765, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, G.; Furmanov, A.; Itshayek, E.; Shoshan, Y.; Singh, V. Assessment of a noninvasive cerebral oxygenation monitor in patients with severe traumatic brain injury: Clinical article. J. Neurosurg. 2014, 120, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Vilke, A.; Bilskiene, D.; Šaferis, V.; Gedminas, M.; Bieliauskaite, D.; Tama&auskas, A.; Macas, A. Predictive value of early near-infrared spectroscopy monitoring of patients with traumatic brain injury. Medicina 2014. [Google Scholar] [CrossRef]
- Durnev, V.; Filipce, V.; Brzanov, A.G.; Mijovska, M.M.; Stevanovska, M.T. CEREBRAL OXYGENATION NON INVASIVE MONITORING IN TRAUMATIC BRAIN INJURY-A PILOT STUDY. Mac Med. Rev. 2017, 71, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Davies, D.J.; Clancy, M.; Dehghani, H.; Lucas, S.J.E.; Forcione, M.; Yakoub, K.M.; Belli, A. Cerebral oxygenation in traumatic brain injury: Can a non-invasive frequency domain near-infrared spectroscopy device detect changes in brain tissue oxygen tension as well as the established invasive monitor? J. Neurotrauma 2019, 36, 1175–1183. [Google Scholar] [CrossRef]
- Fantini, S.; Sassaroli, A. Frequency-Domain Techniques for Cerebral and Functional Near-Infrared Spectroscopy. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, K. Traumatic brain injury: Pathophysiology for neurocritical care. J. Intensive Care 2016, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calviello, L.A.; Donnelly, J.; Zeiler, F.A.; Thelin, E.P.; Smielewski, P.; Czosnyka, M. Cerebral autoregulation monitoring in acute traumatic brain injury: What’s the evidence? Minerva Anestesiol. 2017, 83, 844–857. [Google Scholar] [CrossRef]
- Fabregas, N.; Fernández-Candil, J. Hypercapnia. In Complications in Neuroanesthesia; Elsevier: Amsterdam, The Netherlands, 2016; pp. 157–168. [Google Scholar]
- Bor-Seng-Shu, E.; Kita, W.S.; Figueiredo, E.G.; Paiva, W.S.; Fonoff, E.T.; Teixeira, M.J.; Panerai, R.B. Cerebral hemodynamics: Concepts of clinical importance. Arq. Neuropsiquiatr. 2012, 70, 357–365. [Google Scholar] [CrossRef]
- Chiluwal, A.; Narayan, R.K.; Chaung, W.; Mehan, N.; Wang, P.; Bouton, C.E.; Golanov, E.V.; Li, C. Neuroprotective effects of trigeminal nerve stimulation in severe traumatic brain injury. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Tameemm, A.; Krovvidi, H. Cerebral physiology. Contin. Educ. Anaesthesia, Crit. Care Pain 2013, 13, 113–118. [Google Scholar] [CrossRef]
- Adelson, P.D.; Nemoto, E.; Colak, A.; Painter, M. The Use of Near Infrared Spectroscopy (NIRS) in Children after Traumatic Brain Injury: A Preliminary Report. Acta Neurochir. Suppl. 1998, 1998, 250–254. [Google Scholar] [CrossRef]
- Kreipke, C.W.; Rafols, J.A. Situating Cerebral Blood Flow in the Pathotrajectory of Head Trauma. In Cerebral Blood Flow, Metabolism, and Head Trauma: The Pathotrajectory of Traumatic Brain Injury; Springer: New York, NY, USA, 2013; pp. 29–51. ISBN 9781461441489. [Google Scholar]
- Berry, C.; Ley, E.J.; Bukur, M.; Malinoski, D.; Margulies, D.R.; Mirocha, J.; Salim, A. Redefining hypotension in traumatic brain injury. Injury 2012, 43, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.; Bhardwaj, A. Normal Intracranial Pressure Physiology. In Cerebrospinal Fluid in Clinical Practice; Morrison, B.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 19–25. ISBN 9781416029083. [Google Scholar]
- Ontario Neurotrauma Foundation. Guideline for Concussion/Mild Traumatic Brain Injury and Persistent Symptoms, Healthcare Professional Version Adults (18+ years of age) Third Edition; Ontario Neurotrauma Foundation: Toronto, ON, Canada, 2018. [Google Scholar]
- Guyton, A.C.; Hall, J.E. Cerebral Blood Flow, Cerebrospinal Fluid, and Brain Metabolism. In GUYTON AND HALL Textbook of Medical Physiology; Saunders: Philadelphia, PA, USA, 2011; pp. 764–771. ISBN 9781416045748. [Google Scholar]
