Plasmonic Nanoparticles as Optical Sensing Probes for the Detection of Alzheimer’s Disease
Abstract
:1. Introduction
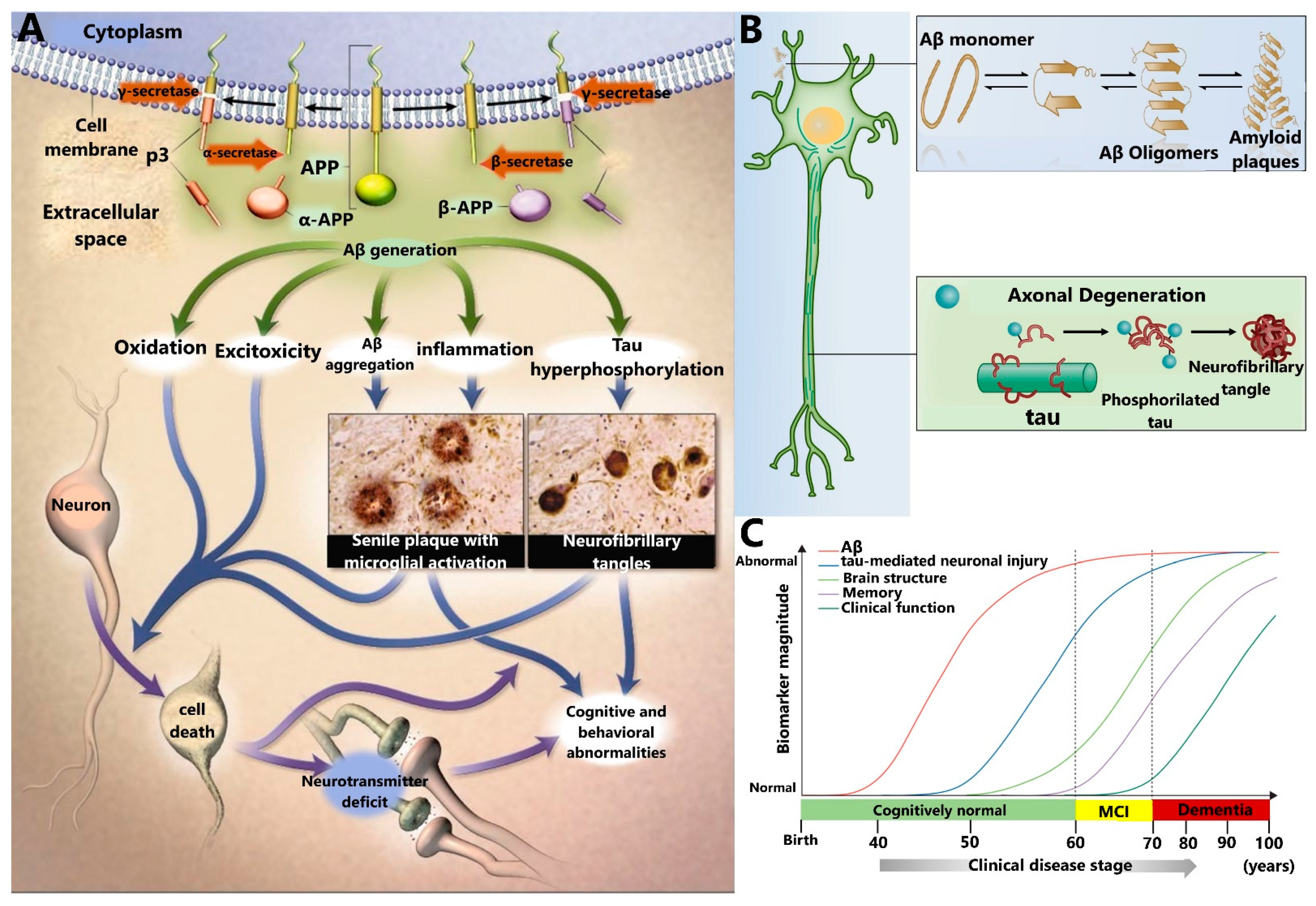
2. Relevant AD Biomarkers
2.1. Amyloid-β Peptide (Aβ)
2.2. Tau Protein
2.3. Other AD Biomarkers
3. Optical Properties of Plasmonic Metal Nanoparticles
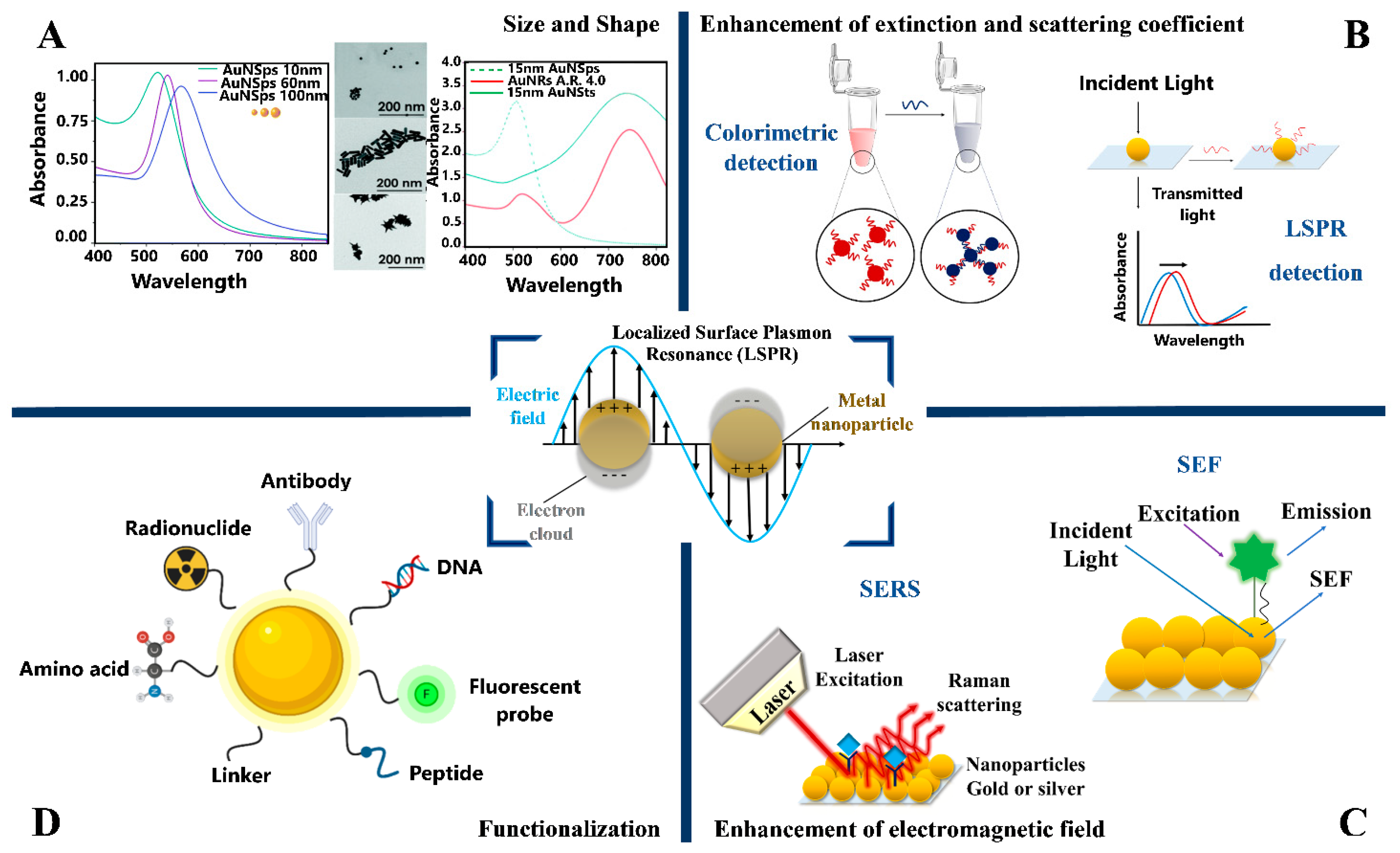
4. Plasmonic Biosensors for In Vitro Optical Detection of AD Biomarkers
4.1. Surface-Enhanced Raman Scattering (SERS) Detection
4.1.1. SERS Biosensors for Aβ Detection
4.1.2. SERS Biosensors for Tau Detection
4.1.3. SERS Biosensors for the Simultaneous Detection of Aβ and Tau
4.1.4. SERS Biosensors for Other Biomarkers
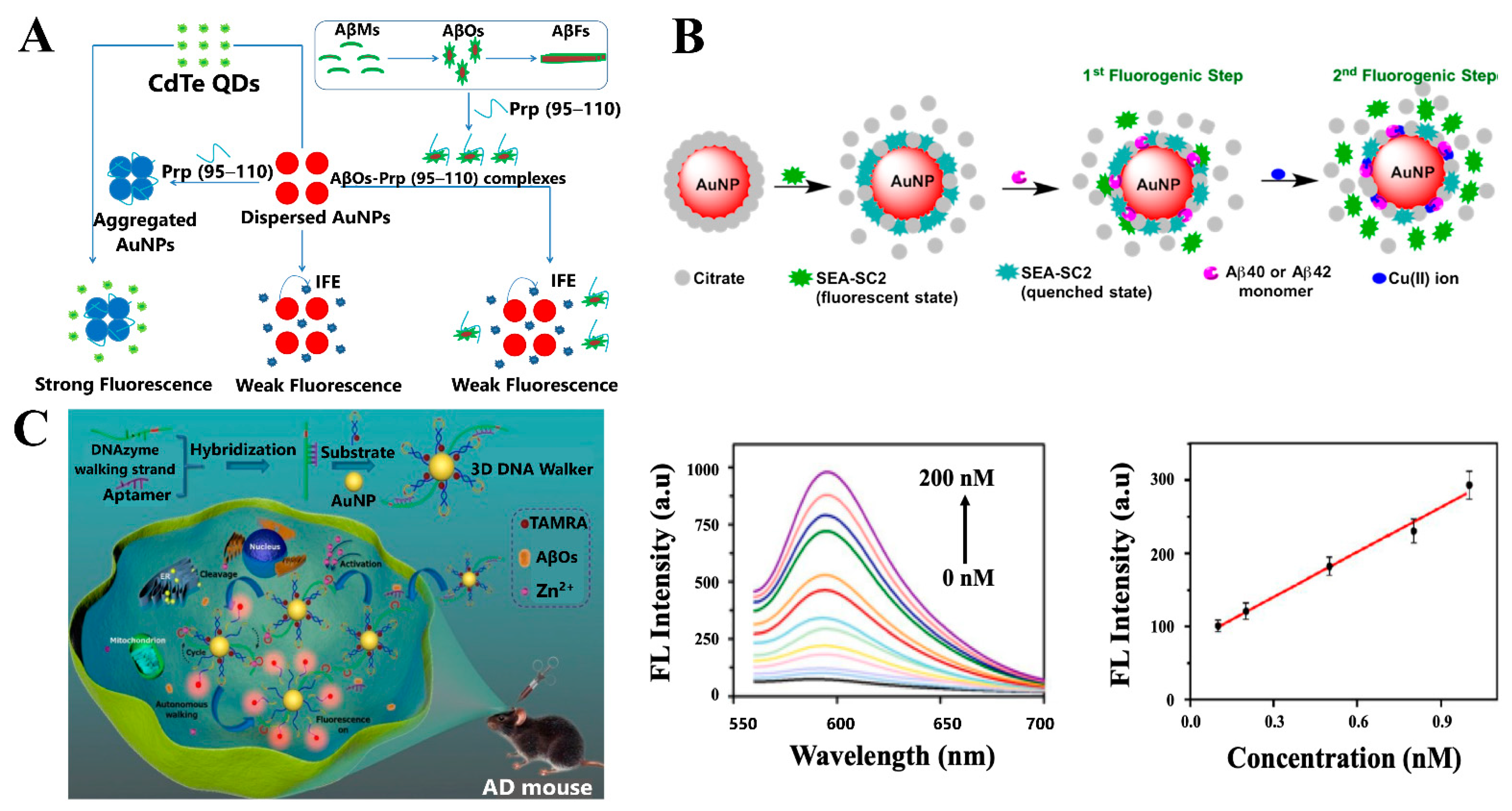
4.2. Fluorescence-Based Sensing Platforms
4.2.1. Fluorescent Biosensors for Aβ Detection
4.2.2. Fluorescent Biosensors for Other AD Biomarkers
4.3. Colorimetric Detection
4.3.1. Colorimetric Biosensors for Aβ Detection
4.3.2. Colorimetric Biosensors for the Detection of Other AD Biomarkers
4.4. Localized Surface Plasmon Resonance Detection Platforms
4.4.1. LSPR Biosensors for Aβ Detection
4.4.2. LSPR Biosensors for Tau Detection
4.4.3. LSPR Biosensors for the Simultaneous Detection of Aβ and Tau
4.4.4. LSPR Biosensors for Kinetic Studies of Aβ Aggregation
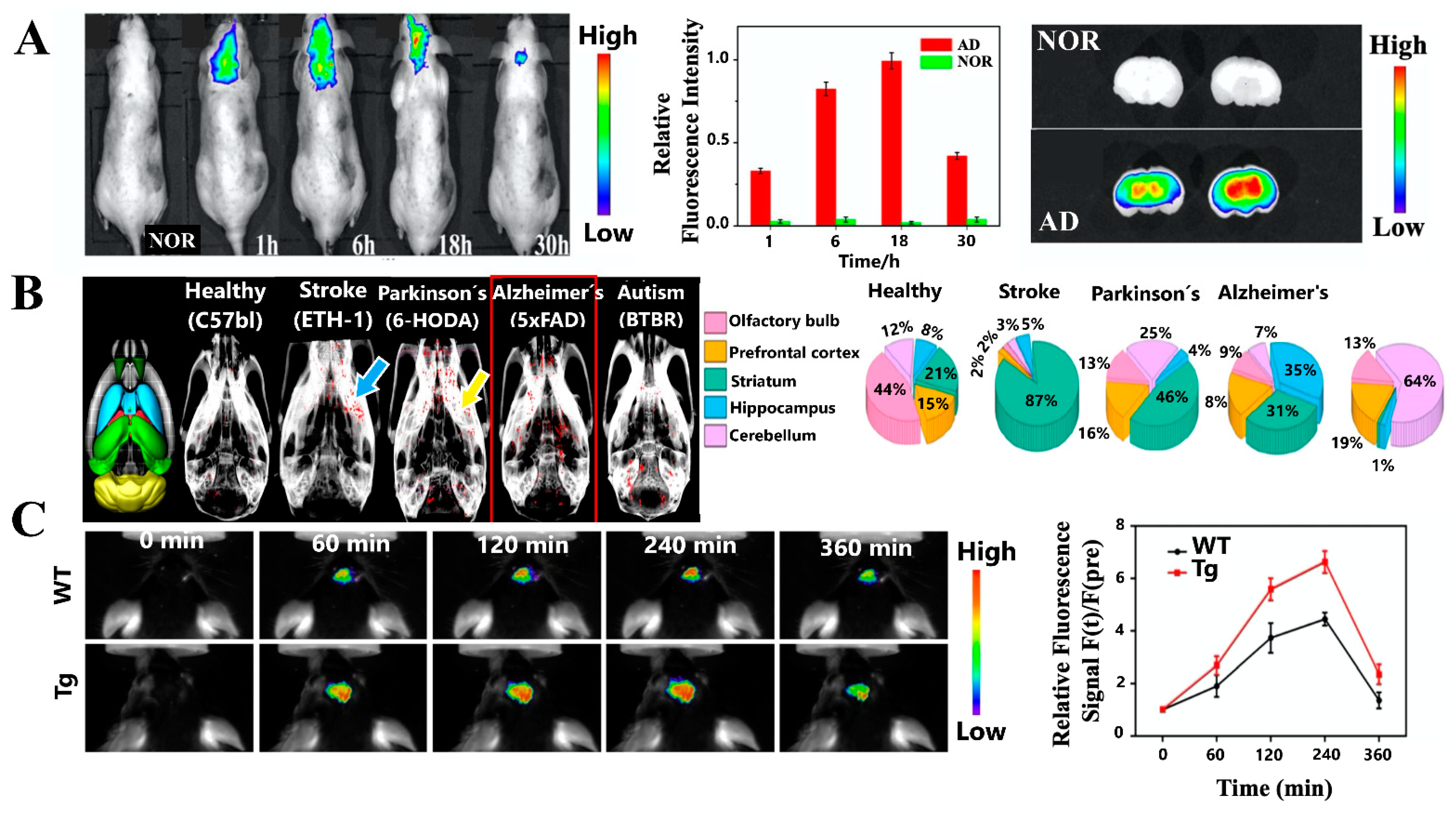
5. In Vivo Imaging Detection Platforms of AD Using Plasmonic Nanoparticles
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wenk, G.L. Neuropathologic changes in Alzheimer’s disease. J. Clin. Psychiatry 2003, 64, 7–10. [Google Scholar] [PubMed]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Carneiro, P.; Morais, S.; Pereira, M.C. Nanomaterials towards biosensing of Alzheimer’s disease biomarkers. Nanomaterials 2019, 9, 1663. [Google Scholar] [CrossRef] [Green Version]
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Ameri, M.; Shabaninejad, Z.; Movahedpour, A.; Sahebkar, A.; Mohammadi, S.; Hosseindoost, S.; Ebrahimi, M.S.; Savardashtaki, A.; Karimipour, M.; Mirzaei, H. Biosensors for detection of Tau protein as an Alzheimer’s disease marker. Int. J. Biol. Macromol. 2020, 162, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Zetterberg, H.; Fagan, A.M. Fluid biomarkers in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezabakhsh, A.; Rahbarghazi, R.; Fathi, F. Surface plasmon resonance biosensors for detection of Alzheimer’s biomarkers; an effective step in early and accurate diagnosis. Biosens. Bioelectron. 2020, 167, 112511. [Google Scholar] [CrossRef]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.S.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, S.; Delaby, C.; Boursier, G.; Catteau, C.; Ginestet, N.; Tiers, L.; Maceski, A.; Navucet, S.; Paquet, C.; Dumurgier, J.; et al. Relevance of Aβ42/40 Ratio for Detection of Alzheimer Disease Pathology in Clinical Routine: The PLM(R) Scale. Front. Aging Neurosci. 2018, 10, 138. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein e and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Liu, C.C.; Qiao, W.; Bu, G. Apolipoprotein E, Receptors, and Modulation of Alzheimer’s Disease. Biol. Psychiatry 2018, 83, 347–357. [Google Scholar] [CrossRef]
- Carneiro, P.; Morais, S.; do Carmo Pereira, M. Biosensors on the road to early diagnostic and surveillance of Alzheimer’s disease. Talanta 2020, 211, 120700. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Duyne, R.P. Van Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 8–10. [Google Scholar] [CrossRef]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.A.; Higgins, G.A. Science Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.L.; Dis, J.A. Synaptotoxic amyloid-β oligomers: A molecular basis for the cause, diagnosis, and treatment of Alzheimer’s disease? J. Alzheimers Dis. 2013, 33, S49–S65. [Google Scholar] [CrossRef]
- Reinhard, C.; Hébert, S.S.; De Strooper, B. The amyloid-beta precursor protein: Integrating structure with biological function. EMBO J. 2005, 24, 3996–4006. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Koo, E.H. The amyloid precursor protein: Beyond amyloid. Mol. Neurodegener. 2006, 1, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Cras, P.; Kawai, M.; Lowery, D.; Gonzalez-DeWhitt, P.; Greenberg, B.; Perry, G. Senile plaque neurites in Alzheimer disease accumulate amyloid precursor protein. Proc. Natl. Acad. Sci. USA 1991, 88, 7552–7556. [Google Scholar] [CrossRef] [Green Version]
