Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends
Abstract
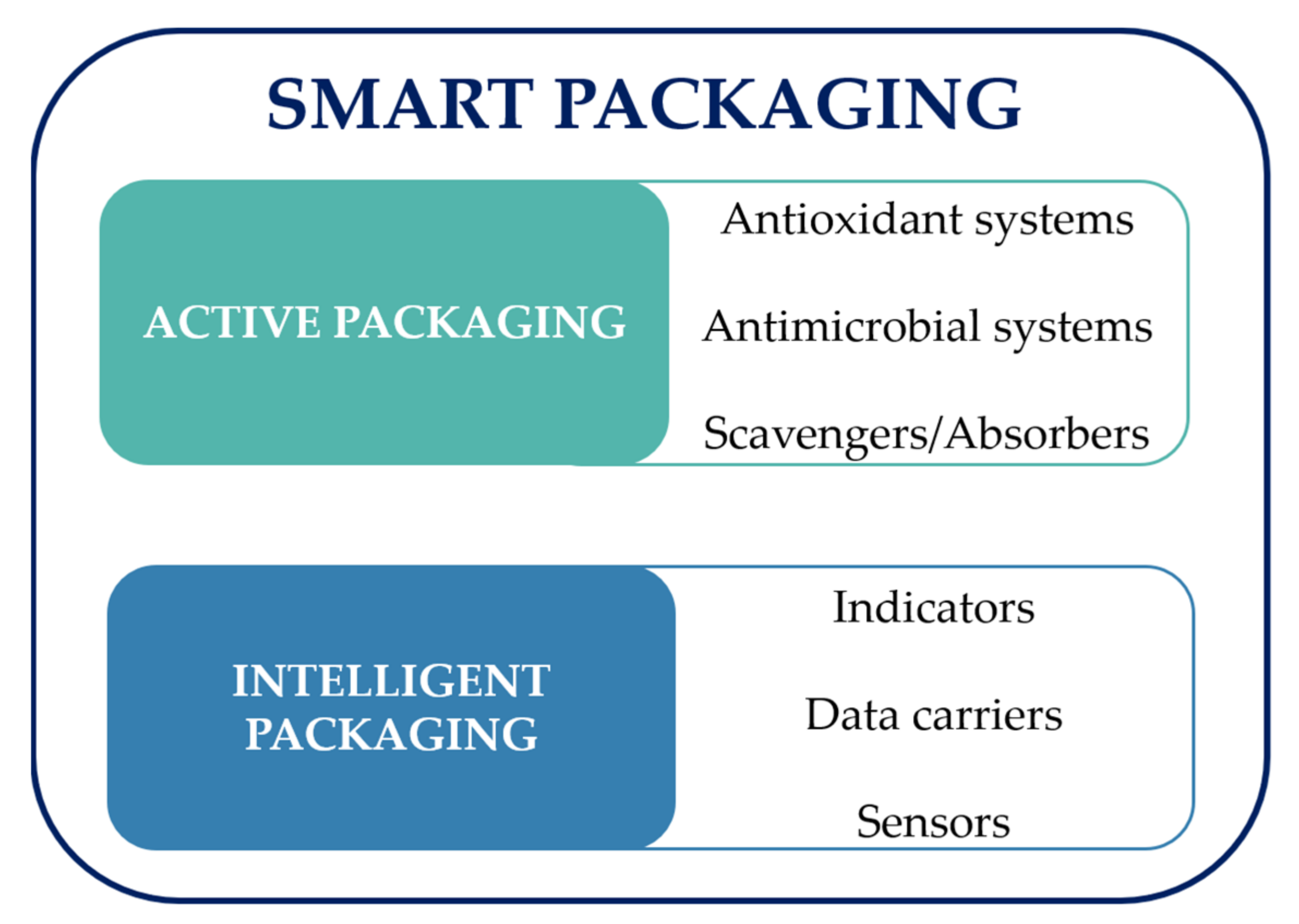
:1. Introduction
2. Developments in Bio-Based Sensors
3. Bio-Based Sensors—Applications
| Matrix | Sensor Material (Source/Type) | Function | Major Results | Food Application | Ref. |
|---|---|---|---|---|---|
| Gelatin-Gellan gum bilayer films | Mulberry fruits—anthocyanins | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Crucian fish | [58] |
| Starch | Grape skin—anthocyanins | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Beef and fish | [59] |
| Agarose | Red cabbage and rose—anthocyanins | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Buffalo meat | [60] |
| Chitin nanofiber and methylcellulose | Red barberry—anthocyanins | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Fish | [61] |
| Pectin | Red cabbage—anthocyanins | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Beef, chicken, shrimp and fish | [62] |
| Chitosan, carboxymethyl cellulose | Blueberry and red grape skin pulp—anthocyanins | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Chicken meat | [50] |
| Carboxymethyl cellulose sodium and Artemisia sphaerocephala Krasch. gum | Red cabbage—anthocyanins | pH and NH3 |
| Not applied | [63] |
| κ—carrageenan | Lycium ruthenicum Murr. —anthocyanins | Sensitivity of the films to volatile nitrogen compounds and to lactic acid production through pH change |
| Milk and Shrimp | [64] |
| Starch and gelatin | Red raddish—anthocyanins | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Prawn and poultry meat | [65] |
| Methylcellulose | Jambolan fruit—anthocyanins | pH |
| Not applied | [66] |
| Chitosan and TiO2 | Apple pomace—anthocyanins | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Salmon | [67] |
| Composite of polyvinyl alcohol, microcrystalline cellulose and polyvinyl pyrrolidone | Red cabbage—anthocyanins | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Prawn and chicken | [68] |
| Chitosan and Oxidized chitin nanocrystals | Black rice—anthocyanins | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Shrimp and promfet | [69] |
| Quaternary ammonium Chitosan-Polyvinyl alcohol | Cactus pears extract (CPE)—betalains | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Shrimp | [70] |
| Quaternary ammonium chitosan—Fish gelatin | Amaranth—betalains | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Shrimp | [71] |
| Starch-Polyvinyl alcohol | Red pitaya—betalains | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Shrimp | [72] |
| Gelatin-Polyvinyl alcohol | Amaranth—betalains | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Chicken and Fish | [73] |
| Cellulose | Arnebia euchroma- Naphthoquinone | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Pork and shrimp | [74] |
| Cellulose paper | Gromwell (Lithospermum erythrorhizon)—Shikonin | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Fish and Pork | [75] |
| Agar—Polyvinyl alcohol | Curcumin | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Shrimp | [76] |
| Tara gum and Polyvinyl alcohol | Curcumin | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Shrimp | [77] |
| Pectin and sulfur nanoparticles | Curcumin | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Shrimp | [78] |
| Lallemantia iberica seed gum | Curcumin | Sensitivity of the films to volatile nitrogen compounds through pH change |
| Shrimp | [79] |
3.1. Anthocyanins Used as Bio-Based Sensors
3.2. Betalains Used as Bio-Based Sensors
3.3. Curcumin Used as Bio-Based Sensor
3.4. Other Potential Applications of Bio-Based Sensors