- Dunham, C.M.; Sosnowski, C.; Porter, J.M.; Siegal, J.; Kohli, C. Correlation of noninvasive cerebral oximetry with cerebral perfusion in the severe head injured patient: A pilot study. J. Trauma 2002, 52, 40–46. [Google Scholar] [CrossRef]
- Zweifel, C.; Castellani, G.; Czosnyka, M.; Helmy, A.; Manktelow, A.; Carrera, E.; Brady, K.M.; Hutchinson, P.J.A.; Menon, D.K.; Pickard, J.D.; et al. Noninvasive monitoring of cerebrovascular reactivity with near infrared spectroscopy in head-injured patients. J. Neurotrauma 2010, 27, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Shafer, R.; Brown, A.; Taylor, C. Correlation between cerebral blood flow and oxygen saturation in patients with subarachnoid hemorrhage and traumatic brain injury. J. Neurointerventional Surg. 2010. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, E.; Diedler, J.; Kasprowicz, M.; Budohoski, K.P.; Haubrich, C.; Smielewski, P.; Outtrim, J.G.; Manktelow, A.; Hutchinson, P.J.; Pickard, J.D.; et al. Critical Thresholds for Cerebrovascular Reactivity After Traumatic Brain Injury. Neurocritical Care 2011. [Google Scholar] [CrossRef] [PubMed]
- Taussky, P.; O’Neal, B.; Daugherty, W.P.; Luke, S.; Thorpe, D.; Pooley, R.A.; Evans, C.; Hanel, R.A.; Freeman, W.D. Validation of frontal near-infrared spectroscopy as noninvasive bedside monitoring for regional cerebral blood flow in brain-injured patients. Neurosurg. Focus 2012, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.N.; Edlow, B.L.; Durduran, T.; Frangos, S.; Mesquita, R.C.; Levine, J.M.; Greenberg, J.H.; Yodh, A.G.; Detre, J.A. Continuous Optical Monitoring of Cerebral Hemodynamics During Head-of-Bed Manipulation in Brain-Injured Adults. Neurocritical Care 2014. [Google Scholar] [CrossRef] [Green Version]
- Highton, D.; Ghosh, A.; Tachtsidis, I.; Panovska-Griffiths, J.; Elwell, C.E.; Smith, M. Monitoring Cerebral Autoregulation After Brain Injury: Multimodal Assessment of Cerebral Slow-Wave Oscillations Using Near-Infrared Spectroscopy. Anesth Analg. 2015. [Google Scholar] [CrossRef] [Green Version]
- Bindra, J.; Pham, P.; Aneman, A.; Chuan, A.; Jaeger, M. Non-invasive Monitoring of Dynamic Cerebrovascular Autoregulation Using Near Infrared Spectroscopy and the Finometer Photoplethysmograph. Neurocrit. Care 2016. [Google Scholar] [CrossRef]
- Diedler, J.; Zweifel, C.; Budohoski, K.P.; Kasprowicz, M.; Sorrentino, E.; Haubrich, C.; Brady, K.M.; Czosnyka, M.; Pickard, J.D.; Smielewski, P. The limitations of near-infrared spectroscopy to assess cerebrovascular reactivity: The role of slow frequency oscillations. Anesth. Analg. 2011, 113, 849–857. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.W. Overview of Mild Brain Injury. In Textbook of Traumatic Brain Injury; Silver, J.M., McAllister, T.W., Arciniegas, D.B., Eds.; American Psychiatric Association Publishing: Washington, DC, USA, 2018; pp. 583–605. ISBN 9781615371129. [Google Scholar]
- Doğan, N.Ö. Bland-Altman analysis: A paradigm to understand correlation and agreement. Turkish J. Emerg. Med. 2018, 18, 139–141. [Google Scholar] [CrossRef] [PubMed]













| Author, Year | N (m:f) | Device (Type) | Output | Control | Conclusion |
|---|---|---|---|---|---|
| (Jöbsis, 1977) [21] | 1 (1:0) | Inhouse NIRS, non commercial (CW; S-D 13.3 cm) | Photon count | Paired measure: hyperventilation | Oxygen sufficiency can be monitored noninvasively. |
| (Tateishi et al., 1995) [62] | 9 (9:0) | NIRO-500, Hamamatsu Photonics, Hamamatsu, Japan. (CW; S-D 4 cm) | HbO2 | SjO2 | Cerebral HbO2 magnitude and direction, measured by NIRS, were similar to changes in invasive measurements of SjvO2. |
| (Kirkpatrick et al., 1995) [63] | 14 (12:2) | NIRO 1000, Hamamatsu Photonics U.K. Ltd., Enfield, UK. (CW; S-D 6 cm) | HbO2 | CPP, ICP and CBFSjO2 | A close correlation between NIRS signals and intracranial parameters strengthens the belief that the observed chromophore concentration changes are derived primarily from cerebral tissues. |
