Potentiometric Response of Solid-State Sensors Based on Ferric Phosphate for Iron(III) Determination
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. XRD Characterization
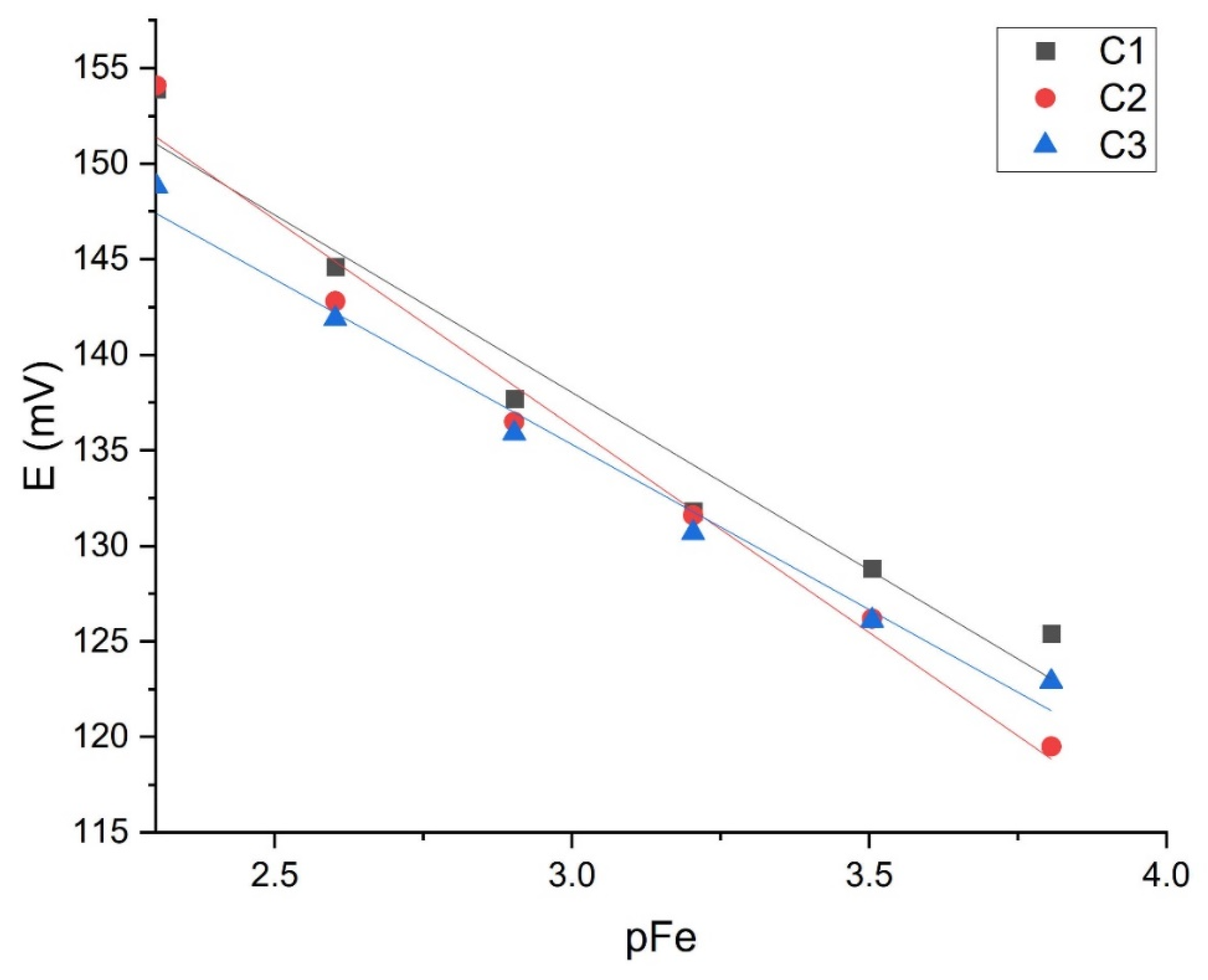
3.2. Membrane Testing Results
3.3. Electrode Selectivity
3.4. Determination of Iron(III) in Pharmaceuticals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Prkic, A.; Vukusic, T.; Giljanovic, J.; Sokol, V.; Boskovic, P.; Lavcevic, M.L.; Mitar, I.; Jakic, M. Development of a New Potentiometric Sensor based on home made Iodide ISE Enriched with ZnO Nanoparticles and its Application for Determination of Penicillamine. Int. J. Electrochem. Sci. 2018, 13, 10894–10903. [Google Scholar] [CrossRef]
- Prkic, A.; Vukusic, T.; Mitar, I.; Giljanovic, J.; Sokol, V.; Boskovic, P.; Jakic, M.; Sedlar, A. New sensor based on AgCl containing Iron Oxide or Zinc Oxide Nanoparticles for Chloride Determination. Int. J. Electrochem. Sci. 2019, 14, 861–874. [Google Scholar] [CrossRef]
- Shao, Y.Z.; Ying, Y.B.; Ping, J.F. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Ozer, T.; Isildak, I. A New Fe (III)-Selective Membrane Electrode Based on Fe (II) Phthalocyanine. J. Electrochem. Sci. Technol. 2019, 10, 321–328. [Google Scholar] [CrossRef]
- Burguera, J.L.; Burguera, M. Flow-Injection Spectrophotometry Followed by Atomic-Absorption Spectrometry for the Determination of Iron(Ii) and Total Iron. Anal. Chim. Acta 1984, 161, 375–379. [Google Scholar] [CrossRef]
- Carbonell, V.; Sanz, A.; Salvador, A.; de la Guardia, M. Flow injection flame atomic spectrometric determination of aluminium, iron, calcium, magnesium, sodium and potassium in ceramic material by on-line dilution in a stirred chamber. J. Anal. At. Spectrom. 1991, 6, 233–238. [Google Scholar] [CrossRef]
- Vanhoe, H.; Vandecasteele, C.; Versieck, J.; Dams, R. Determination of Iron, Cobalt, Copper, Zinc, Rubidium, Molybdenum, and Cesium in Human-Serum by Inductively Coupled Plasma Mass-Spectrometry. Anal. Chem. 1989, 61, 1851–1857. [Google Scholar] [CrossRef]
- Samadi, A.; Amjadi, M. Halloysite Nanotubes as a New Adsorbent for Solid Phase Extraction and Spectrophotometric Determination of Iron in Water and Food Samples. J. Appl. Spectrosc. 2016, 83, 422–428. [Google Scholar] [CrossRef]
- Jha, A.R.; Mishra, R.K. Solvent-Extraction of the Thiocyanato Mixed-Ligand Complexes of Iron(III) with Various Hydroxyamidines and Spectrophotometric Determination of Iron(III) in Various Biochemical and Biological Samples. Analyst 1981, 106, 1150–1156. [Google Scholar] [CrossRef]