- Capetillo-Zarate, E.; Gracia, L.; Tampellini, D.; Gouras, G.K. Intraneuronal Abeta accumulation, amyloid plaques, and synapse pathology in Alzheimer’s disease. Neurodegener. Dis. 2012, 10, 56–59. [Google Scholar] [CrossRef]
- Veerabhadrappa, B.; Delaby, C.; Hirtz, C.; Vialaret, J.; Alcolea, D.; Lleó, A.; Fortea, J.; Santosh, M.S.; Choubey, S.; Lehmann, S. Detection of amyloid beta peptides in body fluids for the diagnosis of alzheimer’s disease: Where do we stand? Crit. Rev. Clin. Lab. Sci. 2020, 57, 99–113. [Google Scholar] [CrossRef]
- Hansson, O.; Lehmann, S.; Otto, M.; Zetterberg, H.; Lewczuk, P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers. Res. Ther. 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Zetterberg, H.; Prog Mol Biol Transl, S. Fluid biomarker-based molecular phenotyping of Alzheimer’s disease patients in research and clinical settings. Prog. Mol. Biol. Transl. Sci. 2019, 169, 3–23. [Google Scholar] [CrossRef]
- Maeda, S.; Mucke, L. Previews Tau Phosphorylation—Much More than a Biomarker. Neuron 2016, 92, 265–267. [Google Scholar] [CrossRef] [Green Version]
- Sunderland, T.; Linker, G.; Mirza, N.; Putnam, K.T.; Friedman, D.L.; Kimmel, L.H.; Bergeson, J.; Manetti, G.J.; Zimmermann, M.; Tang, B.; et al. Decreased β-Amyloid1-42 and Increased Tau Levels in Cerebrospinal Fluid of Patients with Alzheimer Disease. J. Am. Med. Assoc. 2003, 289, 2094–2103. [Google Scholar] [CrossRef] [Green Version]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C. Articles CSF and blood biomarkers for the diagnosis of Alzheimer ’ s disease: A systematic review and meta-analysis. Lancet Neurol 2016, 15, 673–684. [Google Scholar] [CrossRef]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Mantzavinos, V.; Alexiou, A. Biomarkers for Alzheimer’s Disease Diagnosis. Curr. Alzheimer Res. 2017, 14, 1149–1154. [Google Scholar] [CrossRef] [Green Version]
- Brazaca, L.C.; Moreto, J.R.; Martín, A.; Tehrani, F.; Wang, J.; Zucolotto, V. Colorimetric Paper-Based Immunosensor for Simultaneous Determination of Fetuin B and Clusterin toward Early Alzheimer’s Diagnosis. ACS Nano 2019, 13, 13325–13332. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.M. Apolipoprotein E and Alzheimer disease: An update on genetic and functional analyses. J. Neuropathol. Exp. Neurol. 2000. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, T.; Atwal, J.K.; Steinberg, S.; Snaedal, J.; Jonsson, P.V.; Bjornsson, S.; Stefansson, H.; Sulem, P.; Gudbjartsson, D.; Maloney, J.; et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 2012, 488, 96–99. [Google Scholar] [CrossRef]
- Delkhahi, S.; Rahaie, M.; Rahimi, F. Design and Fabrication a Gold Nanoparticle-DNA Based Nanobiosensor for Detection of microRNA Involved in Alzheimer’s Disease. J. Fluoresc. 2017, 27, 603–610. [Google Scholar] [CrossRef]
- Counts, S.E.; Ikonomovic, M.D.; Mercado, N.; Vega, I.E.; Mufson, E.J. Biomarkers for the Early Detection and Progression of Alzheimer’s Disease. Neurotherapeutics 2017, 14, 35–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blennow, K.; Zetterberg, H. Biomarkers for Alzheimer’s disease: Current status and prospects for the future. J. Intern. Med. 2018, 284, 643–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, J.L. Alzheimer’s Disease. N. Engl. J. Med. 2004, 351, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, C.H.; Park, C.B. Chemical sensing platforms for detecting trace-level Alzheimer’s core biomarkers. Chem. Soc. Rev. 2020, 49, 5446–5472. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajczewski, J.; Kołątaj, K.; Kudelski, A. Plasmonic nanoparticles in chemical analysis. RSC Adv. 2017, 7, 17559–17576. [Google Scholar] [CrossRef] [Green Version]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. LSPR-based nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Li, Z.Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Gans, R. Über die Form ultramikroskopischer Goldteilchen. Ann. Phys. 1912, 342, 881–900. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.P.; Sebba, D.S. Surface-Enhanced Raman Scattering (SERS) Cytometry, 2nd ed.; Elsevier Inc.: London, UK, 2011; Volume 102, ISBN 9780123749123. [Google Scholar]