4. Final Remarks and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghoshal, G. Recent Trends in Active, Smart, and Intelligent Packaging for Food Products. In Food Packaging and Preservation; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 343–374. ISBN 9780128115169. [Google Scholar]
- Souza, V.G.L.; Pires, J.R.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh-Sani, M.; Mohammadian, E.; Rhim, J.W.; Jafari, S.M. pH-sensitive (halochromic) smart packaging films based on natural food colorants for the monitoring of food quality and safety. Trends Food Sci. Technol. 2020, 105, 93–144. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Halonen, N.; Pálvölgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-Based Smart Materials for Food Packaging and Sensors—A Review. Front. Mater. 2020, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Mohammadian, E.; Alizadeh-Sani, M.; Jafari, S.M. Smart monitoring of gas/temperature changes within food packaging based on natural colorants. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2885–2931. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780429105401. [Google Scholar]
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Mohanty, F.; Swain, S.K. Bionanocomposites for Food Packaging Applications. In Nanotechnology Applications in Food: Flavor, Stability, Nutrition and Safety; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 363–379. ISBN 9780128119433. [Google Scholar]
- Pilevar, Z.; Bahrami, A.; Beikzadeh, S.; Hosseini, H.; Jafari, S.M. Migration of styrene monomer from polystyrene packaging materials into foods: Characterization and safety evaluation. Trends Food Sci. Technol. 2019, 91, 248–261. [Google Scholar] [CrossRef]
- Videira-Quintela, D.; Martin, O.; Montalvo, G. Recent advances in polymer-metallic composites for food packaging applications. Trends Food Sci. Technol. 2021, 109, 230–244. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Genovese, L.; Lotti, N.; Gazzano, M.; Siracusa, V.; Rosa, M.D.; Munari, A. Novel biodegradable aliphatic copolyesters based on poly(butylene succinate) containing thioether-linkages for sustainable food packaging applications. Polym. Degrad. Stab. 2016, 132, 191–201. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent Advances in Biopolymers and Biopolymer-Based Nanocomposites for Food Packaging Materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Rhim, J.W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2020, ahead-of-print. 1–29. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Gopal, T.K.S. Smart packaging systems for food applications: A review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Wilson, C.T.; Harte, J.; Almenar, E. Effects of sachet presence on consumer product perception and active packaging acceptability—A study of fresh-cut cantaloupe. Lwt 2018, 92, 531–539. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, C.; Ferreira, L.; Pires, J.R.A.; Duarte, M.P.; Coelhoso, I.; Fernando, A.L. In vitro bioactivity of novel chitosan bionanocomposites incorporated with different essential oils. Ind. Crop. Prod. Prod. 2019, 140, 111563. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Andrade, M.A.; Vilarinho, F.; Castanheira, I.; Fernando, A.L.; Loizzo, M.R.; Silva, A.S. A new insight on cardoon: Exploring new uses besides cheese making with a view to zero waste. Foods 2020, 9, 564. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Souza, V.G.L.; Coelhoso, I.M.; Reboleira, J.; Bernardino, S.; Ganhão, R.; Mendes, S.; Fernando, A.L.; Vilarinho, F.; et al. Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat. Coatings 2021, 11, 229. [Google Scholar] [CrossRef]
