Review of Dissolved CO and H2 Measurement Methods for Syngas Fermentation
Abstract
:1. Introduction
2. Dissolved CO Measurement Methods
2.1. Current Status of Dissolved CO Measurement Methods in Syngas Fermentation
2.2. Potential Dissolved CO Measurement Methods
- Highly sensitive to CO due to low CO solubility in the aqueous solution;
- Excellent selectivity to H2 and other chemicals in the fermentation medium;
- Sensor should not dramatically change the conditions of fermentation, such as anaerobic fermentation, pH, and temperature and;
- Sensor should be capable for automatic, rapid, and reversible measurement.
2.2.1. Optical Sensors
Colorimetric Sensors
Fluorescent Sensors
Vacuum Ultraviolet Resonance Fluorescence Sensors
Photoacoustic Sensors
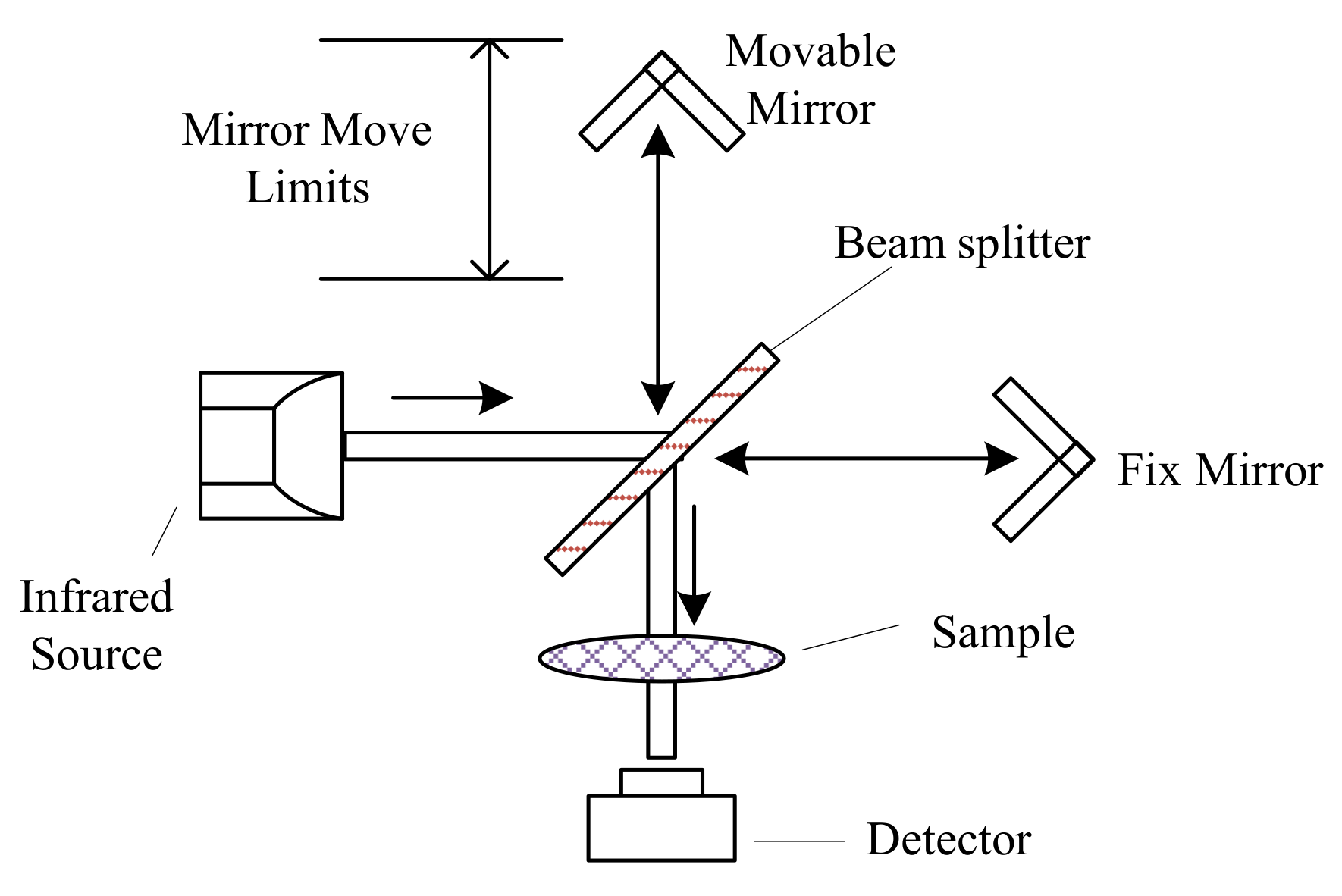
Infrared Sensors
2.2.2. Acoustic Wave Sensors
2.2.3. Electrochemical Sensors
Amperometric Sensors
Potentiometric Sensors
2.2.4. Conductivity Sensors
Metal-Oxide Semiconductor Sensors
Conducting Polymer Semiconductor Sensors
2.2.5. Work Function Type Sensors
Field Effect Transistor Sensors
MOS Capacitor Sensors
The Schottky Diode Sensors
2.2.6. Thermoelectric Sensors
2.3. Summary of the Potential Dissolved CO Measurement Method
| Sensor Type | Direct DCO Measurement Potential | Sensitivity/Detection Limit | Selectivity | Measurement Conditions | Other Challenges |
|---|---|---|---|---|---|
| Colorimetric: transition metal oxide | No report | High, 0.5 ppm [58] | H2 | Room and elevated temperature, May require O2 | Need spectrometer for signal analysis |
| Colorimetric: metallic proteins | CO-myoglobin assay | Moderate, unclear detection limit | NO | Room temperature | Proteins have limited lifespan, Slow recovery time |
| Colorimetric: chromogenic probes | Probes in solutions was used in CO detection | High, 11 ppm [38] | NOX, Require additional selectivity test | Room temperature | Color may be shaded by fermentation media |
| Fluorescent | Living tissue and in aqueous solution | High, ppb level [81] | Unclear for H2, Require additional selectivity test | Room temperature | Irreversible response, Need UV excitation of fluorescent probes |
| Nondispersive infrared | No report | High, ppm level [100] | HCN at 4.6 µm | Room temperature | Need infrared database analysis for proper detection wavelength |
| Tunable diode laser absorption spectroscopy | No report | Very High, ppb level [133] | HCN at 4.6 µm | Room temperature | Require mid-infrared laser for max sensitivity |
| Spectrometer and FTIR | No report | High, unreported detection limit | HCN at 4.6 µm | Room temperature | Complicated and expensive |
| Vacuum ultraviolet resonance fluorescence | No report | High, 1 ppb [85] | CO2, water vapor | Room temperature | Complicated system, Measurement range is small |
| Photoacoustic | No report | Very high, ppb level [97] | HCN at 4.6 µm | Room temperature | Require mid-infrared laser for max sensitivity |
| Bulk acoustic wave | No report | High, 0.91 ppm [152] | Depend on sensing materials | Room and elevated temperature, May require O2 | Irreversible detection Limited CO exposure time |
| Surface acoustic wave | No report | Moderate, 25 ppm [158] | Unclear | Room and elevated temperature. May require O2 | |
| Amperometric | No report | High, 0.01 ppm [165] | Reducing gases NOx, H2, and hydrocarbons | Room and elevated temperature. Require O2 | Measurement affected by relative humidity |
| Potentiometric | No report | High, 1 ppm [169] | Reducing gases NOx, H2, and hydrocarbons | Best sensitivity at elevated temperature, Require O2 | |
| Conductivity: Metal oxide semiconductor | No report | Moderate, 6-18 ppm [228] | Reducing gases NOx, H2, and hydrocarbons | Elevated temperature, May require O2 | Some materials affected by relative humidity |
| Conductivity: Conducting polymer | No report | High, 0.02 ppm [200] | H2, liquid petroleum gases, and CH4 for PANI. Other polymers unclear | Room, temperature, May require O2 | |
| Work function type: Field effect transistor | No report | Moderate, 54 ppm [206] | H2, CH4, NO, and ethanol | Elevated temperature, May require O2 | |
| Work function type: Metal-oxide-semiconductor capacitor | No report | Moderate, 100 ppm [203] | H2 | Elevated temperature, May require O2 | |
| Work function: the Schottky diode | No report | Moderate, 25 ppm [216] | NO and H2 | Elevated temperature, May require O2 | |
| Thermoelectric | No report | High, 1 ppm [220] | Depend on catalyst | Elevated temperature for best sensitivity, Require O2 | Require sophisticated signal processing |
3. Dissolved H2 Sensors
3.1. Current Status of the Dissolved H2 Measurement
3.2. Possible Improved Methods for the DH Measurement
4. Discussion and Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Phillips, J.R.; Huhnke, R.L.; Atiyeh, H.K. Syngas Fermentation: A Microbial Conversion Process of Gaseous Substrates to Various Products. Fermentation 2017, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, M.R.; Atiyeh, H.K. Microbial Production of Ethanol from Carbon Monoxide. Curr. Opin. Biotechnol. 2011, 22, 326–330. [Google Scholar] [CrossRef]
- Maddipati, P.; Atiyeh, H.K.; Bellmer, D.D.; Huhnke, R.L. Ethanol Production from Syngas by Clostridium Strain P11 Using Corn Steep Liquor as a Nutrient Replacement to Yeast Extract. Bioresour. Technol. 2011, 102, 6494–6501. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, P.C.; Khanal, S.K. Biomass-Derived Syngas Fermentation into Biofuels: Opportunities and Challenges. Bioresour. Technol. 2010, 101, 5013–5022. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic Biomass for Bioethanol Production: Current Perspectives, Potential Issues and Future Prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Devarapalli, M.; Atiyeh, H.K. A Review of Conversion Processes for Bioethanol Production with a Focus on Syngas Fermentation. Biofuel Res. J. 2015, 2, 268–280. [Google Scholar] [CrossRef]
- Pauss, A.; Samson, R.; Guiot, S.; Beauchemin, C. Continuous Measurement of Dissolved H2 in an Anaerobic Reactor Using a New Hydrogen/Air Fuel Cell Detector. Biotechnol. Bioeng. 1990, 35, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Sweet, W.J.; Houchins, J.P.; Rosen, P.R.; Arp, D.J. Polarographic Measurement of H2 in Aqueous Solutions. Anal. Biochem. 1980, 107, 337–340. [Google Scholar] [CrossRef]
- Orgill, J.J.; Abboud, M.C.; Atiyeh, H.K.; Devarapalli, M.; Sun, X.; Lewis, R.S. Measurement and Prediction of Mass Transfer Coefficients for Syngas Constituents in a Hollow Fiber Reactor. Bioresour. Technol. 2019, 276, 1–7. [Google Scholar] [CrossRef]
- Kundu, S.; Premer, S.A.; Hoy, J.A.; Trent, J.T.; Hargrove, M.S. Direct Measurement of Equilibrium Constants for High-Affinity Hemoglobins. Biophys. J. 2003, 84, 3931–3940. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.-H.; Ni, S.-Q.; Chang, S.-Y.; Sung, S.; Kim, S.-H. Enhancement of Carbon Monoxide Mass Transfer Using an Innovative External Hollow Fiber Membrane (HFM) Diffuser for Syngas Fermentation: Experimental Studies and Model Development. Chem. Eng. J. 2012, 184, 268–277. [Google Scholar] [CrossRef]
- Ramieri, A.; Jatlow, P.; Seligson, D. New Method for Rapid Determination of Carboxyhemoglobin by Use of Double-Wavelength Spectrophotometry. Clin. Chem. 1974, 20, 278–281. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Tan, L.; Zheng, K.; Huang, W. Lighting up Carbon Monoxide: Fluorescent Probes for Monitoring CO in Living Cells. Angew. Chem. Int. Ed. 2013, 52, 1628–1630. [Google Scholar] [CrossRef] [PubMed]