- Wang, L.; Hasanzadeh Kafshgari, M.; Meunier, M. Optical Properties and Applications of Plasmonic-Metal Nanoparticles. Adv. Funct. Mater. 2020, 2005400, 1–28. [Google Scholar] [CrossRef]
- Young, J.K.; Figueroa, E.R.; Drezek, R.A. Tunable nanostructures as photothermal theranostic agents. Ann. Biomed. Eng. 2012, 40, 438–459. [Google Scholar] [CrossRef] [PubMed]
- Indrasekara, A.S.D.S.; Thomas, R.; Fabris, L. Plasmonic properties of regiospecific core-satellite assemblies of gold nanostars and nanospheres. Phys. Chem. Chem. Phys. 2014, 17, 21133–21142. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef] [Green Version]
- Polavarapu, L.; Pérez-Juste, J.; Xu, Q.H.; Liz-Marzán, L.M. Optical sensing of biological, chemical and ionic species through aggregation of plasmonic nanoparticles. J. Mater. Chem. C 2014, 2, 7460–7476. [Google Scholar] [CrossRef]
- Mody, V.; Siwale, R.; Singh, A.; Mody, H. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282. [Google Scholar] [CrossRef]
- Viswambari Devi, R.; Doble, M.; Verma, R.S. Nanomaterials for early detection of cancer biomarker with special emphasis on gold nanoparticles in immunoassays/sensors. Biosens. Bioelectron. 2015, 68, 688–698. [Google Scholar] [CrossRef]
- Saeed, A.A.; Sánchez, J.L.A.; O’Sullivan, C.K.; Abbas, M.N. DNA biosensors based on gold nanoparticles-modified graphene oxide for the detection of breast cancer biomarkers for early diagnosis. Bioelectrochemistry 2017, 118, 91–99. [Google Scholar] [CrossRef]
- Mieszawska, A.J.; Mulder, W.J.M.; Fayad, Z.A.; Cormode, D.P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef] [Green Version]
- Mahan, M.M.; Doiron, A.L. Gold Nanoparticles as X-Ray, CT, and Multimodal Imaging Contrast Agents: Formulation, Targeting, and Methodology. J. Nanomater. 2018, 2018, 5837276. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Neogi, P.B.; Neogi, A.; Wang, Z. Gold Nanoparticles-enabled Efficient Dual Delivery of Anticancer Therapeutics to HeLa Cells. Sci. Rep. 2018, 8, 2907. [Google Scholar] [CrossRef] [Green Version]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [Green Version]
- Aghaie, T.; Jazayeri, M.H.; Manian, M.; Khani, l.; Erfani, M.; Rezayi, M.; Ferns, G.A.; Avan, A. Gold nanoparticle and polyethylene glycol in neural regeneration in the treatment of neurodegenerative diseases. J. Cell. Biochem. 2019, 120, 2749–2755. [Google Scholar] [CrossRef]
- Credi, C.; Bibikova, O.; Dallari, C.; Tiribilli, B.; Ratto, F.; Centi, S.; Pini, R.; Artyushenko, V.; Cicchi, R.; Pavone, F.S. Fiber-cap biosensors for SERS analysis of liquid samples. J. Mater. Chem. B 2020, 8, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tilley, R.D.; Gooding, J.J. Challenges and Solutions in Developing Ultrasensitive Biosensors. J. Am. Chem. Soc. 2019, 141, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Höller, R.P.M.; Jahn, I.J.; Cialla-May, D.; Chanana, M.; Popp, J.; Fery, A.; Kuttner, C. Biomacromolecular-Assembled Nanoclusters: Key Aspects for Robust Colloidal SERS Sensing. ACS Appl. Mater. Interfaces 2020, 12, 57302–57313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, B.; Zhang, D.; Liu, Y.; Zhang, M.; Zhao, C.; Zheng, J. Design principles and fundamental understanding of biosensors for amyloid-β detection. J. Mater. Chem. B 2020, 8, 6179–6196. [Google Scholar] [CrossRef]
- McFarland, A.D.; Young, M.A.; Dieringer, J.A.; Van Duyne, R.P. Wavelength-Scanned Surface-Enhanced Raman Excitation Spectroscopy. J. Phys. Chem. B 2005, 109, 11279–11285. [Google Scholar] [CrossRef]
- Sivapalan, S.T.; Devetter, B.M.; Yang, T.K.; Van Dijk, T.; Schulmerich, M.V.; Carney, P.S.; Bhargava, R.; Murphy, C.J. Off-resonance surface-enhanced raman spectroscopy from gold nanorod suspensions as a function of aspect ratio: Not what we thought. ACS Nano 2013, 7, 2099–2105. [Google Scholar] [CrossRef] [Green Version]
- Höller, R.P.M.; Kuttner, C.; Mayer, M.; Wang, R.; Dulle, M.; Contreras-Cáceres, R.; Fery, A.; Liz-Marzán, L.M. Colloidal Superstructures with Triangular Cores: Size Effects on SERS Efficiency. ACS Photonics 2020, 7, 1839–1848. [Google Scholar] [CrossRef]
