Evolving Paradigm of Prothrombin Time Diagnostics with Its Growing Clinical Relevance towards Cardio-Compromised and COVID-19 Affected Population
Abstract
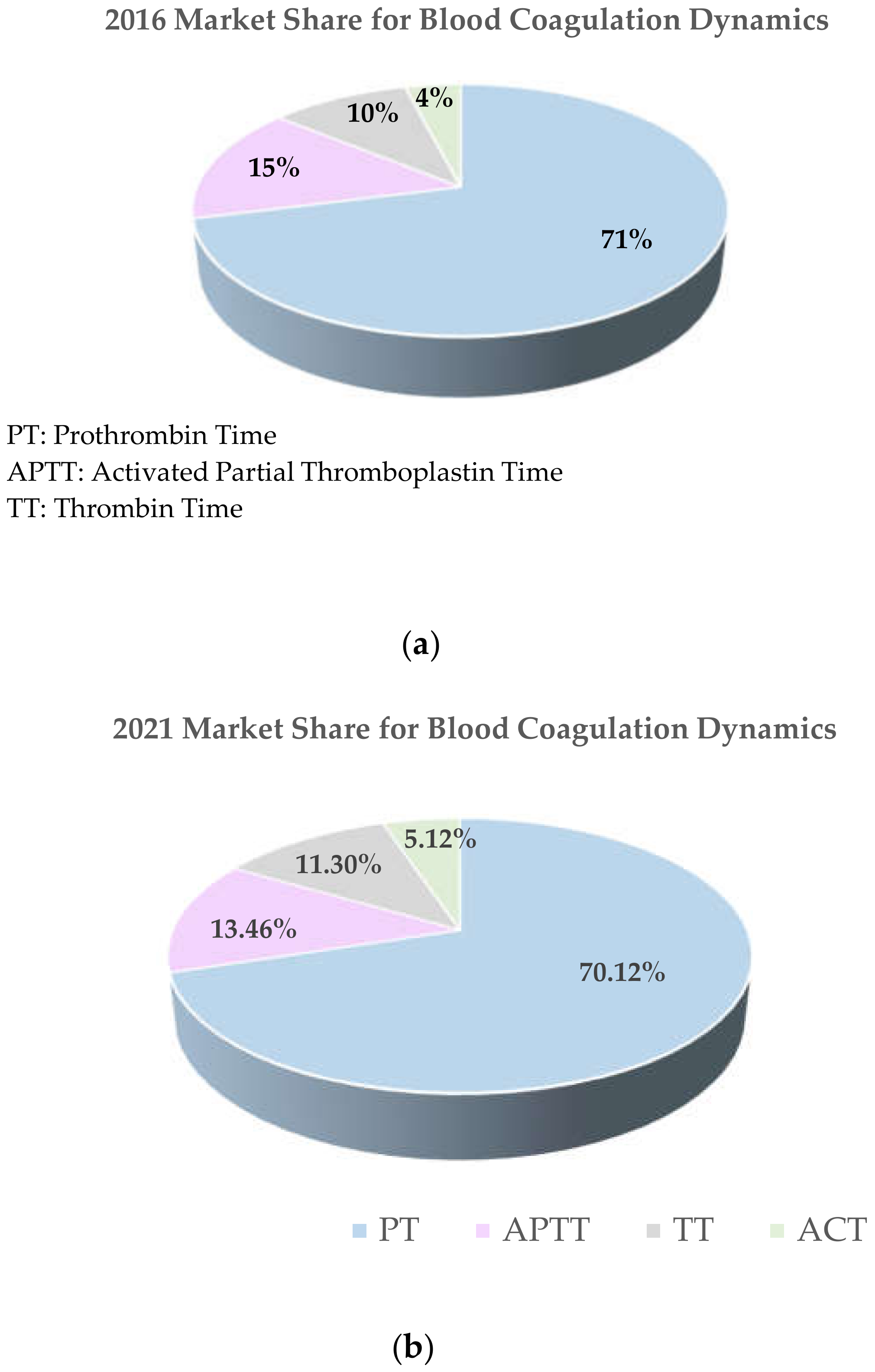
1. Introduction
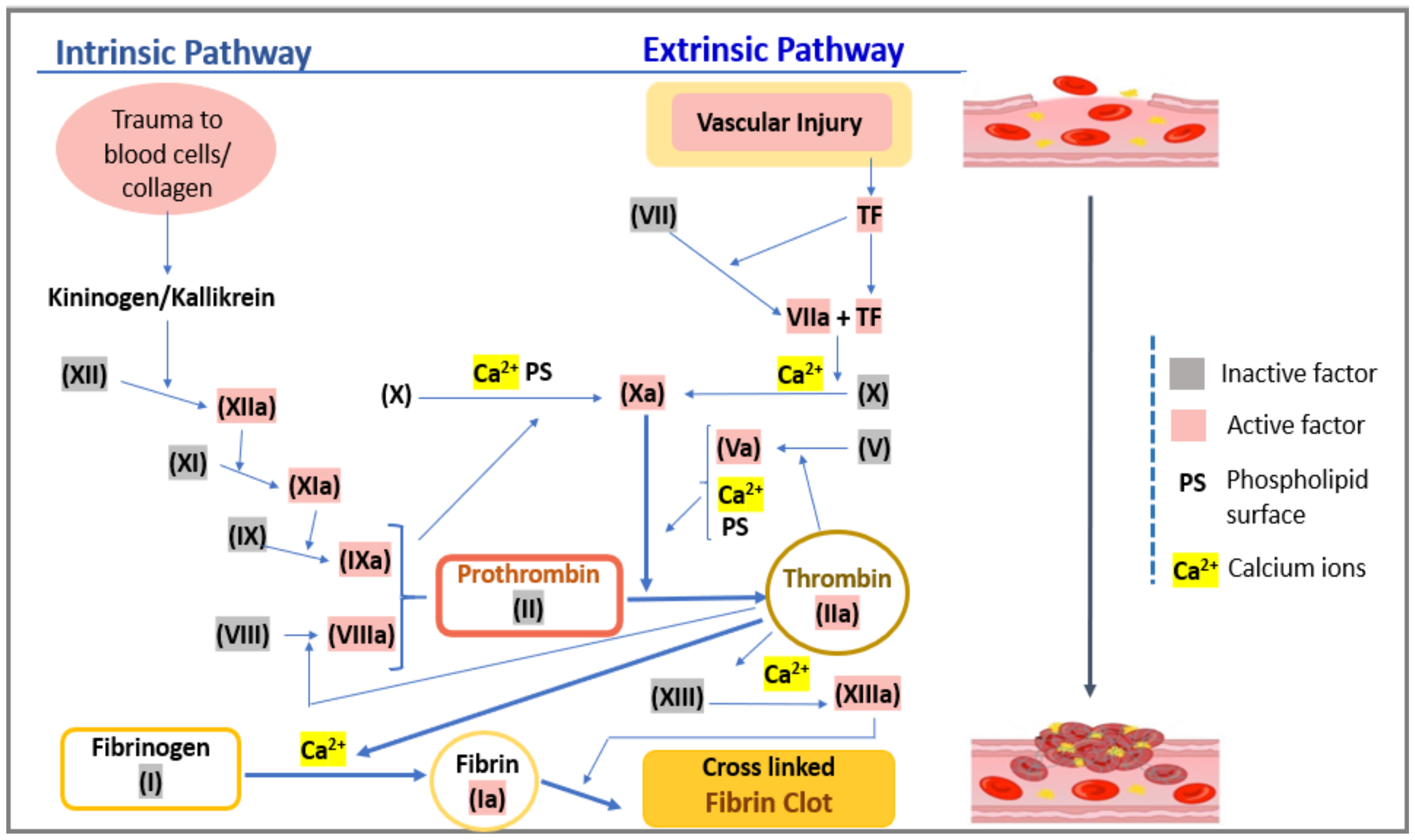
2. Hemostasis and Prothrombin Time
3. Clinical Relevance of PT in Thromboembolic Complications and COVID-19 Associated Coagulopathy CAC
4. Lab-Based PT Monitoring
5. Point of Care PT/INR
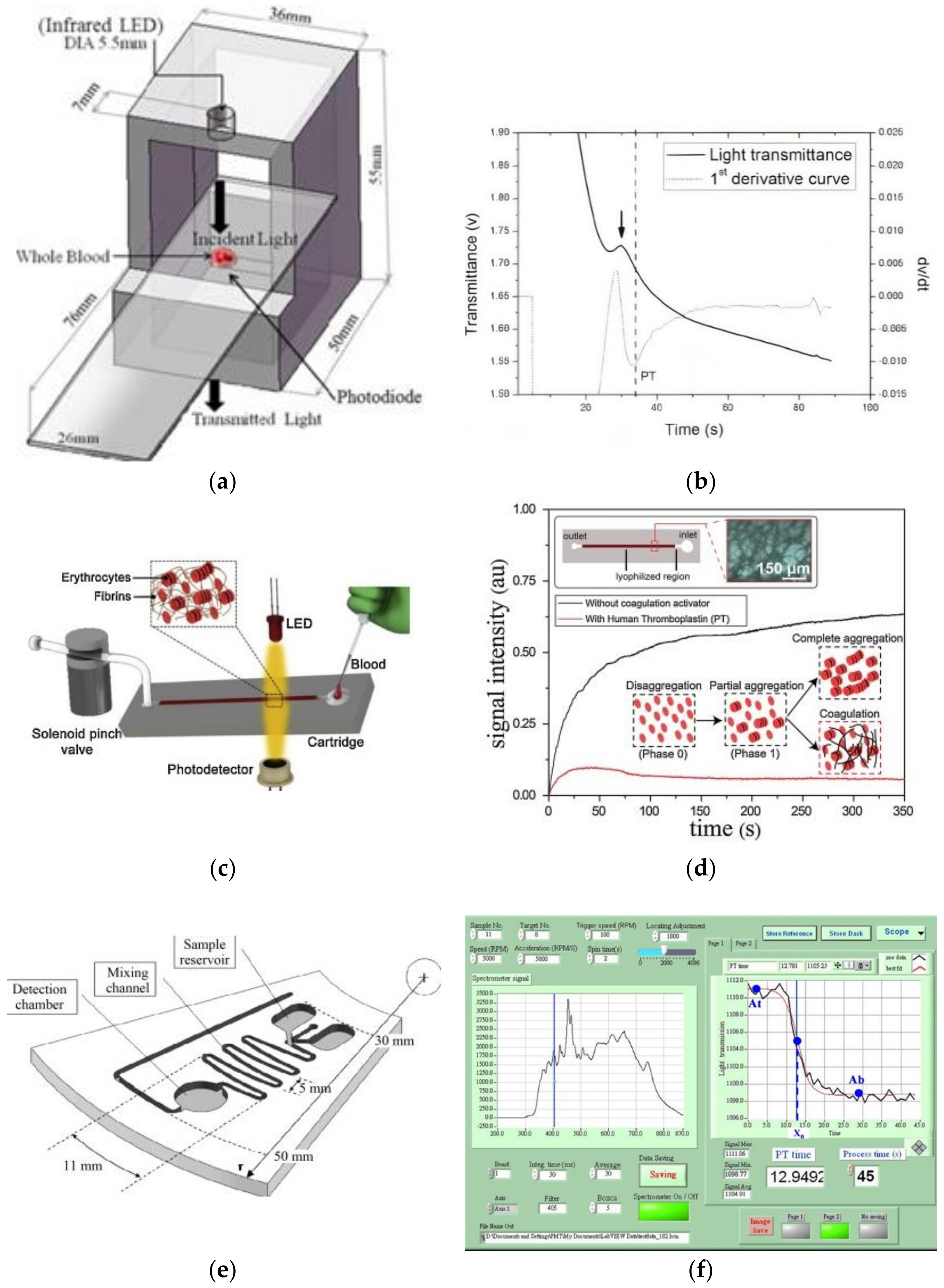
5.1. Optical
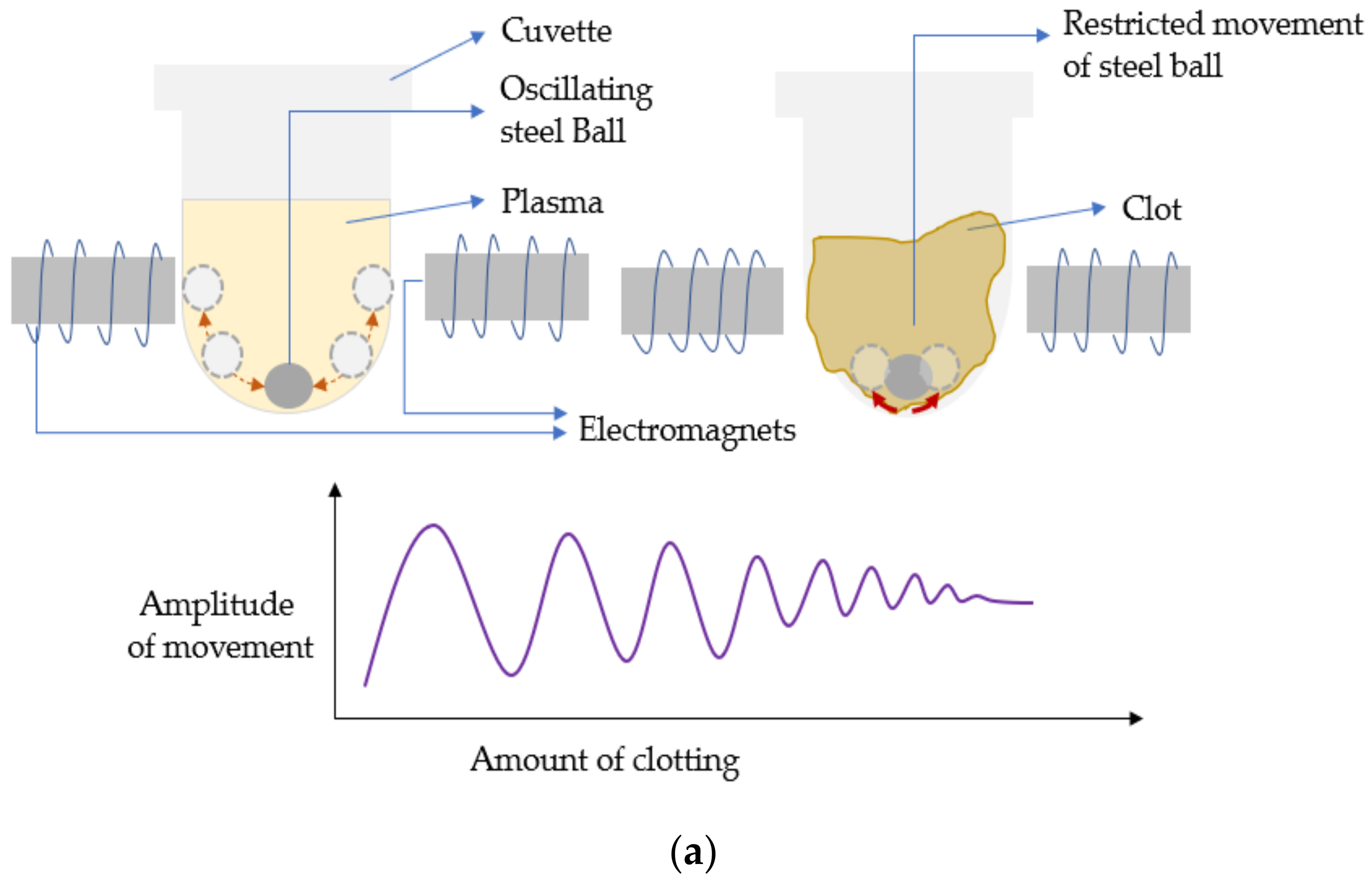
5.2. Acoustic/Electromechanical Resonators
5.3. Electrochemical
5.4. Low-Cost Lateral Flow Assays for Performing PoC PT Diagnosis