- Hara, N.; Macdonald, D.D. Development of Dissolved Hydrogen Sensors Based on Yttria-Stabilized Zirconia Solid Electrolyte with Noble Metal Electrodes. J. Electrochem. Soc. 1997, 144, 4152–4157. [Google Scholar] [CrossRef]
- Kriksunov, L.B.; Macdonald, D.D. Amperometric Hydrogen Sensor for High-Temperature Water. Sens. Actuators B Chem. 1996, 32, 57–60. [Google Scholar] [CrossRef]
- Lovley, D.R.; Chapelle, F.H.; Woodward, J.C. Use of Dissolved H2 Concentrations To Determine Distribution of Microbially Catalyzed Redox Reactions in Anoxic Groundwater. Environ. Sci. Technol. 1994, 28, 1205–1210. [Google Scholar] [CrossRef]
- Lovley, D.R.; Goodwin, S. Hydrogen Concentrations as an Indicator of the Predominant Terminal Electron-Accepting Reactions in Aquatic Sediments. Geochim. Cosmochim. Acta 1988, 52, 2993–3003. [Google Scholar] [CrossRef] [Green Version]
- Björnsson, L.; Hörnsten, E.G.; Mattiasson, B. Utilization of a Palladium-Metal Oxide Semiconductor (Pd-MOS) Sensor for on-Line Monitoring of Dissolved Hydrogen in Anaerobic Digestion. Biotechnol. Bioeng. 2001, 73, 35–43. [Google Scholar] [CrossRef]
- Cord-Ruwisch, R.; Mercz, T.I.; Hoh, C.-Y.; Strong, G.E. Dissolved Hydrogen Concentration as an On-Line Control Parameter for the Automated Operation and Optimization of Anaerobic Digesters. Biotechnol. Bioeng. 1997, 56, 626–634. [Google Scholar] [CrossRef]
- Sorita, R.; Kawano, T. A Highly Selective CO Sensor Using LaMnO3 Electrode-Attached Zirconia Galvanic Cell. Sens. Actuators B Chem. 1997, 40, 29–32. [Google Scholar] [CrossRef]
- van der Wal, P.D.; de Rooij, N.F.; Koudelka-Hep, M. Extremely Stable Nafion Based Carbon Monoxide Sensor. Sens. Actuators B Chem. 1996, 35, 119–123. [Google Scholar] [CrossRef]
- Wu, R.-J.; Chang, W.-C.; Tsai, K.-M.; Wu, J.-G. The Novel CO Sensing Material CoOOH–WO3 with Au and SWCNT Performance Enhancement. Sens. Actuators B Chem. 2009, 138, 35–41. [Google Scholar] [CrossRef]
- Matsumiya, M.; Qiu, F.; Shin, W.; Izu, N.; Matsubara, I.; Murayama, N.; Kanzaki, S. Thermoelectric CO Gas Sensor Using Thin-Film Catalyst of Au and Co3O4. J. Electrochem. Soc. 2004, 151, H7–H10. [Google Scholar] [CrossRef]
- Phillips, J.R.; Atiyeh, H.K.; Tanner, R.S.; Torres, J.R.; Saxena, J.; Wilkins, M.R.; Huhnke, R.L. Butanol and Hexanol Production in Clostridium Carboxidivorans Syngas Fermentation: Medium Development and Culture Techniques. Bioresour. Technol. 2015, 190, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Sander, R. Compilation of Henry’s Law Constants (Version 4.0) for Water as Solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Bay, H.W.; Blurton, K.F.; Sedlak, J.M.; Valentine, A.M. Electrochemical Technique for the Measurement of Carbon Monoxide. Anal. Chem. 1974, 46, 1837–1839. [Google Scholar] [CrossRef]
- Kobayashi, T.; Haruta, M.; Sano, H.; Nakane, M. A Selective CO Sensor Using Ti-Doped α-Fe2O3 with Coprecipitated Ultrafine Particles of Gold. Sens. Actuators 1988, 13, 339–349. [Google Scholar] [CrossRef]
- Riggs, S.S.; Heindel, T.J. Measuring Carbon Monoxide Gas-Liquid Mass Transfer in a Stirred Tank Reactor for Syngas Fermentation. Biotechnol. Prog. 2006, 22, 903–906. [Google Scholar] [CrossRef]
- Ungerman, A.J.; Heindel, T.J. Carbon Monoxide Mass Transfer for Syngas Fermentation in a Stirred Tank Reactor with Dual Impeller Configurations. Biotechnol. Prog. 2008, 23, 613–620. [Google Scholar] [CrossRef]
- Atiyeh, H.K.; Phillips, J.R.; Huhnke, R.L. Fermentation Control for Optimization of Syngas Utilization. U.S. Patent 10,640,792 B2, 13 November 2015. and issued 5 May 2020. [Google Scholar]
- Widdop, B. Analysis of Carbon Monoxide. Ann. Clin. Biochem. 2002, 39, 378–391. [Google Scholar] [CrossRef]
- Wang, J.; Karpus, J.; Zhao, B.S.; Luo, Z.; Chen, P.R.; He, C. A Selective Fluorescent Probe for Carbon Monoxide Imaging in Living Cells. Angew. Chem. Int. Ed. 2012, 51, 9652–9656. [Google Scholar] [CrossRef]
- Collison, H.A.; Rodkey, F.L.; O’Neal, J.D. Determination of Carbon Monoxide in Blood by Gas Chromatography. Clin. Chem. 1968, 14, 162–171. [Google Scholar] [CrossRef]
- Varlet, V.; Lagroy De Croutte, E.; Augsburger, M.; Mangin, P. Accuracy Profile Validation of a New Method for Carbon Monoxide Measurement in the Human Blood Using Headspace-Gas Chromatography–Mass Spectrometry (HS-GC–MS). J. Chromatogr. B 2012, 880, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Michel, B.W.; Lippert, A.R.; Chang, C.J. A Reaction-Based Fluorescent Probe for Selective Imaging of Carbon Monoxide in Living Cells Using a Palladium-Mediated Carbonylation. J. Am. Chem. Soc. 2012, 134, 15668–15671. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yan, J.; Li, J.; Yao, P.; Tan, J.; Zhang, L. A Colorimetric and Fluorescent Turn-on Probe for Carbon Monoxide and Imaging in Living Cells. Tetrahedron Lett. 2016, 57, 2927–2930. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Nan, D.; Lin, C.; Wan, Y.; Pan, Q.; Qi, Z. A Near-Infrared Fluorescent Probe for Rapid Detection of Carbon Monoxide in Living Cells. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2018, 202, 284–289. [Google Scholar] [CrossRef]
- Moragues, M.E.; Montes-Robles, R.; Ros-Lis, J.V.; Alcañíz, M.; Ibañez, J.; Pardo, T.; Martínez-Máñez, R. An Optoelectronic Sensing Device for CO Detection in Air Based on a Binuclear Rhodium Complex. Sens. Actuators B Chem. 2014, 191, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Swinnerton, J.W.; Linnenbom, V.J.; Cheek, C.H. Determination of Dissolved Gases in Aqueous Solutions by Gas Chromatography. Anal. Chem. 1962, 34, 483–485. [Google Scholar] [CrossRef]
- B’Hymer, C. Residual Solvent Testing: A Review of Gas-Chromatographic and Alternative Techniques. Pharm. Res. 2003, 20, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Brix, O. A Modified Van Slyke Apparatus. J. Appl. Physiol. 1981, 50, 1093–1097. [Google Scholar] [CrossRef]
- Van Slyke, D.D.; Neill, J.M. The Determination of Gases in Blood and Other Solutions by Vacuum Extraction and Manometric Measurement. I. J. Biol. Chem. 2002, 277, e16. [Google Scholar] [PubMed]
- Matsumoto, T.; Han, L.-F.; Jaklitsch, M.; Aggarwal, P.K. A Portable Membrane Contactor Sampler for Analysis of Noble Gases in Groundwater. Groundwater 2013, 51, 461–468. [Google Scholar] [CrossRef]
- Probst, P.; Yokochi, R.; Sturchio, N.C. Method for Extraction of Dissolved Gases From Groundwater for Radiokrypton Analysis. In Proceedings of the 4th Mini Conference on Noble Gases in the Hydrosphere and in Natural Gas Reservoirs, GFZ, Potsdam, Germany, 28 March 2007. [Google Scholar]
- Webb, J.R.; Maher, D.T.; Santos, I.R. Automated, in Situ Measurements of Dissolved CO2, CH4, and Δ13C Values Using Cavity Enhanced Laser Absorption Spectrometry: Comparing Response Times of Air-Water Equilibrators. Limnol. Oceanogr. Methods 2016, 14, 323–337. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Valencia, R.; Magana-Rodriguez, F.; Gerardo-Nieto, O.; Sepulveda-Jauregui, A.; Martinez-Cruz, K.; Walter Anthony, K.; Baer, D.; Thalasso, F. In Situ Measurement of Dissolved Methane and Carbon Dioxide in Freshwater Ecosystems by Off-Axis Integrated Cavity Output Spectroscopy. Environ. Sci. Technol. 2014, 48, 11421–11428. [Google Scholar] [CrossRef] [PubMed]