| (Lewis et al., 1996) [64] | 10 (6:4) | INVOS 3100, Medtronic, Minneapolis, MN, USA. (SRS; S-D 3&4 cm) | rSO2 | SjO2 | Tissue oxygen saturation determined by near-infrared spectroscopy does not reflect significant changes in cerebral oxygenation detected by the global measurement of jugular venous bulb oximetry. |
| (Kampfl et al., 1997) [65] | 8 (5:3) | INVOS 3100A, Medtronic, Minneapolis, MN, USA. (SRS; S-D 3&4 cm) | rSO2 | ICP | rSO2 values in patients with an ICP > 25 mmHg were significantly lower than in patients with an ICP < 25 mmHg after the hyperoxygenation period. |
| (Kerr et al., 1998) [66] | 28 (25:3) | INVOS 3100A, Medtronic, Minneapolis, MN, USA. (SRS; S-D 3&4 cm) | rSO2 | SaO2, SjvO2, extracranial SO2 | rSO2 index represented a summation of differential weighting of SaO2 (20%), SjvO2 (75%), and extracranial O2 saturation (5%). |
| (Ter Minassian et al., 1999) [67] | 9 (NR) | INVOS 3100, Medtronic, MN, USA. (SRS; S-D 3&4 cm) | rSO2 | SjO2 | rSO2 assessed by NIRS does not adequately reflect changes in SjvO2 in patients with a severe head injury. |
| (Cheng et al., 2002) [68] | 9 (7:2) | CCD detector system, noncommercial John Wright, UK. (CW; S-D 3.5 cm) | HbO2 oscillations | NR | The presence of oscillations at 0.013–0.033, 0.11, and 0.19–0.28 Hz are compatible with B-waves, vasomotion, and respiratory cycles, respectively. |
| (Brawanski et al., 2002) [69] | 12 (11:1) | INVOS 3100, Medtronic, Minneapolis, MN, USA. (SRS; S-D 3&4 cm) | rSO2 | PbtO2 | rSO2 and PbtO2 contain similar information from a mathematical point of view. |
| (McLeod et al., 2003) [70] | 8 (8:0) | NIRO 300, Hamamatsu Photonics, Hamamatsu City, Japan. (SRS; S-D 4 cm) | TOI | SjO2 and PbtO2 | Altering the fraction of inspired oxygen changes significantly each variable measured of cerebral oxygenation. Each variable represents a different physiologic process. |
| (Kim et al., 2010) [71] | 8 (5:3) | Inhouse, noncommercial (CW; S-D 2.5 cm) | DCS and HbO2 | XeCT | Significant moderate correlations between DCS measurements of relative CBF and NIRS measurements of delta HbO2 were demonstrated. |
| (Leal-Noval et al., 2010) [72] | 22 (NR) | INVOS 5100, Medtronics Inc., MI, USA. (CW; NR) | rSO2 | PbtO2 | PbrO2 and rSO2 were directly and significantly related. However, the diagnostic accuracy of rSO2 was limited, therefore, that measurement by NIRS should not be considered to be an acceptable substitute for PbrO2. |
| (Tachtsidis et al., 2011) [73] | 6 (5:1) | Broadband NIRS, noncommercial (CW; S-D 3.5 cm) | oxCCO | Paired measure: hypercapnia | Despite the increase in total HbO2 in all patients, only four of the six patients showed an increase in the oxidation states. |
| (Ghosh et al., 2013) [74] | 10 (3:7) | Inhouse, noncommercial (SRS; S-D 3.5 cm) | oxCCO | Paired measure: normobaric hyperoxia | Optical measurement of chromophore concentration in the injured brain is not confounded by changes in optical scattering or pathlength. |
| (Rosenthal et al., 2014) [75] | 18 (13:5) | CerOx 3110, Ornim Medical Ltd. Dedham, MA, USA. (UT-NIRS; NR) | rSO2 | SjO2 PbtO2 | The correlation between UT-NIRS measurements and SjvO2 indicate that the CerOx may be able to provide a noninvasive estimation of cerebral oxygenation status in brain-injured patients. However, rSO2 was not correlated with PbtO2. |
| (Vilke et al., 2014) [76] | 61 (42:19) | INVOS, Medtronics Inc., MI, USA. (SRS; NR) | rSO2 | Mortality | rSO2 values were determined as a strong discriminator and predictor of hospital mortality. When rSO2 < 68.0% in the left hemisphere HR = 17.7. |
| (Durnev et al., 2017) [77] | 15 (10:5) | INVOS 5100, Medtronics Inc., MI, USA. (CW; NR) | rSO2 | Paired measure: changes on MAP | NIRS signals of cerebral hypoxygenation reacted first to MAP changes. |