- Kuttner, C.; Mayer, M.; Dulle, M.; Moscoso, A.; López-Romero, J.M.; Förster, S.; Fery, A.; Pérez-Juste, J.; Contreras-Cáceres, R. Seeded Growth Synthesis of Gold Nanotriangles: Size Control, SAXS Analysis, and SERS Performance. ACS Appl. Mater. Interfaces 2018, 10, 11152–11163. [Google Scholar] [CrossRef]
- Kuttner, C. Plasmonics in Sensing: From Colorimetry to SERS Analytics. In Plasmonics; IntechOpen: London, UK, 2018; Chapter 9; ISBN 978-1-78984-435-1. [Google Scholar]
- Yang, J.K.; Hwang, I.J.; Cha, M.G.; Kim, H.I.; Yim, D.B.; Jeong, D.H.; Lee, Y.S.; Kim, J.H. Reaction Kinetics-Mediated Control over Silver Nanogap Shells as Surface-Enhanced Raman Scattering Nanoprobes for Detection of Alzheimer’s Disease Biomarkers. Small 2019, 15, 1900613. [Google Scholar] [CrossRef]
- El-Said, W.A.; Kim, T.H.; Yea, C.H.; Kim, H.; Choi, J.W. Fabrication of gold nanoparticle modified ITO substrate TO detect β-amyloid using surface-enhanced Raman scattering. J. Nanosci. Nanotechnol. 2011, 11, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Huh, Y.S.; Erickson, D. Size-selective concentration and label-free characterization of protein aggregates using a Raman active nanofluidic device. Lab Chip 2011, 11, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Suk, Y.; David, H. Ultra-sensitive, label-free probing of the conformational characteristics of amyloid beta aggregates with a SERS active nanofluidic device. Microfluid. Nanofluid. 2012, 12, 663–669. [Google Scholar] [CrossRef]
- Dallari, C.; Credi, C.; Lenci, E.; Trabocchi, A.; Cicchi, R.; Pavone, F.S. Nanostars—decorated microfluidic sensors for surface enhanced Raman scattering targeting of biomolecules. J. Phys. Photonics 2020, 2, 024008. [Google Scholar] [CrossRef]
- Lin, D.; Wu, Z.; Li, S.; Zhao, W.; Ma, C.; Wang, J.; Jiang, Z.; Zhong, Z.; Zheng, Y.; Yang, X. Large-Area Au-Nanoparticle-Functionalized Si Nanorod Arrays for Spatially Uniform Surface-Enhanced Raman Spectroscopy. ACS Nano 2017, 11, 1478–1487. [Google Scholar] [CrossRef]
- Xia, Y.; Padmanabhan, P.; Sarangapani, S.; Gulyás, B.; Vadakke Matham, M. Bifunctional Fluorescent/Raman Nanoprobe for the Early Detection of Amyloid. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Billings, L.M.; Oddo, S.; Green, K.N.; McGaugh, J.L.; LaFerla, F.M. Intraneuronal Aβ Causes the Onset of Early Alzheimer’s Disease-Related Cognitive Deficits in Transgenic Mice. Neuron 2005, 45, 675–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zengin, A.; Tamer, U.; Caykara, T. A SERS-based sandwich assay for ultrasensitive and selective detection of Alzheimer’s tau protein. Biomacromolecules 2013, 14, 3001–3009. [Google Scholar] [CrossRef]
- Maurer, V.; Frank, C.; Porsiel, J.C.; Zellmer, S.; Garnweitner, G.; Stosch, R. Step-by-step monitoring of a magnetic and SERS-active immunosensor assembly for purification and detection of tau protein. J. Biophotonics 2020, 13, e201960090. [Google Scholar] [CrossRef]
- Sinha, S.S.; Jones, S.; Pramanik, A.; Ray, P.C. Nanoarchitecture Based SERS for Biomolecular Fingerprinting and Label-Free Disease Markers Diagnosis. Acc. Chem. Res. 2016, 49, 2725–2735. [Google Scholar] [CrossRef]
- Demeritte, T.; Priya, B.; Nellore, V.; Kanchanapally, R.; Sinha, S.S.; Pramanik, A.; Chavva, S.R.; Ray, P.C. Hybrid Graphene Oxide Based Plasmonic-Magnetic Multifunctional Nanoplatform for Selective Separation and Label-Free Identifi cation of Alzheimer’s Disease Biomarkers. ACS Appl. Mater. Interfaces 2015, 7, 13693–13700. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Andrade, G.F.S.; Temperini, M.L.A. SERS performance of gold nanotubes obtained by sputtering onto polycarbonate track-etched membranes. Phys. Chem. Chem. Phys. 2013, 15, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Scarabelli, L.; Coronado-Puchau, M.; Giner-Casares, J.J.; Langer, J.; Liz-Marzán, L.M. Monodisperse gold nanotriangles: Size control, large-scale self-assembly, and performance in surface-enhanced raman scattering. ACS Nano 2014, 8, 5833–5842. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Cho, S.; Kim, M.; Jung, Y.S. Carboxylic Acid-Functionalized, Graphitic Layer-Coated Three-Dimensional SERS Substrate for Label-Free Analysis of Alzheimer’s Disease Biomarkers. Nano Lett. 2020, 20, 2576–2584. [Google Scholar] [CrossRef]