- Davey, N.G.; Krogh, E.T.; Gill, C.G. Membrane-Introduction Mass Spectrometry (MIMS). TrAC Trends Anal. Chem. 2011, 30, 1477–1485. [Google Scholar] [CrossRef]
- Kristensen, G.H.; Klausen, M.M.; Hansen, V.A.; Lauritsen, F.R. On-Line Monitoring of the Dynamics of Trihalomethane Concentrations in a Warm Public Swimming Pool Using an Unsupervised Membrane Inlet Mass Spectrometry System with off-Site Real-Time Surveillance. Rapid Commun. Mass Spectrom. RCM 2010, 24, 30–34. [Google Scholar] [CrossRef]
- Wenner, P.G.; Bell, R.J.; van Amerom, F.H.W.; Toler, S.K.; Edkins, J.E.; Hall, M.L.; Koehn, K.; Short, R.T.; Byrne, R.H. Environmental Chemical Mapping Using an Underwater Mass Spectrometer. TrAC Trends Anal. Chem. 2004, 23, 288–295. [Google Scholar] [CrossRef]
- Loose, B.; Stute, M.; Alexander, P.; Smethie, W.M. Design and Deployment of a Portable Membrane Equilibrator for Sampling Aqueous Dissolved Gases. Water Resour. Res. 2009, 45, W00D34. [Google Scholar] [CrossRef]
- Raub, J. (Ed.) Carbon Monoxide, 2nd ed.; Environmental health criteria; World Health Organization: Geneva, Switzerland, 1999; ISBN 978-92-4-157213-2. [Google Scholar]
- Ando, M.; Kobayashi, T.; Haruta, M. Enhancement in the Optical CO Sensitivity of NiO Film by the Deposition of Ultrafine Gold Particles. J. Chem. Soc. Faraday Trans. 1994, 90, 1011–1013. [Google Scholar] [CrossRef]
- Ghodselahi, T.; Zahrabi, H.; Saani, M.H.; Vesaghi, M.A. CO Gas Sensor Properties of Cu@CuO Core–Shell Nanoparticles Based on Localized Surface Plasmon Resonance. J. Phys. Chem. C 2011, 115, 22126–22130. [Google Scholar] [CrossRef]
- Blyth, D.J.; Aylott, J.W.; Richardson, D.J.; Russell, D.A. Sol–Gel Encapsulation of Metalloproteins for the Development of Optical Biosensors for Nitrogen Monoxide and Carbon Monoxide. Analyst 1995, 120, 2725–2730. [Google Scholar] [CrossRef]
- Ando, M.; Kobayashi, T.; Haruta, M. Optical CO Detection by Use of CuO/Au Composite Films. Sens. Actuators B Chem. 1995, 24–25, 851–853. [Google Scholar] [CrossRef]
- Itou, M.; Araki, Y.; Ito, O.; Kido, H. Carbon Monoxide Ligand-Exchange Reaction of Triruthenium Cluster Complexes Induced by Photosensitized Electron Transfer: A New Type of Photoactive CO Color Sensor. Inorg. Chem. 2006, 45, 6114–6116. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Kobayashi, T.; Iijima, S.; Haruta, M. Optical CO Sensitivity of Au–CuO Composite Film by Use of the Plasmon Absorption Change. Sens. Actuators B Chem. 2003, 96, 589–595. [Google Scholar] [CrossRef]
- Paliwal, A.; Sharma, A.; Tomar, M.; Gupta, V. Carbon Monoxide (CO) Optical Gas Sensor Based on ZnO Thin Films. Sens. Actuators B Chem. 2017, 250, 679–685. [Google Scholar] [CrossRef]
- Ando, M.; Kobayashi, T.; Haruta, M. Combined Effects of Small Gold Particles on the Optical Gas Sensing by Transition Metal Oxide Films. Catal. Today 1997, 36, 135–141. [Google Scholar] [CrossRef]
- Cantalini, C.; Post, M.; Buso, D.; Guglielmi, M.; Martucci, A. Gas Sensing Properties of Nanocrystalline NiO and Co3O4 in Porous Silica Sol–Gel Films. Sens. Actuators B Chem. 2005, 108, 184–192. [Google Scholar] [CrossRef]
- Nam, H.-J.; Sasaki, T.; Koshizaki, N. Optical CO Gas Sensor Using a Cobalt Oxide Thin Film Prepared by Pulsed Laser Deposition under Various Argon Pressures. J. Phys. Chem. B 2006, 110, 23081–23084. [Google Scholar] [CrossRef]
- Ando, M.; Chabicovsky, R.; Haruta, M. Optical Hydrogen Sensitivity of Noble Metal–Tungsten Oxide Composite Films Prepared by Sputtering Deposition. Proceeding Eighth Int. Meet. Chem. Sens. IMCS-8 Part 1 2001, 76, 13–17. [Google Scholar] [CrossRef]
- Benito-Garagorri, D.; Puchberger, M.; Mereiter, K.; Kirchner, K. Stereospecific and Reversible CO Binding at Iron Pincer Complexes. Angew. Chem. Int. Ed. 2008, 47, 9142–9145. [Google Scholar] [CrossRef]
- Courbat, J.; Pascu, M.; Gutmacher, D.; Briand, D.; Wöllenstein, J.; Hoefer, U.; Severin, K.; de Rooij, N.F. A Colorimetric CO Sensor for Fire Detection. Procedia Eng. 2011, 25, 1329–1332. [Google Scholar] [CrossRef]
- Esteban, J.; Ros-Lis, J.V.; Martínez-Máñez, R.; Marcos, M.D.; Moragues, M.; Soto, J.; Sancenón, F. Sensitive and Selective Chromogenic Sensing of Carbon Monoxide by Using Binuclear Rhodium Complexes. Angew. Chem. Int. Ed. 2010, 49, 4934–4937. [Google Scholar] [CrossRef]
- Peter, C.; Gutmacher, D.; Schumacher, I.; Schmitt, K.; Wöllenstein, J. 8.5.3 Colorimetric CO and NO2 Gas Sensors for Fire Detection. In Proceedings of the 14th International Meeting on Chemical Sensors—IMCS 2012, Nürnberg/Nuremberg, Germany, 20–23 May 2012; pp. 732–735. [Google Scholar]
- Park, H.-J.; Kim, K.; Chung, Y.K. ReI–IrI Bimetallic Complexes Based on a Bis(Chelating) Ligand Composed of 1,10-Phenanthroline and N-Heterocyclic Carbene: Coordination Chemistry and Their Application for Optical Indicator of CO Gas. Inorg. Chim. Acta 2014, 410, 214–220. [Google Scholar] [CrossRef]
- Moragues, M.E.; Toscani, A.; Sancenón, F.; Martínez-Máñez, R.; White, A.J.P.; Wilton-Ely, J.D.E.T. A Chromo-Fluorogenic Synthetic “Canary” for CO Detection Based on a Pyrenylvinyl Ruthenium(II) Complex. J. Am. Chem. Soc. 2014, 136, 11930–11933. [Google Scholar] [CrossRef]
- Marín-Hernández, C.; Toscani, A.; Sancenón, F.; Wilton-Ely, J.D.E.T.; Martínez-Máñez, R. Chromo-Fluorogenic Probes for Carbon Monoxide Detection. Chem. Commun. 2016, 52, 5902–5911. [Google Scholar] [CrossRef] [Green Version]
- Moragues, M.E.; Esteban, J.; Ros-Lis, J.V.; Martínez-Máñez, R.; Marcos, M.D.; Martínez, M.; Soto, J.; Sancenón, F. Sensitive and Selective Chromogenic Sensing of Carbon Monoxide via Reversible Axial CO Coordination in Binuclear Rhodium Complexes. J. Am. Chem. Soc. 2011, 133, 15762–15772. [Google Scholar] [CrossRef] [Green Version]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold Catalysts Prepared by Coprecipitation for Low-Temperature Oxidation of Hydrogen and of Carbon Monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Feng, W.; Liu, D.; Zhai, Q.; Feng, G. Lighting up Carbon Monoxide in Living Cells by a Readily Available and Highly Sensitive Colorimetric and Fluorescent Probe. Sens. Actuators B Chem. 2017, 240, 625–630. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Wang, R.; Yuan, R.; Liu, C.; Duan, Q.; Zhu, W.; Li, X.; Zhu, B. A Mitochondria-Targetable Colorimetric and Far-Red Fluorescent Probe for the Sensitive Detection of Carbon Monoxide in Living Cells. Anal. Methods 2019, 11, 288–295. [Google Scholar] [CrossRef]
- Feng, W.; Liu, D.; Feng, S.; Feng, G. Readily Available Fluorescent Probe for Carbon Monoxide Imaging in Living Cells. Anal. Chem. 2016, 88, 10648–10653. [Google Scholar] [CrossRef]
- Miloserdov, F.M.; Grushin, V.V. Palladium-Catalyzed Aromatic Azidocarbonylation. Angew. Chem. Int. Ed. 2012, 51, 3668–3672. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, X.; Wang, A.; Lau, C.; Lu, J. Bioluminescence Imaging of Carbon Monoxide in Living Cells and Nude Mice Based on Pd0-Mediated Tsuji–Trost Reaction. Anal. Chem. 2018, 90, 5951–5958. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.W.; Wolf, M.O. Drastic Luminescence Response to Carbon Monoxide from a RuII Complex Containing a Hemilabile Phosphane Pyrene Ether. Angew. Chem. Int. Ed. 2002, 41, 1898–1900. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, J.; Tan, Q.; Zhou, L.; Yao, P.; Lu, Y.; Tan, J.; Zhang, L. Development of a Colorimetric and NIR Fluorescent Dual Probe for Carbon Monoxide. RSC Adv. 2016, 6, 65373–65376. [Google Scholar] [CrossRef]