- Pascoal, A.; Quirantes-Piné, R.; Fernando, A.L.; Alexopoulou, E.; Segura-Carretero, A. Phenolic composition and antioxidant activity of kenaf leaves. Ind. Crops Prod. 2015, 78, 116–123. [Google Scholar] [CrossRef]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.J.; Choi, D.S.; Hur, S.J. Current topics in active and intelligent food packaging for preservation of fresh foods. J. Sci. Food Agric. 2015, 95, 2799–2810. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No. 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Off. J. Eur. Union 2009, 135, 3–11. [Google Scholar]
- Shivakumar, N.; Madhusudan, P.; Daniel, S.C.G.K. Nanomaterials for smart food packaging. Handb. Nanomater. Ind. Appl. 2018, 260–270. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent packaging in the food sector: A brief overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Kalpana, S.; Priyadarshini, S.R.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Dobrucka, R.; Przekop, R. New perspectives in active and intelligent food packaging. J. Food Process. Preserv. 2019, 43, 1–9. [Google Scholar] [CrossRef]
- Chowdhury, E.U.; Morey, A. Intelligent Packaging for Poultry Industry. J. Appl. Poult. Res. 2019, 28, 791–800. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.W.; Zhu, Z. Recent developments in intelligent packaging for enhancing food quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef]
- Griesche, C.; Baeumner, A.J. Biosensors to support sustainable agriculture and food safety. TrAC Trends Anal. Chem. 2020, 128, 115906. [Google Scholar] [CrossRef]
- Kuswandi, B. Freshness Sensors for Food Packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–11. ISBN 9780081005965. [Google Scholar]
- Young, E.; Mirosa, M.; Bremer, P. A systematic review of consumer perceptions of smart packaging technologies for food. Front. Sustain. Food Syst. 2020, 4, 1–20. [Google Scholar] [CrossRef]
- Chen, S.; Brahma, S.; Mackay, J.; Cao, C.; Aliakbarian, B. The role of smart packaging system in food supply chain. J. Food Sci. 2020, 85, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Vanderroost, M.; Ragaert, P.; Devlieghere, F.; De Meulenaer, B. Intelligent food packaging: The next generation. Trends Food Sci. Technol. 2014, 39, 47–62. [Google Scholar] [CrossRef]
- Emanuel, N.; Harloveleen, K.S. Food Packaging Development: Recent Perspective. J. Thin Film. Coat. Sci. Technol. Appl. 2019, 6, 13–29. [Google Scholar]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of polysaccharide-based materials as advanced food packaging. Molecules 2020, 25, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balbinot-Alfaro, E.; Craveiro, D.V.; Lima, K.O.; Costa, H.L.G.; Lopes, D.R.; Prentice, C. Intelligent Packaging with pH Indicator Potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A European Strategy for Plastics in a Circular Economy; COM (2018); European Commission: Brussels, Belgium, 2018; Volume 28. [Google Scholar]
- Poyatos-Racionero, E.; Ros-Lis, J.V.; Vivancos, J.L.; Martínez-Máñez, R. Recent advances on intelligent packaging as tools to reduce food waste. J. Clean. Prod. 2018, 172, 3398–3409. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Emerging trends in food packaging. Nutr. Food Sci. 2018, 48, 764–779. [Google Scholar] [CrossRef]