6. Prothrombin Time, Oral Anticoagulation Therapy, and Related Standardization Protocols for Designing/Calibrating and Usage of PT Assays (Especially at PoC Settings)
7. Discussion and Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, P.; Gao, Y.; Zheng, M.; Xu, T.; Schoenhagen, P.; Jin, Z. Recent progress and market analysis of anticoagulant drugs. J. Thorac. Dis. 2018, 10, 2011–2025. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 19 October 2020).
- About Cardiovascular Diseases. Available online: http://www.who.int/cardiovascular_diseases/about_cvd/en/ (accessed on 19 October 2020).
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; De Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation 2016, 133, e38–e48. [Google Scholar] [CrossRef] [PubMed]
- Laoprasopwattana, K.; Binsaai, J.; Pruekprasert, P.; Geater, A. Prothrombin time prolongation was the most important indicator of severe bleeding in children with severe dengue viral infection. J. Trop. Pediatr. 2017, 63, 314–320. [Google Scholar] [CrossRef]
- Harter, K.; Levine, M.; Henderson, S.O. Anticoagulation drug therapy: A review. West. J. Emerg. Med. 2015, 16, 11–17. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Connors, J.M.; Warkentin, T.E.; Thachil, J.; Levi, M. The unique characteristics of COVID-19 coagulopathy. Crit. Care 2020, 24, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Middeldorp, S.; Coppens, M.; van Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Müller, M.C.A.; Bouman, C.C.S.; Beenen, L.F.M.; Kootte, R.S.; Heijmans, J.; et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1995–2002. [Google Scholar] [CrossRef]
- Llitjos, J.F.; Leclerc, M.; Chochois, C.; Monsallier, J.M.; Ramakers, M.; Auvray, M.; Merouani, K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020, 18, 1743–1746. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef]
- The Lancet Haematology; Elsevier Ltd: Amsterdam, The Netherlands, May 2020; p. e425.