- Isildak, I.; Attar, A.; Demir, E.; Kemer, B.; Aboul-Enein, H.Y. A Novel all Solid-State Contact PVC-Membrane Beryllium-Selective Electrode Based on 4-Hydroxybenzo-15-Crown-5 Ether Ionophore. Curr. Anal. Chem. 2018, 14, 43–48. [Google Scholar] [CrossRef]
- Mizani, F.; Ganjali, M.R.; Faridbod, F.; Esmaeilnia, S. A Novel Iron(III) Selective Potentiometric Sensor Based on 9-Ethylacenaphtho [1, 2-B]Quinoxaline. Int. J. Electrochem. Sci. 2013, 8, 10473–10486. [Google Scholar]
- Bita, S.; Sadati, S.O.; Soleymanpour, A.; Amouzad, F. Highly Selective Solid Contact Sensor for Low Level Concentration Measurements of Iron(III) in Pharmaceutical and Biological Media. J. Anal. Chem. 2018, 73, 1202–1208. [Google Scholar] [CrossRef]
- Yari, A.; Bagheri, M.; Ghazizadeh, M. A Novel Iron(III) Potentiometric Sensor Based on (E)-N ’-((2-hydroxynaphthalen-3-yl)methylene)benzohydrazide. Int. J. Electrochem. Sci. 2016, 11, 6597–6608. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Shoaei, I.S.; Monadi, N. A novel ion selective membrane potentiometric sensor for direct determination of Fe(III) in the presence of Fe(II). Talanta 2004, 64, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Ekmekci, G.; Uzun, D.; Somer, G.; Kalayci, S. A novel iron(III) selective membrane electrode based on benzo-18-crown-6 crown ether and its applications. J. Membr. Sci. 2007, 288, 36–40. [Google Scholar] [CrossRef]
- Vlascici, D.; Fagadar-Cosma, E.; Popa, I.; Chiriac, V.; Gil-Agusti, M. A Novel Sensor for Monitoring of Iron(III) Ions Based on Porphyrins. Sensors 2012, 12, 8193–8203. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sethi, B.; Upadhyay, N.; Kumar, S.; Singh, R.; Singh, L.P. Iron (III) Selective Electrode Based on S-Methyl N-(Methylcarbamoyloxy) Thioacetimidate as a Sensing Material. Int. J. Electrochem. Sci. 2011, 6, 650–663. [Google Scholar]
- Fakhari, A.R.; Alaghemand, M.; Shamsipur, M. Iron(III)-selective membrane potentiometric sensor based on 5,10,15,20-tetrakis(pentafluorophenyl)-21H, 23H-porphyrin. Anal. Lett. 2001, 34, 1097–1106. [Google Scholar] [CrossRef]
- Saber, A.L.; Hameed, A.M.; Sayqal, A.A.; Alessa, H.; Alharbi, A. Iron-selective Poly(Vinyl Chloride) Membrane Electrode Based on Norfloxacin as a Neutral Carrier. Int. J. Electrochem. Sci. 2018, 13, 10076–10087. [Google Scholar] [CrossRef]
- Ozer, T.; Isildak, I. Potentiometric Studies of a New Solid-state Contact Iron(III)-Selective Electrode Based on Morin-Fe2+ Shiff Base Complex. Int. J. Electrochem. Sci. 2018, 13, 11375–11387. [Google Scholar] [CrossRef]