- Nemes, S.A.; Szabo, K.; Vodnar, D.C. Applicability of agro-industrial by-products in intelligent food packaging. Coatings 2020, 10, 550. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Kurek, M.; Hlupić, L.; Ščetar, M.; Bosiljkov, T.; Galić, K. Comparison of Two pH Responsive Color Changing Bio-Based Films Containing Wasted Fruit Pomace as a Source of Colorants. J. Food Sci. 2019, 84, 2490–2498. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Pacheco-Hernández, M.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. The application of natural food colorants as indicator substances in intelligent biodegradable packaging materials. Food Chem. Toxicol. 2020, 135, 110975. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Stratakos, A.C. Application of Modified Atmosphere Packaging and Active/Smart Technologies to Red Meat and Poultry: A Review. Food Bioprocess Technol. 2012, 5, 1423–1446. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A comprehensive review on freshness of fish and assessment: Analytical methods and recent innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Rodrigues, P.F.; Lopes, A.; Silva, R.J.; Caldeira, J.; Duarte, M.P.; Fernandes, F.B.; Coelhoso, I.M.; et al. Physical and Morphological Characterization of Chitosan/Montmorillonite Films Incorporated with Ginger Essential Oil. Coatings 2019, 9, 700. [Google Scholar] [CrossRef] [Green Version]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B.; Duarte, M.P.; Coelhoso, I.M.; Fernando, A.L. Bionanocomposites of chitosan/montmorillonite incorporated with Rosmarinus officinalis essential oil: Development and physical characterization. Food Packag. Shelf Life 2018, 16, 148–156. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, X.; Zou, X.; Shi, J.; Huang, X.; Li, Z.; Gong, Y.; Holmes, M.; Povey, M.; Xiao, J. Bilayer pH-sensitive colorimetric films with light-blocking ability and electrochemical writing property: Application in monitoring crucian spoilage in smart packaging. Food Chem. 2021, 336, 127634. [Google Scholar] [CrossRef]
- Vedove, T.M.A.R.D.; Maniglia, B.C.; Tadini, C.C. Production of sustainable smart packaging based on cassava starch and anthocyanin by an extrusion process. J. Food Eng. 2021, 289, 110274. [Google Scholar] [CrossRef]
- Shukla, V.; Kandeepan, G.; Vishnuraj, M.R.; Soni, A. Anthocyanins based indicator sensor for intelligent packaging application. Agric. Res. 2016, 5, 205–209. [Google Scholar] [CrossRef]
- Sani, M.A.; Tavassoli, M.; Hamishehkar, H.; McClements, D.J. Carbohydrate-based films containing pH-sensitive red barberry anthocyanins: Application as biodegradable smart food packaging materials. Carbohydr. Polym. 2021, 255, 117488. [Google Scholar] [CrossRef]
- Dudnyk, I.; Janeček, E.R.; Vaucher-Joset, J.; Stellacci, F. Edible sensors for meat and seafood freshness. Sens. Actuators B Chem. 2018, 259, 1108–1112. [Google Scholar] [CrossRef]
- Liang, T.; Sun, G.; Cao, L.; Li, J.; Wang, L. A pH and NH3 sensing intelligent film based on Artemisia sphaerocephala Krasch. gum and red cabbage anthocyanins anchored by carboxymethyl cellulose sodium added as a host complex. Food Hydrocoll. 2019, 87, 858–868. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Guo, M.; Li, L.; Chen, M.; Jiang, S.; Li, X.; Jiang, S. Extract from Lycium ruthenicum Murr. Incorporating κ-carrageenan colorimetric film with a wide pH–sensing range for food freshness monitoring. Food Hydrocoll. 2019, 94, 1–10. [Google Scholar] [CrossRef]
- Chayavanich, K.; Thiraphibundet, P.; Imyim, A. Biocompatible film sensors containing red radish extract for meat spoilage observation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117601. [Google Scholar] [CrossRef]
- Da Filipini, G.S.; Romani, V.P.; Martins, V.G. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium cumini) skins extract for food packaging. Food Hydrocoll. 2020, 109, 106139. [Google Scholar] [CrossRef]
- Lan, W.; Wang, S.; Zhang, Z.; Liang, X.; Liu, X.; Zhang, J. Development of red apple pomace extract/chitosan-based films reinforced by TiO2 nanoparticles as a multifunctional packaging material. Int. J. Biol. Macromol. 2021, 168, 105–115. [Google Scholar] [CrossRef]