- Pei, H.; Li, F.; Wan, Y.; Wei, M.; Liu, H.; Su, Y.; Chen, N.; Huang, Q.; Fan, C. Designed Diblock Oligonucleotide for the Synthesis of Spatially Isolated and Highly Hybridizable Functionalization of DNA–Gold Nanoparticle Nanoconjugates. J. Am. Chem. Soc. 2012, 134, 11876–11879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, S.; Song, X.; Wang, H.; Wang, J.; Wang, Y.; Huang, J.; Yu, J. Robust and Universal SERS Sensing Platform for Multiplexed Detection of Alzheimer’s Disease Core Biomarkers Using PAapt-AuNPs Conjugates. ACS Sens. 2019, 4, 2140–2149. [Google Scholar] [CrossRef]
- Carlomagno, C.; Cabinio, M.; Picciolini, S.; Gualerzi, A.; Baglio, F.; Bedoni, M. SERS-based biosensor for Alzheimer disease evaluation through the fast analysis of human serum. J. Biophotonics 2020, 13, e201960033. [Google Scholar] [CrossRef]
- Xia, N.; Zhou, B.; Huang, N.; Jiang, M.; Zhang, J.; Liu, L. Visual and fluorescent assays for selective detection of beta-amyloid oligomers based on the inner filter effect of gold nanoparticles on the fluorescence of CdTe quantum dots. Biosens. Bioelectron. 2016, 85, 625–632. [Google Scholar] [CrossRef]
- Saini, M.; Sadhu, K.K. Two instantaneous fluorogenic steps for detection of nanomolar amyloid beta monomer and its interaction with stoichiometric copper(II) ion. Sens. Actuators B Chem. 2020, 303, 127086. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, G.; Gong, L.; Ge, K.; Pan, W.; Li, N.; Machuki, J.O.; Yu, Y.; Geng, D.; Dong, H.; et al. DNAzyme-Powered Three-Dimensional DNA Walker Nanoprobe for Detection Amyloid β-Peptide Oligomer in Living Cells and in Vivo. Anal. Chem. 2020, 92, 9247–9256. [Google Scholar] [CrossRef]
- Jara-Guajardo, P.; Cabrera, P.; Celis, F.; Soler, M.; Berlanga, I.; Parra-Muñoz, N.; Acosta, G.; Albericio, F.; Guzman, F.; Campos, M.; et al. Gold nanoparticles mediate improved detection of β-amyloid aggregates by fluorescence. Nanomaterials 2020, 10, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Man, Z.; Xu, S.; Cong, L.; Wang, Y.; Wang, X.; Du, Y.; Zhang, Q.; Tang, S.; Liu, Z.; et al. A gold nanocluster chemical tongue sensor array for Alzheimer’s disease diagnosis. Colloids Surf. B Biointerfaces 2019, 173, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chen, C.P.; Wu, T.H.; Yang, C.H.; Lin, C.W.; Chen, C.Y. Gold nanoparticle-based colorimetric strategies for chemical and biological sensing applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, L.; Sun, X.; Li, C.; Qiu, Y.; Sun, H.; Tang, D.; Liu, Y.; Yin, X. A sensitive colorimetric strategy for monitoring cerebral β-amyloid peptides in AD based on dual-functionalized gold nanoplasmonic particles. Chem. Commun. 2015, 51, 8880–8883. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, H.; Liu, L.; Xu, M. Simple colorimetric detection of amyloid β-peptide (1-40) based on aggregation of gold nanoparticles in the presence of copper ions. Small 2015, 11, 2144–2149. [Google Scholar] [CrossRef]
- Ghasemi, F.; Reza Hormozi-Nezhad, M.; Mahmoudi, M. Label-free detection of β-amyloid peptides (Aβ40 and Aβ42): A colorimetric sensor array for plasma monitoring of Alzheimer’s disease. Nanoscale 2018, 10, 6361–6368. [Google Scholar] [CrossRef]
- Hu, T.; Lu, S.; Chen, C.; Sun, J.; Yang, X. Colorimetric sandwich immunosensor for Aβ(1-42) based on dual antibody-modified gold nanoparticles. Sens. Actuators B Chem. 2017, 243, 792–799. [Google Scholar] [CrossRef]
- Hu, T.; Chen, C.; Huang, G.; Yang, X. Antibody modified-silver nanoparticles for colorimetric immuno sensing of Aβ(1-40/1-42) based on the interaction between β-amyloid and Cu2+. Sens. Actuators B Chem. 2016, 234, 63–69. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, N.; Zhang, Y.; Liu, B.; Chang, Z.; Zhou, Y.; Hao, Y.; Ye, B.; Xu, M. A sensitive gold nanoparticle-based aptasensor for colorimetric detection of Aβ1-40 oligomers. Anal. Methods 2018, 10, 641–645. [Google Scholar] [CrossRef]
- Li, M.; Guan, Y.; Zhao, A.; Ren, J.; Qu, X. Using multifunctional peptide conjugated Au nanorods for monitoring β-amyloid aggregation and chemo-photothermal treatment of Alzheimer’s disease. Theranostics 2017, 7, 2996–3006. [Google Scholar] [CrossRef]