- Ali, T.A.; Mahmoud, W.H.; Mohamed, G.G. Construction and characterization of nano iron complex ionophore for electrochemical determination of Fe(III) in pure and various real water samples. Appl. Organomet. Chem. 2019, 33. [Google Scholar] [CrossRef]
- Ali, T.A.; Mohamed, G.G.; El-Dessouky, M.M.I.; Abou El Ella, S.M.; Mohamed, R.T.F. Modified Carbon Paste Ion Selective Electrodes for the Determination of Iron (III) in Water, Soil and Fish Tissue Samples. Int. J. Electrochem. Sci. 2013, 8, 1469–1486. [Google Scholar]
- Duzgun, E.; Tastekin, M.; Atakol, O. A new modified Fe(III)-selective solid membrane electrode. Rev. Anal. Chem. 2008, 27, 83–90. [Google Scholar] [CrossRef]
- Prkic, A.; Giljanovic, J.; Bralic, M. Direct Potentiometric Determination of N-acetyl-L-cysteine (NAC) in Real Samples by Using “home made” Iodide ISE. Int. J. Electrochem. Sci. 2011, 6, 5388–5395. [Google Scholar]
- Bralić, M.; Prkić, A.; Radić, J.; Pleslić, I. Preparation of Phosphate Ion-Selective Membrane Based on Silver Salts Mixed with PTFE or Carbon Nanotubes. Int. J. Electrochem. Sci. 2018, 13, 1390–1399. [Google Scholar] [CrossRef]
- Umezawa, Y.; Umezawa, K.; Sato, H. Selectivity Coefficients for Ion-Selective Electrodes—Recommended Methods for Reporting K-a,B(Pot) Values—(Technical Report). Pure Appl. Chem. 1995, 67, 507–518. [Google Scholar] [CrossRef]










| Sensor Name | Membrane Composition Ratio | ||
|---|---|---|---|
| FePO4 | Ag2S | PTFE | |
| M1 | 1 | 1 | 2 |
| M2 | 1 | 2 | 3 |
| M3 | 1 | 3 | 4 |
| M4 | 1 | 4 | 5 |
| M5 | 1 | 5 | 6 |
| M6 | 1 | 4 | 3.33 |
| M7 | 1 | 4 | 2.14 |
| M8 | 1 | 4 | 1.25 |
| M9 | 1 | 4 | 0.56 |
| M10 | 1 | 4 | 0 |
| M11 | 1 | 1 | 0 |
| M12 | 2 | 1 | 0 |
| M13 | 3 | 1 | 0 |
| M14 | 1 | 0 | 0 |
| M15 | 1 | 0 | 1 |
| Testing Solution | Linear Curve No. | Slope ± SD | LOD | LOQ | R2 |
|---|---|---|---|---|---|
| FeCl3 (pH = 1.00) | C1 | −20.525 ± 1.56 | 2.64 × 10−5 | 8.97 × 10−5 | 0.9720 |
| C2 | −20.528 ± 0.63 | 2.41 × 10−5 | 3.97 × 10−5 | 0.9925 | |
| C3 | −16.948 ± 0.72 | 1.31 × 10−5 | 2.63 × 10−5 | 0.9836 | |
| Fe(NO3)3 (pH = 1.00) | C1 | −18.271 ± 1.57 | 1.13 × 10−4 | 4.52 × 10−4 | 0.9783 |
| C2 | −18.755 ± 1.01 | 4.54 × 10−4 | 1.08 × 10−3 | 0.9885 | |
| C3 | −13.587 ± 0.54 | 1.55 × 10−4 | 8.20 × 10−4 | 0.9954 |
| Testing Solution | FeCl3 (pH = 1.00) | Fe(NO3)3 (pH = 1.00) | ||
|---|---|---|---|---|
| Sensor | Slope/mV dec−1 | R2 | Slope/mV dec−1 | R2 |
| M2 | 11.49 | 0.9306 | −4.95 | 0.7662 |
| M3 | 3.09 | 0.3982 | 18.38 | 0.9176 |