- Zia, J.; Mancini, G.; Bustreo, M.; Zych, A.; Donno, R.; Athanassiou, A.; Fragouli, D. Porous pH natural indicators for acidic and basic vapor sensing. Chem. Eng. J. 2021, 403, 126373. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an intelligent film based on chitosan/oxidized chitin nanocrystals incorporating black rice bran anthocyanins for seafood spoilage monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef]
- Yao, X.; Hu, H.; Qin, Y.; Liu, J. Development of antioxidant, antimicrobial and ammonia-sensitive films based on quaternary ammonium chitosan, polyvinyl alcohol and betalains-rich cactus pears (Opuntia ficus-indica) extract. Food Hydrocoll. 2020, 106, 105896. [Google Scholar] [CrossRef]
- Hu, H.; Yao, X.; Qin, Y.; Yong, H.; Liu, J. Development of multifunctional food packaging by incorporating betalains from vegetable amaranth (Amaranthus tricolor L.) into quaternary ammonium chitosan/fish gelatin blend films. Int. J. Biol. Macromol. 2020, 159, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Dong, H.; Ling, Z.; Zhang, X.; Zhang, X.; Ramaswamy, S.; Xu, F. Smart colorimetric sensing films with high mechanical strength and hydrophobic properties for visual monitoring of shrimp and pork freshness. Sens. Actuators B Chem. 2020, 309, 127752. [Google Scholar] [CrossRef]
- Ezati, P.; Bang, Y.J.; Rhim, J.W. Preparation of a shikonin-based pH-sensitive color indicator for monitoring the freshness of fish and pork. Food Chem. 2021, 337, 127995. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zou, X.; Shi, J.; Zhai, X.; Liu, L.; Li, Z.; Holmes, M.; Gong, Y.; Povey, M.; et al. A visual indicator based on curcumin with high stability for monitoring the freshness of freshwater shrimp, Macrobrachium rosenbergii. J. Food Eng. 2021, 292, 110290. [Google Scholar] [CrossRef]
- Ma, Q.; Du, L.; Wang, L. Tara gum/polyvinyl alcohol-based colorimetric NH3 indicator films incorporating curcumin for intelligent packaging. Sens. Actuators B Chem. 2017, 244, 759–766. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W. pH-responsive pectin-based multifunctional films incorporated with curcumin and sulfur nanoparticles. Carbohydr. Polym. 2020, 230, 115638. [Google Scholar] [CrossRef] [PubMed]
- Taghinia, P.; Abdolshahi, A.; Sedaghati, S.; Shokrollahi, B. Smart edible films based on mucilage of lallemantia iberica seed incorporated with curcumin for freshness monitoring. Food Sci. Nutr. 2021, 9, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.; Garofulić, I.E.; Bakić, M.T.; Ščetar, M.; Uzelac, V.D.; Galić, K. Development and evaluation of a novel antioxidant and pH indicator film based on chitosan and food waste sources of antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef]
- Merz, B.; Capello, C.; Leandro, G.C.; Moritz, D.E.; Monteiro, A.R.; Valencia, G.A. A novel colorimetric indicator film based on chitosan, polyvinyl alcohol and anthocyanins from jambolan (Syzygium cumini) fruit for monitoring shrimp freshness. Int. J. Biol. Macromol. 2020, 153, 625–632. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of physicochemical and thermal properties of starch films by incorporation of TiO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef]
- Mushtaq, M.; Gani, A.; Gani, A.; Punoo, H.A.; Masoodi, F.A. Use of pomegranate peel extract incorporated zein film with improved properties for prolonged shelf life of fresh Himalayan cheese (Kalari/kradi). Innov. Food Sci. Emerg. Technol. 2018, 48, 25–32. [Google Scholar] [CrossRef]