- Yan, T.; Chen, J.; Wu, S.; Mao, Z.; Liu, Z. A Rationally Designed Fluorescence Chemosensor for On-Site Monitoring of Carbon Monoxide in Air. Org. Lett. 2014, 16, 3296–3299. [Google Scholar] [CrossRef]
- Feng, W.; Hong, J.; Feng, G. Colorimetric and Ratiometric Fluorescent Detection of Carbon Monoxide in Air, Aqueous Solution, and Living Cells by a Naphthalimide-Based Probe. Sens. Actuators B Chem. 2017, 251, 389–395. [Google Scholar] [CrossRef]
- Sun, M.; Yu, H.; Zhang, K.; Wang, S.; Hayat, T.; Alsaedi, A.; Huang, D. Palladacycle Based Fluorescence Turn-On Probe for Sensitive Detection of Carbon Monoxide. ACS Sens. 2018, 3, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Yoon, T.; Cha, S.; Kim, S.; Yousuf, M.; Ahmed, N.; Kim, D.; Kang, H.-W.; Kim, K.S. Turn-on and Turn-off Fluorescent Probes for Carbon Monoxide Detection and Blood Carboxyhemoglobin Determination. ACS Sens. 2018, 3, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Lin, W.; Tan, L.; Chen, H.; Cui, H. A Unique Carbazole–Coumarin Fused Two-Photon Platform: Development of a Robust Two-Photon Fluorescent Probe for Imaging Carbon Monoxide in Living Tissues. Chem. Sci. 2014, 5, 3439–3448. [Google Scholar] [CrossRef]
- Gulino, A.; Gupta, T.; Altman, M.; Lo Schiavo, S.; Mineo, P.G.; Fragalà, I.L.; Evmenenko, G.; Dutta, P.; van der Boom, M.E. Selective Monitoring of Parts per Million Levels of CO by Covalently Immobilized Metal Complexes on Glass. Chem. Commun. 2008, 2900–2902. [Google Scholar] [CrossRef]
- Scharffe, D.; Slemr, F.; Brenninkmeijer, C.A.M.; Zahn, A. Carbon Monoxide Measurements Onboard the CARIBIC Passenger Aircraft Using UV Resonance Fluorescence. Atmos. Meas. Tech. 2012, 5, 1753–1760. [Google Scholar] [CrossRef] [Green Version]
- Volz, A.; Kley, D. A Resonance-Fluorescence Instrument for the in-Situ Measurement of Atmospheric Carbon Monoxide. J. Atmos. Chem. 1985, 2, 345–357. [Google Scholar] [CrossRef]
- Gerbig, C.; Kley, D.; Volz-Thomas, A.; Kent, J.; Dewey, K.; McKenna, D.S. Fast Response Resonance Fluorescence CO Measurements Aboard the C-130: Instrument Characterization and Measurements Made during North Atlantic Regional Experiment 1993. J. Geophys. Res. Atmos. 1996, 101, 29229–29238. [Google Scholar] [CrossRef] [Green Version]
- Hodgkinson, J.; Tatam, R.P. Optical Gas Sensing: A Review. Meas. Sci. Technol. 2013, 24, 012004. [Google Scholar] [CrossRef] [Green Version]
- Tam, A.C. Applications of Photoacoustic Sensing Techniques. Rev. Mod. Phys. 1986, 58, 381–431. [Google Scholar] [CrossRef]
- Harren, F.; Cotti, G.; Oomens, J.; te LintelHekkert, S. Photoacoustic Spectroscopy in Trace Gas Monitoring. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006; pp. 1–23. ISBN 978-0-470-02731-8. [Google Scholar]
- Patimisco, P.; Scamarcio, G.; Tittel, F.; Spagnolo, V. Quartz-Enhanced Photoacoustic Spectroscopy: A Review. Sensors 2014, 14, 6165–6206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vansteenkiste, T.H.; Faxvog, F.R.; Roessler, D.M. Photoacoustic Measurement of Carbon Monoxide Using a Semiconductor Diode Laser. Appl. Spectrosc. 1981, 35, 194–196. [Google Scholar] [CrossRef]
- Kosterev, A.A.; Bakhirkin, Y.A.; Curl, R.F.; Tittel, F.K. Quartz-Enhanced Photoacoustic Spectroscopy. Opt. Lett. 2002, 27, 1902–1904. [Google Scholar] [CrossRef] [Green Version]
- Bozóki, Z.; Pogány, A.; Szabó, G. Photoacoustic Instruments for Practical Applications: Present, Potentials, and Future Challenges. Appl. Spectrosc. Rev. 2011, 46, 1–37. [Google Scholar] [CrossRef]
- Gerlach, R.; Amer, N.M. Sensitive Optoacoustic Detection of Carbon Monoxide by Resonance Absorption. Appl. Phys. Lett. 1978, 32, 228–231. [Google Scholar] [CrossRef] [Green Version]
- Kosterev, A.A.; Bakhirkin, Y.A.; Tittel, F.K. Ultrasensitive Gas Detection by Quartz-Enhanced Photoacoustic Spectroscopy in the Fundamental Molecular Absorption Bands Region. Appl. Phys. B 2005, 80, 133–138. [Google Scholar] [CrossRef]
- Stefanski, P.; Lewicki, R.; Tarka, J.; Ma, Y.; Jahjah, M.; Tittel, F.K. Sensitive Detection of Carbon Monoxide Using a Compact High Power CW DFB-QCL Based QEPAS Sensor. In Proceedings of the CLEO: 2013; OSA: San Jose, CA, USA, 2013; p. JW2A.68. [Google Scholar]
- Stuart, B.H. Introduction. In Infrared Spectroscopy: Fundamentals and Applications; Ando, D.J., Stuart, B.H., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 1–13. ISBN 978-0-470-01114-0. [Google Scholar]
- Dinh, T.-V.; Choi, I.-Y.; Son, Y.-S.; Kim, J.-C. A Review on Non-Dispersive Infrared Gas Sensors: Improvement of Sensor Detection Limit and Interference Correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Silver, J.; Chen, S.-J. Carbon Monoxide Sensor for Combustion Feedback Control; American Institute of Aeronautics and Astronautics: Reno, Nevada, 9 January 2006. [Google Scholar]
- Werle, P.; Slemr, F.; Maurer, K.; Kormann, R.; Mücke, R.; Jänker, B. Near- and Mid-Infrared Laser-Optical Sensors for Gas Analysis. Opt. Lasers Eng. 2002, 37, 101–114. [Google Scholar] [CrossRef]
- Childers, J.W.; Thompson, E.L.; Harris, D.B.; Kirchgessner, D.A.; Clayton, M.; Natschke, D.F.; Phillips, W.J. Multi-Pollutant Concentration Measurements around a Concentrated Swine Production Facility Using Open-Path FTIR Spectrometry. Atmos. Environ. 2001, 35, 1923–1936. [Google Scholar] [CrossRef]
- Twiss, S.B.; Teague, D.M.; Bozek, J.W.; Sink, M.V. Application of Infrared Spectroscopy to Exhaust Gas Analysis. J. Air Pollut. Control Assoc. 1955, 5, 75–83. [Google Scholar] [CrossRef]
- Zhao, Y.; Strong, K.; Kondo, Y.; Koike, M.; Matsumi, Y.; Irie, H.; Rinsland, C.P.; Jones, N.B.; Suzuki, K.; Nakajima, H.; et al. Spectroscopic Measurements of Tropospheric CO, C2H6, C2H2, and HCN in Northern Japan. J. Geophys. Res. Atmos. 2002, 107, 4343. [Google Scholar] [CrossRef]
- Li, G.-L.; Sui, Y.; Dong, M.; Ye, W.-L.; Zheng, C.-T.; Wang, Y.-D. A Carbon Monoxide Detection Device Based on Mid-Infrared Absorption Spectroscopy at 4.6 μm. Appl. Phys. B 2015, 119, 287–296. [Google Scholar] [CrossRef]
- Williams, P.; Norris, K. (Eds.) Near-Infrared Technology in the Agricultural and Food Industries; American Association of Cereal Chemists, Inc.: St. Paul, Minnesota, USA, 1988. [Google Scholar]
- Barron-Jimenez, R.; Caton, J.A.; Anderson, T.N.; Lucht, R.P.; Walther, T.; Roy, S.; Brown, M.S.; Gord, J.R. Application of a Difference-Frequency-Mixing Based Diode-Laser Sensor for Carbon Monoxide Detection in the 4.4–4.8 μm Spectral Region. Appl. Phys. B 2006, 85, 185–197. [Google Scholar] [CrossRef]
- Rothman, L.S.; Gordon, I.E.; Barbe, A.; Benner, D.C.; Bernath, P.F.; Birk, M.; Boudon, V.; Brown, L.R.; Campargue, A.; Champion, J.-P.; et al. The HITRAN 2008 Molecular Spectroscopic Database. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 533–572. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.-V.; Ahn, J.-W.; Choi, I.-Y.; Song, K.-Y.; Chung, C.-H.; Kim, J.-C. Limitations of Gas Filter Correlation: A Case Study on Carbon Monoxide Non-Dispersive Infrared Analyzer. Sens. Actuators B Chem. 2017, 243, 684–689. [Google Scholar] [CrossRef]