| M4 | 11.46 | 0.9900 | 17.47 | 0.4835 |
| M5 | 24.68 | 0.7874 | - | - |
| M6 | 11.27 | 0.9740 | 5.71 | 0.9881 |
| M7 | −4.42 | 0.0384 | 13.09 | 0.9496 |
| M8 | 20.56 | 0.9813 | - | - |
| M9 | 34.91 | 0.9193 | 1.69 | 0.8627 |
| M10 | 22.00 | 0.9612 | 3.29 | 0.8586 |
| M11 | - | - | 5.81 | 0.8828 |
| M12 | - | - | 17.19 | 0.9744 |
| M13 | −9.01 | 0.9392 | −27.74 | 0.8946 |
| M14 | –6.48 | 0.2376 | - | - |
| M15 | −6.91 | 0.9397 | −11.02 | 0.9221 |
| Intraday Results | Interday Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Curve | Slope ± SD/ mV dec−1 | R2 | LOD/ mol L−1 | LOQ/ mol L−1 | Curve | Slope ± SD/ mV dec−1 | R2 | LOD/ mol L−1 | LOQ/ mol L−1 |
| C1 | −18.58 ± 1.99 | 0.9563 | 3.27 × 10−4 | 1.83 × 10−3 | C1 (day 1) | −18.53 ± 1.83 | 0.9761 | 1.21 × 10−4 | 3.40 × 10−4 |
| C2 | −19.421 ± 1.70 | 0.9631 | 1.43 × 10−4 | 5.86 × 10−4 | C2 (day 2) | −24.78 ± 1.87 | 0.9724 | 1.31 × 10−4 | 4.43 × 10−4 |
| C3 | −17.28 ± 1.04 | 0.9855 | 2.38 × 10−4 | 6.31 × 10−4 | C3 (day 3) | −19.421 ± 1.7 | 0.9631 | 1.43 × 10−4 | 5.86 × 10−4 |
| Interfering Species (B) | |
|---|---|
| Al3+ | −0.74 |
| Ba2+ | −1.16 |
| Ca2+ | <−1.45 |
| Mg2+ | −0.82 |
| Added m(Fe3+)/mg | Determined m(Fe3+)/mg | Recovery (%) |
|---|---|---|
| 0.84 | 0.85 | 101.2 |
| 1.68 | 1.65 | 98.2 |
| 8.37 | 8.28 | 98.9 |
| Pharmaceutical | Determined m(Fe3+) by M1/mg | Determined m(Fe3+) by UV/VIS/mg | Recovery (%) |
|---|---|---|---|
| Tardyferon | 0.925 | 0.938 | 98.6 |
| Heferol | 1.283 | 1.205 | 106.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paut, A.; Prkić, A.; Mitar, I.; Bošković, P.; Jozić, D.; Jakić, M.; Vukušić, T. Potentiometric Response of Solid-State Sensors Based on Ferric Phosphate for Iron(III) Determination. Sensors 2021, 21, 1612. https://doi.org/10.3390/s21051612
Paut A, Prkić A, Mitar I, Bošković P, Jozić D, Jakić M, Vukušić T. Potentiometric Response of Solid-State Sensors Based on Ferric Phosphate for Iron(III) Determination. Sensors. 2021; 21(5):1612. https://doi.org/10.3390/s21051612
Chicago/Turabian StylePaut, Andrea, Ante Prkić, Ivana Mitar, Perica Bošković, Dražan Jozić, Miće Jakić, and Tina Vukušić. 2021. "Potentiometric Response of Solid-State Sensors Based on Ferric Phosphate for Iron(III) Determination" Sensors 21, no. 5: 1612. https://doi.org/10.3390/s21051612
APA StylePaut, A., Prkić, A., Mitar, I., Bošković, P., Jozić, D., Jakić, M., & Vukušić, T. (2021). Potentiometric Response of Solid-State Sensors Based on Ferric Phosphate for Iron(III) Determination. Sensors, 21(5), 1612. https://doi.org/10.3390/s21051612