- Koosha, M.; Hamedi, S. Intelligent Chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog. Org. Coatings 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Sun, J.; Lu, Y.; Tong, C.; Wang, L.; Yan, Z.; Pang, J. Novel konjac glucomannan films with oxidized chitin nanocrystals immobilized red cabbage anthocyanins for intelligent food packaging. Food Hydrocoll. 2020, 98, 105245. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Rein, M.J.; Heinonen, M. Stability and Enhancement of Berry Juice Color. J. Agric. Food Chem. 2004, 52, 3106–3114. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L. Preparation of a visual pH-sensing film based on tara gum incorporating cellulose and extracts from grape skins. Sens. Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J.; Qin, Y.; Bai, R.; Zhang, X.; Liu, J. Antioxidant and pH-sensitive films developed by incorporating purple and black rice extracts into chitosan matrix. Int. J. Biol. Macromol. 2019, 137, 307–316. [Google Scholar] [CrossRef]
- Cazón, P.; Velasquez, G.; Ramirez, J.A.; Vásquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Melgar, B.; Inês, M.; Ciric, A.; Sokovic, M.; Garcia-castello, E.M.; Rodriguez-lopez, A.D.; Barros, L.; Ferreira, I. By-product recovery of Opuntia spp. peels: Betalainic and phenolic profiles and bioactive properties. Ind. Crops Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural plant pigments with potential application in functional foods. LWT Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Del Santos-Díaz, M.S.; de la Rosa, A.P.B.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia spp.: Characterization and Benefits in Chronic Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Castro-Enríquez, D.D.; Montaño-Leyva, B.; Del Toro-Sánchez, C.L.; Juaréz-Onofre, J.E.; Carvajal-Millan, E.; Burruel-Ibarra, S.E.; Tapia-Hernández, J.A.; Barreras-Urbina, C.G.; Rodríguez-Félix, F. Stabilization of betalains by encapsulation—A review. J. Food Sci. Technol. 2020, 57, 1587–1600. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Jacquot, M.; Jasniewski, J.; Jacquot, C.; Imran, M.; Jamshidian, M.; Paris, C.; Desobry, S. Antioxidant capacity and light-aging study of HPMC films functionalized with natural plant extract. Carbohydr. Polym. 2012, 89, 1150–1158. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Guzmán, R.; Jaramillo, C.M.; Famá, L. Effect of beet flour on films made from biological macromolecules: Native and modified plantain flour. Int. J. Biol. Macromol. 2016, 82, 395–403. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, N.; Varaprasad, K.; Reddy, G.V.S.; Rajulu, A.V.; Zhang, J. Preparation and characterization of cellulose/curcumin composite films. RSC Adv. 2012, 2, 8483–8488. [Google Scholar] [CrossRef]
- Cho, M.H.; Paik, Y.S.; Hahn, T.R. Physical stability of shikonin derivatives from the roots of Lithospermum erythrorhizon cultivated in Korea. J. Agric. Food Chem. 1999, 47, 4117–4120. [Google Scholar] [CrossRef]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Couladouros, E.A.; Hepworth, D.; Nicolaou, K.C. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew. Chem. Int. Ed. 1999, 38, 270–301. [Google Scholar] [CrossRef]
- Papageorgiou, V.; Assimopoulou, A.; Samanidou, V.; Papadoyannis, I. Analytical Methods for the Determination of Alkannins and Shikonins. Curr. Org. Chem. 2006, 10, 583–622. [Google Scholar] [CrossRef]
- Jedermann, R.; Praeger, U.; Geyer, M.; Lang, W. Remote quality monitoring in the banana chain. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130303. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 1–19. [Google Scholar] [CrossRef] [Green Version]
- RipeSense. Available online: https://product.statnano.com/product/6730/ripesense (accessed on 1 March 2021).