- Ren, X.; Yan, J.; Wu, D.; Wei, Q.; Wan, Y. Nanobody-Based Apolipoprotein E Immunosensor for Point-of-Care Testing. ACS Sens. 2017, 2, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Haes, A.J.; Chang, L.; Klein, W.L.; Van Duyne, R.P. Detection of a biomarker for Alzheimer’s disease from synthetic and clinical samples using a nanoscale optical biosensor. J. Am. Chem. Soc. 2005, 127, 2264–2271. [Google Scholar] [CrossRef]
- Kang, M.K.; Lee, J.; Nguyen, A.H.; Sim, S.J. Label-free detection of ApoE4-mediated β-amyloid aggregation on single nanoparticle uncovering Alzheimer’s disease. Biosens. Bioelectron. 2015, 72, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.U.; Kim, S.; Song, S.; Sim, S.J. A Nanoplasmonic Biosensor for Ultrasensitive Detection of Alzheimer’s Disease Biomarker Using a Chaotropic Agent. ACS Sens. 2019, 4, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.U.; Song, S.; Kim, S.; Sim, S.J. A shape-code nanoplasmonic biosensor for multiplex detection of Alzheimer’s disease biomarkers. Biosens. Bioelectron. 2018, 101, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Špringer, T.; Hemmerová, E.; Finocchiaro, G.; Krištofiková, Z.; Vyhnálek, M.; Homola, J. Surface plasmon resonance biosensor for the detection of tau-amyloid β complex. Sens. Actuators B Chem. 2020, 316. [Google Scholar] [CrossRef]
- Stefani, M.; Dobson, C.M. Protein aggregation and aggregate toxicity: New insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. 2003, 81, 678–699. [Google Scholar] [CrossRef]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10 (Suppl. S10–S17). [Google Scholar] [CrossRef]
- Dobson, C.M. The structural basis of protein folding and its links with human disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 133–145. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, U.; Nilson, A.N.; Kayed, R. The Role of Amyloid-β Oligomers in Toxicity, Propagation, and Immunotherapy. EBioMedicine 2016, 6, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Serra-Batiste, M.; Ninot-Pedrosa, M.; Bayoumi, M.; Gairí, M.; Maglia, G.; Carulla, N. Aβ42 assembles into specific β-barrel pore-forming oligomers in membrane-mimicking environments. Proc. Natl. Acad. Sci. USA 2016, 113, 10866–10871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.; Davis, J.; Aucoin, D.; Sato, T.; Ahuja, S.; Aimoto, S.; Elliott, J.I.; Van Nostrand, W.E.; Smith, S.O. Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat. Struct. Mol. Biol. 2010, 17, 561–567. [Google Scholar] [CrossRef] [Green Version]
- Arosio, P.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P. Chemical kinetics for drug discovery to combat protein aggregation diseases. Trends Pharmacol. Sci. 2014, 35, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Knowles, T.P.; Linse, S. On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 2015, 17, 7606–7618. [Google Scholar] [CrossRef] [Green Version]
- Arosio, P.; Michaels, T.C.; Linse, S.; Månsson, C.; Emanuelsson, C.; Presto, J.; Johansson, J.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P. Kinetic analysis reveals the diversity of microscopic mechanisms through which molecular chaperones suppress amyloid formation. Nat. Commun. 2016, 7, 10948. [Google Scholar] [CrossRef] [Green Version]
- Hellstrand, E.; Boland, B.; Walsh, D.M.; Linse, S. Amyloid β-protein aggregation produces highly reproducible kinetic data and occurs by a two-phase process. ACS Chem. Neurosci. 2010, 1, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Novo, M.; Freire, S.; Al-Soufi, W. Critical aggregation concentration for the formation of early Amyloid-β (1-42) oligomers. Sci. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Elbassal, E.A.; Morris, C.; Kent, T.W.; Lantz, R.; Ojha, B.; Wojcikiewicz, E.P.; Du, D. Gold Nanoparticles as a Probe for Amyloid-β Oligomer and Amyloid Formation. J. Phys. Chem. C 2017, 121, 20007–20015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, R.V.; Yi, P.J.; Padmanabhan, P.; Gulyás, B.; Murukeshan, V.M. Au nano-urchins enabled localized surface plasmon resonance sensing of beta amyloid fibrillation. Nanoscale Adv. 2020. [Google Scholar] [CrossRef]