- Koppius, O.G. Analysis of Mixtures with Double-Beam Nondispersive Infrared Instrument. Anal. Chem. 1951, 23, 554–559. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Smith, R.; Ho, W.O.; Saffell, J.R.; Tatam, R.P. Non-Dispersive Infra-Red (NDIR) Measurement of Carbon Dioxide at 4.2μm in a Compact and Optically Efficient Sensor. Sens. Actuators B Chem. 2013, 186, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.L.C.; Soljačić, M.; Joannopoulos, J.D. Thermal Emission and Design in 2D-Periodic Metallic Photonic Crystal Slabs. Opt. Express 2006, 14, 8785–8796. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, S.E.; Gavrilov, G.A.; Kapralov, A.A.; Karandashev, S.A.; Matveev, B.A.; Sotnikova, G.Y.; Stus’, N.M. Portable Optoelectronic Gas Sensors Operating in the Mid-IR Spectral Range (λ=3~5 μm). In Proceedings of the Second International Conference on Lasers for Measurement and Information Transfer, St. Petersburg, Russia, 6–8 June 2001; p. 188. [Google Scholar]
- Haigh, M.K.; Nash, G.R.; Terakado, T.; Bando, N.; Yamagishi, Y.; Smith, S.J.; Buckle, L.; Emeny, M.T.; Ashley, T. Mid-Infrared AlxIn1-xSb Components for Gas Sensing. J. Phys. Conf. Ser. 2007, 76, 012003. [Google Scholar] [CrossRef] [Green Version]
- Sotnikova, G.Y.; Gavrilov, G.A.; Aleksandrov, S.E.; Kapralov, A.A.; Karandashev, S.A.; Matveev, B.A.; Remennyy, M.A. Low Voltage CO2-Gas Sensor Based on III–V Mid-IR Immersion Lens Diode Optopairs: Where We Are and How Far We Can Go? IEEE Sens. J. 2010, 10, 225–234. [Google Scholar] [CrossRef]
- MacIsaac, D.; Kanner, G.; Anderson, G. Basic Physics of the Incandescent Lamp (Lightbulb). Phys. Teach. 1999, 37, 520–525. [Google Scholar] [CrossRef]
- Lin, S.Y.; Moreno, J.; Fleming, J.G. Three-Dimensional Photonic-Crystal Emitter for Thermal Photovoltaic Power Generation. Appl. Phys. Lett. 2003, 83, 380–382. [Google Scholar] [CrossRef] [Green Version]
- Popov, A.A.; Sherstnev, V.V.; Yakovlev, Y.P.; Baranov, A.N.; Alibert, C. Powerful Mid-Infrared Light Emitting Diodes for Pollution Monitoring. Electron. Lett. 1997, 33, 86–88. [Google Scholar] [CrossRef]
- Inoue, T.; De Zoysa, M.; Asano, T.; Noda, S. Filter-Free Nondispersive Infrared Sensing Using Narrow-Bandwidth Mid-Infrared Thermal Emitters. Appl. Phys. Express 2014, 7, 012103. [Google Scholar] [CrossRef]
- Abell, J.; Kim, C.S.; Bewley, W.W.; Merritt, C.D.; Canedy, C.L.; Vurgaftman, I.; Meyer, J.R.; Kim, M. Mid-Infrared Interband Cascade Light Emitting Devices with Milliwatt Output Powers at Room Temperature. Appl. Phys. Lett. 2014, 104, 261103. [Google Scholar] [CrossRef]
- Fanchenko, S.; Baranov, A.; Savkin, A.; Sleptsov, V. LED-Based NDIR Natural Gas Analyzer. IOP Conf. Ser. Mater. Sci. Eng. 2016, 108, 012036. [Google Scholar] [CrossRef] [Green Version]
- Downs, C.; Vandervelde, T.E. Progress in Infrared Photodetectors since 2000. Sensors 2013, 13, 5054–5098. [Google Scholar] [CrossRef]
- Rogalski, A. History of Infrared Detectors. Opto-Electron. Rev. 2012, 20, 279–308. [Google Scholar] [CrossRef]
- Rogalski, A. Infrared Detectors: An Overview. Infrared Phys. Technol. 2002, 43, 187–210. [Google Scholar] [CrossRef] [Green Version]
- Rogalski, A. Recent Progress in Infrared Detector Technologies. Infrared Phys. Technol. 2011, 54, 136–154. [Google Scholar] [CrossRef]
- Rogalski, A. HgCdTe Infrared Detector Material: History, Status and Outlook. Rep. Prog. Phys. 2005, 68, 2267–2336. [Google Scholar] [CrossRef] [Green Version]
- Schiff, H.I.; Mackay, G.I.; Bechara, J. The Use of Tunable Diode Laser Absorption Spectroscopy for Atmospheric Measurements. Res. Chem. Intermed. 1994, 20, 525–556. [Google Scholar] [CrossRef]
- Miller, J.H.; Elreedy, S.; Ahvazi, B.; Woldu, F.; Hassanzadeh, P. Tunable Diode-Laser Measurement of Carbon Monoxide Concentration and Temperature in a Laminar Methane–Air Diffusion Flame. Appl. Opt. 1993, 32, 6082–6089. [Google Scholar] [CrossRef]
- Ebert, V.; Giesemann, C.; Koeth, J.; Teichert, H. New Room-Temperature 2.3μm DFB-Diode Lasers: First Spectroscopic Characterization and CO-Detection. Optical Society of America: Annapolis, MD, USA, 9 February 2004; p. TuF9. [Google Scholar]
- Wang, J.; Maiorov, M.; Jeffries, J.B.; Garbuzov, D.Z.; Connolly, J.C.; Hanson, R.K. A Potential Remote Sensor of CO in Vehicle Exhausts Using 2.3 μm Diode Lasers. Meas. Sci. Technol. 2000, 11, 1576–1584. [Google Scholar] [CrossRef]
- Cai, T.; Gao, G.; Chen, W.; Liu, G.; Gao, X. Simultaneous Measurements of CO2 and CO Using a Single Distributed-Feedback (DFB) Diode Laser near 1.57 μm at Elevated Temperatures. Appl. Spectrosc. 2011, 65, 108–112. [Google Scholar] [CrossRef]
- Li, J.; Yu, Z.; Du, Z.; Ji, Y.; Liu, C. Standoff Chemical Detection Using Laser Absorption Spectroscopy: A Review. Remote Sens. 2020, 12, 2771. [Google Scholar] [CrossRef]
- Tittel, F.K.; Richter, D.; Fried, A. Mid-Infrared Laser Applications in Spectroscopy. In Solid-State Mid-Infrared Laser Sources; Sorokina, I.T., Vodopyanov, K.L., Eds.; Topics in Applied Physics; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2003; Volume 89, pp. 458–529. ISBN 978-3-540-00621-3. [Google Scholar]
- Kosterev, A.A.; Tittel, F.K. Chemical Sensors Based on Quantum Cascade Lasers. IEEE J. Quantum Electron. 2002, 38, 582–591. [Google Scholar] [CrossRef]
- Godard, A. Infrared (2–12 μm) Solid-State Laser Sources: A Review. C. R. Phys. 2007, 8, 1100–1128. [Google Scholar] [CrossRef]
- Capasso, F.; Gmachl, C.; Sivco, D.L.; Cho, A.Y. Quantum Cascade Lasers. Phys. World 1999, 12, 27–34. [Google Scholar] [CrossRef]
- Chao, X.; Jeffries, J.B.; Hanson, R.K. Real-Time, in Situ, Continuous Monitoring of CO in a Pulverized-Coal-Fired Power Plant with a 2.3 μm Laser Absorption Sensor. Appl. Phys. B 2013, 110, 359–365. [Google Scholar] [CrossRef]
- Chen, J.; Hangauer, A.; Strzoda, R.; Fleischer, M.; Amann, M.-C. Miniaturized Laser Spectroscopic CO Sensor for Industrial and Safety Applications. Procedia Chem. 2009, 1, 1383–1386. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Parchatka, U.; Königstedt, R.; Fischer, H. Real-Time Measurements of Atmospheric CO Using a Continuous-Wave Room Temperature Quantum Cascade Laser Based Spectrometer. Opt. Express 2012, 20, 7590–7601. [Google Scholar] [CrossRef]
- Petrov, K.P.; Curl, R.F.; Tittel, F.K. Compact Laser Difference-Frequency Spectrometer for Multicomponent Trace Gas Detection. Appl. Phys. B 1998, 66, 531–538. [Google Scholar] [CrossRef]
- Mulrooney, J.; Clifford, J.; Fitzpatrick, C.; Chambers, P.; Lewis, E. A Mid-Infrared Optical Fibre Sensor for the Detection of Carbon Monoxide Exhaust Emissions. Sens. Actuators Phys. 2008, 144, 13–17. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Zhang, G.; Sa, J. A Near-Infrared Optical Fiber Sensor for Carbon Monoxide Concentration Monitoring. Microw. Opt. Technol. Lett. 2010, 52, 2192–2195. [Google Scholar] [CrossRef]
- Tools for infrared and Raman spectroscopy. In Infrared and Raman Spectroscopy; Schrader, B. (Ed.) John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1995; pp. 63–188. ISBN 978-3-527-61543-8. [Google Scholar]
- Saptari, V. Chapter 1: Spectroscopy Instrumentation. In Fourier-Transform Spectroscopy Instrumentation Engineering; SPIE: Bellingham, WA, USA, 2003. [Google Scholar]
- Bacsik, Z.; Mink, J.; Keresztury, G. FTIR Spectroscopy of the Atmosphere. I. Principles and Methods. Appl. Spectrosc. Rev. 2004, 39, 295–363. [Google Scholar] [CrossRef]