- Esser, B.; Schnorr, J.M.; Swager, T.M. Selective detection of ethylene gas using carbon nanotube-based devices: Utility in determination of fruit ripeness. Angew. Chem. Int. Ed. 2012, 51, 5752–5756. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Oveissi, F.; Chandrawati, R.; Dehghani, F.; Naficy, S. Naked-Eye Detection of Ethylene Using Thiol-Functionalized Polydiacetylene-Based Flexible Sensors. ACS Sens. 2020, 5, 1921–1928. [Google Scholar] [CrossRef]
- Puligundla, P.; Jung, J.; Ko, S. Carbon dioxide sensors for intelligent food packaging applications. Food Control 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Saliu, F.; Pergola, R.D. Carbon dioxide colorimetric indicators for food packaging application: Applicability of anthocyanin and poly-lysine mixtures. Sens. Actuators B Chem. 2018, 258, 1117–1124. [Google Scholar] [CrossRef]
- Alshamrani, A.K.; Vanderveen, J.R.; Jessop, P.G. A guide to the selection of switchable functional groups for CO2-switchable compounds. Phys. Chem. Chem. Phys. 2016, 18, 19276–19288. [Google Scholar] [CrossRef]
- Alam, A.U.; Rathi, P.; Beshai, H.; Sarabha, G.K.; Deen, M.J. Fruit quality monitoring with smart packaging. Sensors 2021, 21, 1509. [Google Scholar] [CrossRef]
- Banerjee, S.; Kelly, C.; Kerry, J.P.; Papkovsky, D.B. High throughput non-destructive assessment of quality and safety of packaged food products using phosphorescent oxygen sensors. Trends Food Sci. Technol. 2016, 50, 85–102. [Google Scholar] [CrossRef] [Green Version]
- Chemical, M.G. Ageless Eye, Oxygen Indicator. Available online: https://www.mgc.co.jp/eng/products/sc/ageless-eye.html (accessed on 1 March 2021).
- Sharma, C.; Dhiman, R.; Rokana, N.; Panwar, H. Nanotechnology: An untapped resource for food packaging. Front. Microbiol. 2017, 8, 1735. [Google Scholar] [CrossRef] [Green Version]
- Primožič, M.; Knez, Ž.; Leitgeb, M. (Bio)Nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Alahi, M.E.E.; Mukhopadhyay, S.C. Detection methodologies for pathogen and toxins: A review. Sensors 2017, 17, 1885. [Google Scholar] [CrossRef] [Green Version]
- Shafiq, M.; Anjum, S.; Hano, C.; Anjum, I.; Abbasi, B.H. An overview of the applications of nanomaterials and nanodevices in the food industry. Foods 2020, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Lorite, G.S.; Selkälä, T.; Sipola, T.; Palenzuela, J.; Jubete, E.; Viñuales, A.; Cabañero, G.; Grande, H.J.; Tuominen, J.; Uusitalo, S.; et al. Novel, smart and RFID assisted critical temperature indicator for supply chain monitoring. J. Food Eng. 2017, 193, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Sothornvit, R. Nanostructured materials for food packaging systems: New functional properties. Curr. Opin. Food Sci. 2019, 25, 82–87. [Google Scholar] [CrossRef]
- Ribeiro, H.L.; Brito, E.S.; De, M.; Filho, M.S.; Azeredo, H.M.C. Applied Clay Science Montmorillonite as a reinforcement and color stabilizer of gelatin fi lms containing acerola juice. Appl. Clay Sci. 2018, 165, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Barandun, G.; Soprani, M.; Naficy, S.; Grell, M.; Kasimatis, M.; Chiu, K.L.; Ponzoni, A.; Güder, F. Cellulose Fibers Enable Near-Zero-Cost Electrical Sensing of Water-Soluble Gases. ACS Sens. 2019, 4, 1662–1669. [Google Scholar] [CrossRef]
- Fernando, A.L.; Godovikova, V.; Oliveira, J.F.S. Miscanthus x Giganteus: Contribution to a Sustainable Agriculture of a Future/Present—Oriented Biomaterial. Mater. Sci. Forum 2004, 455, 437–441. [Google Scholar] [CrossRef]
- Fernando, A.L.; Barbosa, B.; Costa, J.; Papazoglou, E.G. Giant Reed (Arundo donax L.). In Bioremediation and Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–95. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. https://doi.org/10.3390/s21062148
Rodrigues C, Souza VGL, Coelhoso I, Fernando AL. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors. 2021; 21(6):2148. https://doi.org/10.3390/s21062148
Chicago/Turabian StyleRodrigues, Carolina, Victor Gomes Lauriano Souza, Isabel Coelhoso, and Ana Luísa Fernando. 2021. "Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends" Sensors 21, no. 6: 2148. https://doi.org/10.3390/s21062148