- Ahmad, J.; Akhter, S.; Rizwanullah, M.; Khan, M.A.; Pigeon, L.; Addo, R.T.; Greig, N.H.; Midoux, P.; Pichon, C.; Kamal, M.A. Nanotechnology Based Theranostic Approaches in Alzheimer’s Disease Management: Current Status and Future Perspective. Curr. Alzheimer Res. 2017, 14, 1164–1181. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Pervushin, K. Magnetic nanoparticles as in vivo tracers for alzheimer’s disease. Magnetochemistry 2020, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Ulanova, M.; Poljak, A.; Wen, W.; Bongers, A.; Gloag, L.; Gooding, J.; Tilley, R.; Sachdev, P.; Braidy, N. Nanoparticles as contrast agents for the diagnosis of Alzheimer’s disease: A systematic review. Nanomedicine 2020, 15, 725–743. [Google Scholar] [CrossRef]
- Pietrzak, K.; Czarnecka, K.; Mikiciuk-Olasik, E.; Szymanski, P. New Perspectives of Alzheimer Disease Diagnosis—The Most Popular and Future Methods. Med. Chem. 2018, 14, 34–43. [Google Scholar] [CrossRef]
- Ajetunmobi, A.; Prina-Mello, A.; Volkov, Y.; Corvin, A.; Tropea, D. Nanotechnologies for the study of the central nervous system. Prog. Neurobiol. 2014, 123, 18–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanifum, E.A.; Ghaghada, K.; Vollert, C.; Head, E.; Eriksen, J.L.; Annapragada, A. A Novel Liposomal Nanoparticle for the Imaging of Amyloid Plaque by Magnetic Resonance Imaging. J. Alzheimers. Dis. 2016, 52, 731–745. [Google Scholar] [CrossRef] [Green Version]
- Badachhape, A.A.; Working, P.K.; Srivastava, M.; Bhandari, P.; Stupin, I.V.; Devkota, L.; Tanifum, E.A.; Annapragada, A.V.; Ghaghada, K.B. Pre-clinical dose-ranging efficacy, pharmacokinetics, tissue biodistribution, and toxicity of a targeted contrast agent for MRI of amyloid deposition in Alzheimer’s disease. Sci. Rep. 2020, 10, 16185. [Google Scholar] [CrossRef] [PubMed]
- Aouidat, F.; Boumati, S.; Khan, M.; Tielens, F.; Doan, B.T.; Spadavecchia, J. Design and synthesis of gold-gadolinium-coreshell nanoparticles as contrast agent: A smart way to future nanomaterials for nanomedicine applications. Int. J. Nanomed. 2019, 14, 9309–9324. [Google Scholar] [CrossRef] [Green Version]
- Faucher, L.; Tremblay, M.; Lagueux, J.; Gossuin, Y.; Fortin, M.-A. Rapid Synthesis of PEGylated Ultrasmall Gadolinium Oxide Nanoparticles for Cell Labeling and Tracking with MRI. ACS Appl. Mater. Interfaces 2012, 4, 4506–4515. [Google Scholar] [CrossRef]
- Guerrero, S.; Araya, E.; Fiedler, J.L.; Arias, J.I.; Adura, C.; Albericio, F.; Giralt, E.; Arias, J.L.; Fernndez, M.S.; Kogan, M.J. Improving the brain delivery of gold nanoparticles by conjugation with an amphipathic peptide. Nanomedicine 2010, 5, 897–913. [Google Scholar] [CrossRef]
- Lai, L.; Zhao, C.; Li, X.; Liu, X.; Jiang, H.; Selke, M.; Wang, X. Fluorescent gold nanoclusters for in vivo target imaging of Alzheimer’s disease. RSC Adv. 2016, 6, 30081–30088. [Google Scholar] [CrossRef]
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef]
- Perets, N.; Betzer, O.; Shapira, R.; Brenstein, S.; Angel, A.; Sadan, T.; Ashery, U.; Popovtzer, R.; Offen, D. Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett. 2019, 19, 3422–3431. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Sensing using localised surface plasmon resonance sensors. Chem. Commun. 2012, 48, 8999–9010. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O. (Eds.) Advantages, Disadvantages and Modifications of Conventional ELISA BT—Enzyme-Linked Immunosorbent Assay (ELISA): From A to Z; Springer: Singapore, 2018; pp. 67–115. ISBN 978-981-10-6766-2. [Google Scholar]
- Kang, H.; Jeong, S.; Yang, J.K.; Jo, A.; Lee, H.; Heo, E.H.; Jeong, D.H.; Jun, B.H.; Chang, H.; Lee, Y.S. Template-assisted plasmonic nanogap shells for highly enhanced detection of cancer biomarkers. Int. J. Mol. Sci. 2021, 22, 1752. [Google Scholar] [CrossRef]
- Quan, L.; Wu, J.; Lane, L.A.; Wang, J.; Lu, Q.; Gu, Z.; Wang, Y. Enhanced Detection Specificity and Sensitivity of Alzheimer’s Disease Using Amyloid-β-Targeted Quantum Dots. Bioconjug. Chem. 2016, 27, 809–814. [Google Scholar] [CrossRef]
- Hong, S.; Lee, C. The current status and future outlook of quantum dot-based biosensors for plant virus detection. Plant Pathol. J. 2018, 34, 85–92. [Google Scholar] [CrossRef]