- Downing, H.D.; Williams, D. Optical Constants of Water in the Infrared. J. Geophys. Res. 1975, 80, 1656–1661. [Google Scholar] [CrossRef]
- Fietzek, P.; Fiedler, B.; Steinhoff, T.; Körtzinger, A. In Situ Quality Assessment of a Novel Underwater PCO2 Sensor Based on Membrane Equilibration and NDIR Spectrometry. J. Atmos. Ocean. Technol. 2014, 31, 181–196. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.-G.; Lee, S.-H.; Zhou, T.; Shieh, J.-J.; Chung, T.-S. A Study on Pilot-Scale Degassing by Polypropylene (PP) Hollow Fiber Membrane Contactors. Desalination 2008, 234, 316–322. [Google Scholar] [CrossRef]
- Ippolito, S.J.; Trinchi, A.; Powell, D.A.; Wlodarski, W. Acoustic Wave Gas and Vapor Sensors. In Solid State Gas Sensing; Comini, E., Faglia, G., Sberveglieri, G., Eds.; Springer: Boston, MA, USA, 2009; pp. 1–44. ISBN 978-0-387-09665-0. [Google Scholar]
- Gomes, M.T.S.R.; Sérgio, P.; Nogueira, T.; Duarte, A.C.; Oliveira, J.A.B.P. Development of a Methodology for the Determination of Carbon Monoxide Using a Quartz Crystal Microbalance. Analyst 1999, 124, 1449–1453. [Google Scholar] [CrossRef]
- Ho, M.H.; Guilbault, G.G.; Scheide, E.P. Detection of Carbon Monoxide in Ambient Air with a Piezoelectric Crystal. Anal. Chem. 1982, 54, 1998–2002. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Shih, J.-S. Cryptand/Metal Ion Coated Piezoelectric Quartz Crystal Sensors with Artificial Back Propagation Neural Network Analysis for Nitrogen Dioxide and Carbon Monoxide. Sens. Actuators B Chem. 2005, 106, 468–476. [Google Scholar] [CrossRef]
- Alder, J.F.; McCallum, J.J. Piezoelectric Crystals for Mass and Chemical Measurements. A Review. Analyst 1983, 108, 1169–1189. [Google Scholar] [CrossRef]
- Miura, N.; Minamoto, H.; Sakai, G.; Yamazoe, N. New-Type Calorimetric Gas Sensor Using Temperature Characteristics of Piezoelectric Quartz Crystal Fitted with Noble Metal Catalyst Film. Sens. Actuators B Chem. 1991, 5, 211–217. [Google Scholar] [CrossRef]
- Fung, Y.S.; Wong, C.C.W. Determination of Carbon Monoxide in Ambient Air Using Piezoelectric Crystal Sorption Detection. Anal. Chim. Acta 2002, 456, 227–239. [Google Scholar] [CrossRef]
- Guimarães, O.M.; Zaniquelli, M.E.D.; Castro, J.R.M.; Balbo, V.R.; Andrade, J.F. Determination of Carbon Monoxide Using a Coated Quartz Crystal Sensor. Eclet. Quim. 2006, 31, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Sadek, A.Z.; Wlodarski, W.; Kalantar-zadeh, K. A ZnO Nanorod Based 64° YX LiNbO3 Surface Acoustic Wave CO Sensor. In Proceedings of the 2006 5th IEEE Conference on Sensors, Daegu, Korea, 22–25 October 2006; pp. 691–694. [Google Scholar]
- Hejczyk, T.; Urbańczyk, M.; Pustelny, T.; Jakubik, W. Numerical and Experimental Analysis of the Response of a SAW Structure with WO3 Layers on Action of Carbon Monoxide. Arch. Acoust. 2015, 40, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Arsat, R.; Breedon, M.; Shafiei, M.; Spizziri, P.G.; Gilje, S.; Kaner, R.B.; Kalantar-zadeh, K.; Wlodarski, W. Graphene-like Nano-Sheets for Surface Acoustic Wave Gas Sensor Applications. Chem. Phys. Lett. 2009, 467, 344–347. [Google Scholar] [CrossRef]
- Vanotti, M.; Blondeau-Patissier, V.; Ballandras, S. Development of Love Wave Devices Based on Delay Line Configuration for the High Detection and Monitoring of Carbon Monoxide. In Proceedings of the Acoustics 2012, Nantes, France, 23 April 2012. [Google Scholar]
- Blondeau-Patissier, V.; Vanotti, M.; Rabus, D.; Ballandras, S.; Chkounda, M.; Barbe, J.M.; Rauch, J.Y. Development of Accurate System of Gas Detection Based on Love Wave Sensors Functionalized with Cobalt Corroles Applied to the Detection of Carbon Monoxide. In Proceedings of the 2011 IEEE SENSORS Proceedings, Limerick, Ireland, 28–31 October 2011; pp. 1078–1081. [Google Scholar]
- Wei, C.; Peng, Z. Study on Wireless Intelligent Surface Acoustic Wave CO Gas Sensor Based on Grid Computing and Web Service. In Proceedings of the 2010 International Conference on Networking and Digital Society, Wenzhou, China, 30–31 May 2010; pp. 121–125. [Google Scholar]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical Sensors and Biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [Green Version]
- Stetter, J.R.; Li, J. Amperometric Gas Sensors A Review. Chem. Rev. 2008, 108, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, P.; Manesh, K.M.; Gopalan, A.; Lee, K.-P. Novel Amperometric Carbon Monoxide Sensor Based on Multi-Wall Carbon Nanotubes Grafted with Polydiphenylamine—Fabrication and Performance. Sens. Actuators B Chem. 2007, 125, 92–99. [Google Scholar] [CrossRef]
- Yan, H.; Liu, C.-C. A Solid Polymer Electrolyte-Bases Electrochemical Carbon Monoxide Sensor. Sens. Actuators B Chem. 1994, 17, 165–168. [Google Scholar] [CrossRef]
- Yasuda, A.; Yamaga, N.; Doi, K.; Fujioka, T.; Kusanagi, S. A Planar Electrochemical Carbon Monoxide Sensor. J. Electrochem. Soc. 1992, 139, 1091–1095. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Palombari, R. Amperometric Sensor for Carbon Monoxide Based on Solid State Protonic Conduction. Solid State Ion. 1993, 61, 241–244. [Google Scholar] [CrossRef]
- Takeda, H.; Ueda, T.; Kamada, K.; Matsuo, K.; Hyodo, T.; Shimizu, Y. CO-Sensing Properties of a NASICON-Based Gas Sensor Attached with Pt Mixed with Bi2O3 as a Sensing Electrode. Electrochim. Acta 2015, 155, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Can, Z.Y.; Narita, H.; Mizusaki, J.; Tagawa, H. Detection of Carbon Monoxide by Using Zirconia Oxygen Sensor. Solid State Ion. 1995, 79, 344–348. [Google Scholar] [CrossRef]
- Yasuda, A.; Yamaga, K.; Doi, K.; Fujioka, T.; Kusanagi, S. Electrochemical Characteristics of the Planar Electrochemical Carbon Monoxide Sensor with a Perfluorosulfonate Ionomer Film. Solid State Ion. 1990, 40–41, 476–479. [Google Scholar] [CrossRef]
- Mukundan, R. A Low Temperature Sensor for the Detection of Carbon Monoxide in Hydrogen. Solid State Ion. 2004, 175, 497–501. [Google Scholar] [CrossRef]
- Chen, C.; He, J.; Xu, D.; Tan, X.; Zhou, X.; Wang, X. Study of Nano-Au-Assembled Amperometric CO Gas Sensor. Sens. Actuators B Chem. 2005, 107, 866–871. [Google Scholar] [CrossRef]
- Fergus, J.W. Solid Electrolyte Based Sensors for the Measurement of CO and Hydrocarbon Gases. Sens. Actuators B Chem. 2007, 122, 683–693. [Google Scholar] [CrossRef]
- Okamoto, H.; Obayashi, H.; Kudo, T. Carbon Monoxide Gas Sensor Made of Stabilized Zirconia. Solid State Ion. 1980, 1, 319–326. [Google Scholar] [CrossRef]
- Li, N.; Tan, T.C.; Zeng, H.C. High-Temperature Carbon Monoxide Potentiometric Sensor. J. Electrochem. Soc. 1993, 140, 1068–1073. [Google Scholar] [CrossRef]
- Miura, N.; Raisen, T.; Lu, G.; Yamazoe, N. Highly Selective CO Sensor Using Stabilized Zirconia and a Couple of Oxide Electrodes. Sens. Actuators B Chem. 1998, 47, 84–91. [Google Scholar] [CrossRef]
- Wu, R.-J.; Hu, C.-H.; Yeh, C.-T.; Su, P.-G. Nanogold on Powdered Cobalt Oxide for Carbon Monoxide Sensor. Sens. Actuators B Chem. 2003, 96, 596–601. [Google Scholar] [CrossRef]
- Goto, T.; Hyodo, T.; Ueda, T.; Kamada, K.; Kaneyasu, K.; Shimizu, Y. CO-Sensing Properties of Potentiometric Gas Sensors Using an Anion-Conducting Polymer Electrolyte and Au-Loaded Metal Oxide Electrodes. Electrochim. Acta 2015, 166, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef] [Green Version]