| (Davies et al., 2019) [78] | 16 (9:3) | Oxiplex TS, ISS, Il, USA. (PMS; NR) | rSO2 | PbtO2 | A clear predictive relationship between NIRS and invasively measured PbtO2 has been established. However, FD enhances NIRS device tested did not demonstrate sufficient reproducibility in its ability to predict changes in PbtO2 to replace the current invasive gold standard. |
| Author, Year | N (m:f) | Device (Type) | Output | Control | Conclusion |
|---|---|---|---|---|---|
| (Adelson et al., 1998) [86] | 10 (6:4) | INV03100A, Medtronic, MN, USA and NIRO500, Hamamatsu Photonics, Hamamatsu, Japan. (SRS and CW respectively; S-D NR) | THb, HbO2, Hb, and rSO2 | CPP, MAP, ICP and PaCO2 | High ICP and decreased CPP correlated with increased THb and HbO2 indicating raised CBV and hyperemia. MAP was not associative. NIRS positively predicted cerebral oxygen desaturations with hyperventilation. |
| (Dunham et al., 2002) [92] | 4 (3:1) | INVOS 4100, Medtronic, MN, USA. (SRS; S-D NR) | rSO2 | CPP, MAP, ICP | Cerebral oximetry correlated significantly with CPP. As such, it could be an adjunct to CPP management. |
| (Zweifel et al., 2010) [93] | 40 (31:9) | NIRO 200, Hamamatsu Photonics U.K. Ltd., Hertfordshire, UK. (SRS; S-D NR) | THx | PRx | THx showed a significant correlation with the validated volume reactivity index PRx. |
| (Shafer et al., 2010) [94] | 22 (10:12) | INVOS 5100, Medtronic, MN, USA. (CW; S-D NR) | rSO2 | XeCT | The relationship between either the left or right NIRS values and Xe/CT scan was not significant. |
| (Diedler et al., 2011) [95] | 37 (NR) | NIRO 200, Hamamatsu Photonics U.K. Ltd., Hertfordshire, UK. (SRS; S-D NR) | THx | PRx | The agreement between PRx and THx is a function of the power of slow oscillations in the input signals. |
| (Taussky et al., 2012) [96] | 8 (2:6) | Bifrontal NIRS optodes, Casmed, Branford, CT, USA. (CW; S-D 4.5 cm) | rSO2 | CBF | CT perfusion CBF has a significant linear correlation with NIRS derived rSO2. |
| (Kim et al., 2014) [97] | 10 (7:3) | Inhouse, DCS and NIRS system, Noncommercial. (SRS; S-D 2.5 cm) | CBF, ΔHbO2, ΔHb and ΔTHb in 10 TBI patients | CBF, ΔHbO2, ΔHb and THb in 10 healthy controls | HbO2, Hb, and THb concentration increased significantly in the brain-injured cohort with head-of-bed lowering. Accordingly, DCS/NIRS hybrid device is well-suited to provide non- invasive, continuous hemodynamic monitoring. |
| (Highton et al., 2015) [98] | 27 (13:14) | NIRO 100, Hamamatsu Photonics U.K. Ltd., Hertfordshire, UK. (SRS; S-D 4 cm) | THx, TOx | PRx, Mx | Significant agreement among PRx and THx, and between Mx and TOx. However, the strength of the interrelationship between ICP or TCD and NIRS signals, THI or rSo2, limits the degree of agreement between these reactivity indices. |
| (Bindra et al., 2015) [99] | 19 (12:7) | ForeSight, Casmed, Connecticut, USA. (CW; S-D NR) | nTOx | iTOx | nTOx from Finometer photoplethysmography and NIRS gives a similar measurement of cerebrovascular autoregulation to iTOx. |
STRENGTHS OF NIRS
|
LIMITATIONS OF NIRS
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roldán, M.; Kyriacou, P.A. Near-Infrared Spectroscopy (NIRS) in Traumatic Brain Injury (TBI). Sensors 2021, 21, 1586. https://doi.org/10.3390/s21051586
Roldán M, Kyriacou PA. Near-Infrared Spectroscopy (NIRS) in Traumatic Brain Injury (TBI). Sensors. 2021; 21(5):1586. https://doi.org/10.3390/s21051586
Chicago/Turabian StyleRoldán, María, and Panayiotis A. Kyriacou. 2021. "Near-Infrared Spectroscopy (NIRS) in Traumatic Brain Injury (TBI)" Sensors 21, no. 5: 1586. https://doi.org/10.3390/s21051586
APA StyleRoldán, M., & Kyriacou, P. A. (2021). Near-Infrared Spectroscopy (NIRS) in Traumatic Brain Injury (TBI). Sensors, 21(5), 1586. https://doi.org/10.3390/s21051586