- Mirsalari, M.; Elhami, S. Colorimetric detection of insulin in human serum using GO/AuNPs/TX-100 nanocomposite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118617. [Google Scholar] [CrossRef]
- Yoo, Y.K.; Kim, G.; Park, D.; Kim, J.; Kim, Y.; Yun Kim, H.; Yang, S.H.; Lee, J.H.; Hwang, K.S. Gold nanoparticles assisted sensitivity improvement of interdigitated microelectrodes biosensor for amyloid-β detection in plasma sample. Sens. Actuators B Chem. 2020, 308, 127710. [Google Scholar] [CrossRef]
- Altuna-Azkargorta, M.; Mendioroz-Iriarte, M. Blood biomarkers in Alzheimer’s disease. Neurología 2020. In Press. [Google Scholar] [CrossRef]
- Kasturirangan, S.; Li, L.; Emadi, S.; Boddapati, S.; Schulz, P.; Sierks, M.R. Nanobody specific for oligomeric beta-amyloid stabilizes nontoxic form. Neurobiol. Aging 2012, 33, 1320–1328. [Google Scholar] [CrossRef]
- Lertvachirapaiboon, C.; Baba, A.; Shinbo, K.; Kato, K. A smartphone-based surface plasmon resonance platform. Anal. Methods 2018, 10, 4732–4740. [Google Scholar] [CrossRef]
- Zeng, F.; Duan, W.; Zhu, B.; Mu, T.; Zhu, L.; Guo, J.; Ma, X. Paper-Based Versatile Surface-Enhanced Raman Spectroscopy Chip with Smartphone-Based Raman Analyzer for Point-of-Care Application. Anal. Chem. 2019, 91, 1064–1070. [Google Scholar] [CrossRef]
- Zeng, F.; Mou, T.; Zhang, C.; Huang, X.; Wang, B.; Ma, X.; Guo, J. Paper-based SERS analysis with smartphones as Raman spectral analyzers. Analyst 2019, 144, 137–142. [Google Scholar] [CrossRef]





| Type of Nanoparticle | Detected Biomarker | Sensing Method | Analytical Parameters | Application | Ref. |
|---|---|---|---|---|---|
| Hybrid (MNPs/AuNPs) | tau | Antibody sandwich SERS assay | LR: 25 fM–500 nM LOD: <25 fM | - | [80] |
| AgNGSs | Aβ1–40 Aβ1–42 | Antibody sandwich SERS assay | LOD: 0.25 pg mL−1 0.33 pg mL−1 | Human serum | [72] |
| MNPs@Au-HGO | Aβ1–42 tau | Antibody SERS assay | LOD: <100 fg mL−1 | Blood | [83] |
| AuNPs | AβOs (Aβ1–42) tau | Aptamer SERS detection | LOD: AβOs: 3.7 × 10−2 nM Tau: 4.2 × 10−4 pM | aCSF | [88] |
| AuNPs | Aβ1–42 | SERS detection with a fluorescent probe | L.R: 0–2 μM | - | [78] |
| AuNPs | Aβ1–16, Aβ1–40, Aβ1–42 monomers | Colorimetric label-free detection | L.R: 0.2–5000 ng mL−1 LOD: 40 pg mL−1 (as total monomers) | CSF/AD rat brain tissues | [96] |
| AgNPs | Aβ1–40/1–42 | Colorimetric immunosensor | LR: 0.25–150 nM LOD: 86 pM | Blood | [100] |
| AuNPs | AβOs (Aβ1–40) | Colorimetric aptasensor | LR: 1–600 nM LOD: 0.56 nM | aCSF | [101] |
| AuNPs | miRNA-137 | DNA based biosensor | LOD: 0.25 nM | Blood | [36] |
| AuNPs | Clusterin Fetuin B | Colorimetric microfluidic device | LOD: 0.24 nM 0.12 nM | - | [33] |
| AuNPs | AβOs | Fluorescence detection | LR: 0.1~1.0 nM LOD: 22.3 pM | - | [92] |
| AuNPs/CdTe QDs | AβOs | Fluorescence detection | LR: 1–60 nM LOD: 0.2–0.5 nM | - | [90] |
| AuNPs | Aβ1–42 Aggregates | Surface-Enhanced Fluorescence | μM level | PBS | [93] |
| AuNRs | Aβ1–42, Aβ1–40, tau | Shape-code LSPR sensor | LR: 1 × 101–1 × 108 fM LOD: 26 fM | - | [107] |
| AuNPs | Aβ-tau complex | Antibody sandwich LSPR assay | LOD: 1 pM | CSF | [108] |
| AuNPs | Aβ1–42 Aggregates | LSPR sensor | LOD: 1.5 pM | aCSF | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyarzún, M.P.; Tapia-Arellano, A.; Cabrera, P.; Jara-Guajardo, P.; Kogan, M.J. Plasmonic Nanoparticles as Optical Sensing Probes for the Detection of Alzheimer’s Disease. Sensors 2021, 21, 2067. https://doi.org/10.3390/s21062067
Oyarzún MP, Tapia-Arellano A, Cabrera P, Jara-Guajardo P, Kogan MJ. Plasmonic Nanoparticles as Optical Sensing Probes for the Detection of Alzheimer’s Disease. Sensors. 2021; 21(6):2067. https://doi.org/10.3390/s21062067
Chicago/Turabian StyleOyarzún, María Paz, Andreas Tapia-Arellano, Pablo Cabrera, Pedro Jara-Guajardo, and Marcelo J. Kogan. 2021. "Plasmonic Nanoparticles as Optical Sensing Probes for the Detection of Alzheimer’s Disease" Sensors 21, no. 6: 2067. https://doi.org/10.3390/s21062067
APA StyleOyarzún, M. P., Tapia-Arellano, A., Cabrera, P., Jara-Guajardo, P., & Kogan, M. J. (2021). Plasmonic Nanoparticles as Optical Sensing Probes for the Detection of Alzheimer’s Disease. Sensors, 21(6), 2067. https://doi.org/10.3390/s21062067