- Windischmann, H.; Mark, P. A Model for the Operation of a Thin-Film SnOx Conductance-Modulation Carbon Monoxide Sensor. J. Electrochem. Soc. 1979, 126, 627–633. [Google Scholar] [CrossRef]
- Yamaura, H.; Tamaki, J.; Moriya, K.; Mirua, N.; Yamazoe, N. Selective CO Detection by Using Indium Oxide-Based Semiconductor Gas Sensor. J. Electrochem. Soc. 1996, 143, L36–L37. [Google Scholar] [CrossRef]
- Morrison, S.R. Semiconductor Gas Sensors. Sens. Actuators 1981, 2, 329–341. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, R.-J.; Wu, J.-G.; Yu, M.-R.; Tsai, T.-K.; Yeh, C.-T. Promotive Effect of CNT on Co3O4–SnO2 in a Semiconductor-Type CO Sensor Working at Room Temperature. Sens. Actuators B Chem. 2008, 131, 306–312. [Google Scholar] [CrossRef]
- Ghosh, S.; Chowdhury, U.; Roy, S.; Bandyopadhyay, R. Detection of Low Ppm Carbon Monoxide with Charge Ordered LuFe2O4 Gas Sensor – A Novel Sensing Mechanism. Ceram. Int. 2016, 42, 14944–14948. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal Oxides for Solid-State Gas Sensors: What Determines Our Choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Yamaura, H.; Moriya, K.; Miura, N.; Yamazoe, N. Mechanism of Sensitivity Promotion in CO Sensor Using Indium Oxide and Cobalt Oxide. Sens. Actuators B Chem. 2000, 65, 39–41. [Google Scholar] [CrossRef]
- Ansari, Z.A.; Ansari, S.G.; Ko, T.; Oh, J.-H. Effect of MoO3 Doping and Grain Size on SnO2-Enhancement of Sensitivity and Selectivity for CO and H2 Gas Sensing. Sens. Actuators B Chem. 2002, 87, 105–114. [Google Scholar] [CrossRef]
- More, P.S.; Khollam, Y.B.; Deshpande, S.B.; Sainkar, S.R.; Date, S.K.; Karekar, R.N.; Aiyer, R.C. High-Performance Temperature-Selective SnO2:Cu-Based Sensor. Mater. Lett. 2003, 57, 2177–2184. [Google Scholar] [CrossRef]
- Chang, J.F.; Kuo, H.H.; Leu, I.C.; Hon, M.H. The Effects of Thickness and Operation Temperature on ZnO:Al Thin Film CO Gas Sensor. Sens. Actuators B Chem. 2002, 84, 258–264. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal Oxide-Based Gas Sensor Research: How To? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Yamaura, H.; Nakaoka, M.; Yahiro, H. Effect of Supported Transition Metal on CO Sensing Performance Using SnO2 in Reducing Atmosphere. Adv. Mater. Res. 2008, 47–50, 1518–1521. [Google Scholar] [CrossRef]
- Pandey, S. Highly Sensitive and Selective Chemiresistor Gas/Vapor Sensors Based on Polyaniline Nanocomposite: A Comprehensive Review. J. Sci. Adv. Mater. Devices 2016, 1, 431–453. [Google Scholar] [CrossRef] [Green Version]
- Dixit, V.; Misra, S.C.K.; Sharma, B.S. Carbon Monoxide Sensitivity of Vacuum Deposited Polyaniline Semiconducting Thin Films. Sens. Actuators B Chem. 2005, 104, 90–93. [Google Scholar] [CrossRef]
- Ram, M.K.; Yavuz, Ö.; Lahsangah, V.; Aldissi, M. CO Gas Sensing from Ultrathin Nano-Composite Conducting Polymer Film. Sens. Actuators B Chem. 2005, 106, 750–757. [Google Scholar] [CrossRef]
- Liu, C.; Noda, Z.; Sasaki, K.; Hayashi, K. Development of a Polyaniline Nanofiber-Based Carbon Monoxide Sensor for Hydrogen Fuel Cell Application. Int. J. Hydrogen Energy 2012, 37, 13529–13535. [Google Scholar] [CrossRef]
- Sen, T.; Shimpi, N.G.; Mishra, S. Room Temperature CO Sensing by Polyaniline/Co3O4 Nanocomposite. J. Appl. Polym. Sci. 2016, 133, 44115. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, X.; Huang, C.; Chen, X.; Dai, W.; Fu, X. CO Gas Sensitivity and Its Oxidation over TiO2 Modified by PANI under UV Irradiation at Room Temperature. Appl. Catal. B Environ. 2017, 219, 379–390. [Google Scholar] [CrossRef]
- Misra, S.C.K.; Mathur, P.; Srivastava, B.K. Vacuum-Deposited Nanocrystalline Polyaniline Thin Film Sensors for Detection of Carbon Monoxide. Sens. Actuators Phys. 2004, 114, 30–35. [Google Scholar] [CrossRef]
- Javadpour, S.; Gharavi, A.; Feizpour, A.; Khanehzar, A.; Panahi, F. Morpholine Doped Poly(3,4-Ethylenedioxy)Thiophene–Poly(Styrenesulfonate) as a Low Temperature and Quick Carbon Monoxide Sensor. Sens. Actuators B Chem. 2009, 142, 152–158. [Google Scholar] [CrossRef]
- Krey, D.; Dobos, K.; Zimmer, G. An Integrated Co-Sensitive Mos Transistor. Sens. Actuators 1982, 3, 169–177. [Google Scholar] [CrossRef]
- Formoso, M.A.; Maclay, G.J. The Effect of Hydrogen and Carbon Monoxide on the Interface State Density in MOS Gas Sensors with Ultra-Thin Palladium Gates. Sens. Actuators B Chem. 1990, 2, 11–22. [Google Scholar] [CrossRef]
- Campos, M.; Bulhões, L.O.S.; Lindino, C.A. Gas-Sensitive Characteristics of Metal/Semiconductor Polymer Schottky Device. Sens. Actuators Phys. 2000, 87, 67–71. [Google Scholar] [CrossRef]
- Dobos, K.; Zimmer, G. Performance of Carbon Monoxide-Sensitive MOSFET’s with Metal-Oxide Semiconductor Gates. IEEE Trans. Electron Devices 1985, 32, 1165–1169. [Google Scholar] [CrossRef]
- Fukuda, H.; Kasama, K.; Nomura, S. Highly Sensitive MISFET Sensors with Porous Pt–SnO2 Gate Electrode for CO Gas Sensing Applications. Sens. Actuators B Chem. 2000, 64, 163–168. [Google Scholar] [CrossRef]
- Fukuda, H.; Zohnishi, R.; Nomura, S. Highly Sensitive Metal-Insulator-Semiconductor Field-Effect Transistor Sensors for Detecting Carbon Monoxide Gas Using Porous Platinum and Tungsten Oxide Thin Films. Jpn. J. Appl. Phys. 2001, 40, 2782–2786. [Google Scholar] [CrossRef]
- Fleischer, M.; Ostrick, B.; Pohle, R.; Simon, E.; Meixner, H.; Bilger, C.; Daeche, F. Low-Power Gas Sensors Based on Work-Function Measurement in Low-Cost Hybrid Flip–Chip Technology. Sens. Actuators B Chem. 2001, 80, 169–173. [Google Scholar] [CrossRef]
- Maclay, G.J.; Jelley, K.W.; Nowroozi-Esfahani, S.; Formosa, M. The Response of MOS Sensors with Ultrathin Palladium Gates to Carbon Monoxide and Methane. Sens. Actuators 1988, 14, 331–348. [Google Scholar] [CrossRef]
- Jelley, K.W.; Maclay, G.J. A Dual-Mechanism Solid-State Carbon-Monoxide and Hydrogen Sensor Utilizing an Ultrathin Layer of Palladium. IEEE Trans. Electron Devices 1987, 34, 2086–2097. [Google Scholar] [CrossRef]
- Hunter, J.C. Carbon Monoxide Gas Sensing Properties of Metal-Oxide-Semiconductor Capacitor Sensor. Ph.D. Thesis, Oregon Health & Science University, Portland, OR, USA, 2003. [Google Scholar]
- Kang, B.S.; Kim, S.; Ren, F.; Ip, K.; Heo, Y.W.; Gila, B.P.; Abernathy, C.R.; Norton, D.P.; Pearton, S.J. Detection of CO Using Bulk ZnO Schottky Rectifiers. Appl. Phys. A 2005, 80, 259–261. [Google Scholar] [CrossRef]
- Juang, F.-R.; Fang, Y.-K.; Chiang, Y.-T.; Chou, T.-H.; Lin, C.-I.; Lin, C.-W.; Liou, Y.-W. Comparative Study of Carbon Monoxide Gas Sensing Mechanism for the LTPS MOS Schottky Diodes With Various Metal Oxides. IEEE Sens. J. 2011, 11, 1227–1232. [Google Scholar] [CrossRef]
- Salehi, A.; Nikfarjam, A. Room Temperature Carbon Monoxide Sensor Using ITO/n-GaAs Schottky Contact. Sens. Actuators B Chem. 2004, 101, 394–400. [Google Scholar] [CrossRef]
- Feng, C.; Wang, X.-L.; Yang, C.-B.; Xiao, H.-L.; Zhang, M.-L.; Jiang, L.-J.; Tang, J.; Hu, G.-X.; Wang, J.-X.; Wang, Z.-G. Effect of CO on Characteristics of AlGaN/GaN Schottky Diode. Chin. Phys. Lett. 2008, 25, 3025–3027. [Google Scholar] [CrossRef]
- Duan, B.K.; Bohn, P.W. Response of Nanostructured Pt/GaN Schottky Barriers to Carbon Monoxide. Sens. Actuators Phys. 2013, 194, 220–227. [Google Scholar] [CrossRef]
- Okada, H.; Naruse, A.; Furukawa, Y.; Wakahara, A. Study of Electrical Response in Pt/GaN Schottky Barrier Diode to CO Gas for High Temperature Gas Sensor. Jpn. J. Appl. Phys. 2011, 50, 01AD08. [Google Scholar] [CrossRef]
- Salehi, A.; Jamshidi Kalantari, D. Characteristics of Highly Sensitive Au/Porous-GaAs Schottky Junctions as Selective CO and NO Gas Sensors. Sens. Actuators B Chem. 2007, 122, 69–74. [Google Scholar] [CrossRef]
- Gurbuz, Y.; Kang, W.P.; Davidson, J.L.; Kerns, D.V. A New Diode-Based Carbon Monoxide Gas Sensor Utilizing Pt–SnOx/Diamond. Sens. Actuators B Chem. 1999, 56, 151–154. [Google Scholar] [CrossRef]
- Nagai, D.; Nakashima, T.; Nishibori, M.; Itoh, T.; Izu, N.; Shin, W. Thermoelectric Gas Sensor with CO Combustion Catalyst for Ppm Level Carbon Monoxide Detection. Sens. Actuators B Chem. 2013, 182, 789–794. [Google Scholar] [CrossRef]
- Goto, T.; Itoh, T.; Akamatsu, T.; Izu, N.; Shin, W. CO Sensing Properties of Au/SnO2 –Co3O4 Catalysts on a Micro Thermoelectric Gas Sensor. Sens. Actuators B Chem. 2016, 223, 774–783. [Google Scholar] [CrossRef]
- Nishibori, M.; Shin, W.; Tajima, K.; Houlet, L.F.; Izu, N.; Itoh, T.; Tsubota, S.; Matsubara, I. Thermoelectric Gas Sensor Using Au Loaded Titania CO Oxidation Catalyst. J. Ceram. Soc. Jpn. 2007, 115, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Luan, W.L.; Wang, T.C.; Su, W.X.; Zhang, L.X. Room-Temperature CO Thermoelectric Gas Sensor Based on Au/Co3O4 Catalyst Tablet. Nanotechnology 2017, 28, 075501. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Huang, H.; Luan, W.; Qi, Y.; Tu, S. Thermoelectric Carbon Monoxide Sensor Using Co-Ce Catalyst. Sens. Actuators B Chem. 2008, 133, 70–77. [Google Scholar] [CrossRef]
- Samotaev, N.; Vasiliev, A. Mixed Cerium/Zirconium Oxide as a Material for Carbon Monoxide Thermocatalytic Gas Sensor. In Proceedings of the Eurosensors 2018 Conference, Graz, Austria, 9–12 September 2018; Volume 2, p. 841. [Google Scholar]
- Chen, T.; Su, G.; Yuan, H. In Situ Gas Filter Correlation: Photoacoustic CO Detection Method for Fire Warning. Sens. Actuators B Chem. 2005, 109, 233–237. [Google Scholar] [CrossRef]
- Gabelman, A.; Hwang, S.-T. Hollow Fiber Membrane Contactors. J. Membr. Sci. 1999, 159, 61–106. [Google Scholar] [CrossRef]
- Kim, B.; Lu, Y.; Hannon, A.; Meyyappan, M.; Li, J. Low Temperature Pd/SnO2 Sensor for Carbon Monoxide Detection. Sens. Actuators B Chem. 2013, 177, 770–775. [Google Scholar] [CrossRef]
- Ding, K.; Seyfried, W.E. In-Situ Measurement of Dissolved H2 in Aqueous Fluid at Elevated Temperatures and Pressures. Geochim. Cosmochim. Acta 1995, 59, 4769–4773. [Google Scholar] [CrossRef]
- Baek, T.-S.; Lee, J.-G.; Kang, S.-W.; Choi, S.-Y. Detection Of Hydrogen From Dissolved Gases In Transformer Oil By Pd/Pt Misfet. In Proceedings of the International Solid-State Sensors and Actuators Conference—TRANSDUCERS ’95, Stockholm, Sweden, 25 June 1995; Volume 1, pp. 749–751. [Google Scholar]
- Kuroda, K.; Gaiger Silveira, R.; Nishio, N.; Sunahara, H.; Nagai, S. Measurement of Dissolved Hydrogen in an Anaerobic Digestion Process by a Membrane-Covered Electrode. J. Ferment. Bioeng. 1991, 71, 418–423. [Google Scholar] [CrossRef]
- Takeshita, T.; Tanaka, K.; Ishizaki, A.; Stanbury, P.F. Development of a Dissolved Hydrogen Sensor and Its Application to Evaluation of Hydrogen Mass Transfer. J. Ferment. Bioeng. 1993, 76, 148–150. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.A.; Teal, P.D.; Swanson, A.; Hunze, A. Development of Hydrogen Sensors Based on Fiber Bragg Grating with a Palladium Foil for Online Dissolved Gas Analysis in Transformers; Lehmann, P., Osten, W., Albertazzi Gonçalves, A., Eds.; SPIE Optical Metrology: Munich, Germany, 26 June 2017; p. 103292P. [Google Scholar]
- Yang, F.; Jung, D.; Penner, R.M. Trace Detection of Dissolved Hydrogen Gas in Oil Using a Palladium Nanowire Array. Anal. Chem. 2011, 83, 9472–9477. [Google Scholar] [CrossRef]
- Mišlov, D.; Cifrek, M.; Krois, I.; Džapo, H. Measurement of Dissolved Hydrogen Concentration with Clark Electrode. In Proceedings of the 2015 IEEE Sensors Applications Symposium (SAS), Zadar, Croatia, 13 April 2015; pp. 1–5. [Google Scholar]
- Bodzenta, J.; Burak, B.; Gacek, Z.; Jakubik, W.P.; Kochowski, S.; Urbańczyk, M. Thin Palladium Film as a Sensor of Hydrogen Gas Dissolved in Transformer Oil. Sens. Actuators B Chem. 2002, 87, 82–87. [Google Scholar] [CrossRef]
- Sandvik, P.; Babes-Dornea, E.; Roy Trudel, A.; Georgescu, M.; Tilak, V.; Renaud, D. GaN-Based Schottky Diodes for Hydrogen Sensing in Transformer Oil. Phys. Status Solidi C 2006, 3, 2283–2286. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen Sensors—A Review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Palmisano, V.; Bader, M.A. Developments in Gas Sensor Technology for Hydrogen Safety. Int. J. Hydrogen Energy 2014, 39, 20474–20483. [Google Scholar] [CrossRef]
- Slaman, M.; Dam, B.; Pasturel, M.; Borsa, D.M.; Schreuders, H.; Rector, J.H.; Griessen, R. Fiber Optic Hydrogen Detectors Containing Mg-Based Metal Hydrides. Sens. Actuators B Chem. 2007, 123, 538–545. [Google Scholar] [CrossRef]
- Baselt, D.R.; Fruhberger, B.; Klaassen, E.; Cemalovic, S.; Britton, C.L.; Patel, S.V.; Mlsna, T.E.; McCorkle, D.; Warmack, B. Design and Performance of a Microcantilever-Based Hydrogen Sensor. Sens. Actuators B Chem. 2003, 88, 120–131. [Google Scholar] [CrossRef]
- Kiefer, T.; Salette, A.; Villanueva, L.G.; Brugger, J. Large Arrays of Chemo-Mechanical Nanoswitches for Ultralow-Power Hydrogen Sensing. J. Micromech. Microeng. 2010, 20, 105019. [Google Scholar] [CrossRef]
- Buric, M.; Chen, K.P.; Bhattarai, M.; Swinehart, P.R.; Maklad, M. Active Fiber Bragg Grating Hydrogen Sensors for All-Temperature Operation. IEEE Photonics Technol. Lett. 2007, 19, 255–257. [Google Scholar] [CrossRef]
- Okazaki, S.; Nakagawa, H.; Asakura, S.; Tomiuchi, Y.; Tsuji, N.; Murayama, H.; Washiya, M. Sensing Characteristics of an Optical Fiber Sensor for Hydrogen Leak. Proc. Ninth Int. Meet. Chem. Sens. 2003, 93, 142–147. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, L.; Wang, G.; Xiang, F.; Qin, Y.; Wang, M.; Yang, M. Optical Fiber Grating Hydrogen Sensors: A Review. Sensors 2017, 17, 577. [Google Scholar] [CrossRef] [PubMed]
- Caucheteur, M.C.; Debliquy, D.; Lahem, P. Megret Catalytic Fiber Bragg Grating Sensor for Hydrogen Leak Detection in Air. IEEE Photonics Technol. Lett. 2008, 20, 96–98. [Google Scholar] [CrossRef]
- Ma, G.; Li, C.; Luo, Y.; Mu, R.; Wang, L. High Sensitive and Reliable Fiber Bragg Grating Hydrogen Sensor for Fault Detection of Power Transformer. Sens. Actuators B Chem. 2012, 169, 195–198. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, J.; Wang, N.; Atiyeh, H.K. Review of Dissolved CO and H2 Measurement Methods for Syngas Fermentation. Sensors 2021, 21, 2165. https://doi.org/10.3390/s21062165
Dang J, Wang N, Atiyeh HK. Review of Dissolved CO and H2 Measurement Methods for Syngas Fermentation. Sensors. 2021; 21(6):2165. https://doi.org/10.3390/s21062165
Chicago/Turabian StyleDang, Jie, Ning Wang, and Hasan K. Atiyeh. 2021. "Review of Dissolved CO and H2 Measurement Methods for Syngas Fermentation" Sensors 21, no. 6: 2165. https://doi.org/10.3390/s21062165
APA StyleDang, J., Wang, N., & Atiyeh, H. K. (2021). Review of Dissolved CO and H2 Measurement Methods for Syngas Fermentation. Sensors, 21(6), 2165. https://doi.org/10.3390/s21062165