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- World Health Organization Weekly Epidemiological Update on COVID-19. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20201012-weekly-epi-update-9.pdf (accessed on 23 November 2020).
- Krayem, O.R. Ben COVID-19: Diagnosis, Evaluation and Treatment Strategies. Res. Rev. J. Med. Health Sci. 2020, 9, 55–60. [Google Scholar]
- Becker, R.C. COVID-19 update: Covid-19-associated coagulopathy. J. Thromb. Thrombolysis 2020, 50, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rojas, R.M.; Pérez-Rus, G.; Delgado-Pinos, V.E.; Domingo-González, A.; Regalado-Artamendi, I.; Alba-Urdiales, N.; Demelo-Rodríguez, P.; Monsalvo, S.; Rodríguez-Macías, G.; Ballesteros, M.; et al. COVID-19 coagulopathy: An in-depth analysis of the coagulation system. Eur. J. Haematol. 2020, 741–750. [Google Scholar] [CrossRef]
- Long, H.; Nie, L.; Xiang, X.; Li, H.; Zhang, X.; Fu, X.; Ren, H.; Liu, W.; Wang, Q.; Wu, Q. D-Dimer and Prothrombin Time Are the Significant Indicators of Severe COVID-19 and Poor Prognosis. Biomed Res. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Miconi, L.; Barbierato, E.; Marinaro, G.; Targhetta, S.; D’agata, M.; Rinaldi, D. Labile PT-INR in a Covid-19 Patient Under Long-term Vitamin K Antagonist Therapy: A Case Report. SN Compr. Clin. Med. 2020, 2, 1680–1682. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Song, F.; Van Cott, E.M.; Kuter, D.J.; Rosovsk, R. Evaluation of the prothrombin fragment 1.2 in patients with coronavirus disease 2019 (COVID-19). Am. J. Hematol. 2020, 95, 1479–1485. [Google Scholar] [CrossRef]
- Luo, H.-C.; You, C.-Y.; Lu, S.-W.; Fu, Y.-Q. Characteristics of coagulation alteration in patients with COVID-19. Ann. Hematol. 2020, 100, 45–52. [Google Scholar] [CrossRef]
- Yang, D.T.; Robetorye, R.S.; Rodgers, G.M. Home Prothrombin Time Monitoring: A Literature Analysis. Am. J. Hematol. 2004, 77, 177–186. [Google Scholar] [CrossRef]
- Mr. Shah Global Coagulation Analyzer Market to Surpass US$ 13.17 Billion by 2025. Available online: https://www.globenewswire.com/news-release/2018/01/29/1313614/0/en/Global-Coagulation-Analyzer-Market-to-Surpass-US-13-17-Billion-by-2025.html (accessed on 19 October 2020).
- Jackson, C.M.; Esnouf, M.P. Has the Time Arrived to Replace the Quick Prothrombin Time Test for Monitoring Oral Anticoagulant Therapy? Clin. Chem. 2005, 51, 483–485. [Google Scholar] [CrossRef]
- Tripathi, M.M.; Egawa, S.; Wirth, A.G.; Tshikudi, D.M.; Van Cott, E.M.; Nadkarni, S.K. Clinical evaluation of whole blood prothrombin time (PT) and international normalized ratio (INR) using a Laser Speckle Rheology sensor. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Jesse Maida Global Coagulation Testing Market Size is Expected to Reach USD 2.69 Billion by 2021: Technavio. Available online: https://www.businesswire.com/news/home/20170209005070/en/Global-Coagulation-Testing-Market-Size-is-Expected-to-Reach-USD-2.69-Billion-by-2021-Technavio (accessed on 3 October 2020).
- Hall, J.E. Textbook of Medical Physiology, 12th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2010; ISBN 9781416045748. [Google Scholar]
- Rogers, K. (Ed.) Blood Physiology and Circulation; Britannica Educational Publishing: New York, NY, USA, 2011; ISBN 978-1-61530-250-5. [Google Scholar]
- Coagulation—Wikipedia. Available online: https://en.wikipedia.org/wiki/Coagulation (accessed on 10 October 2020).
- Kitchen, S.; McGraw, A.; Echenagucia, M. Diagnosis of Hemophilia and Other Bleeding Disorders, 2nd ed.; World Federation of Haemophilia: Montréal, QC, Canada, 2010. [Google Scholar]
- Shih, C.; Lu, C.; Wu, J.; Lin, C.; Wang, J.; Lin, C. Prothrombin time tests on a microfluidic disc analyzer. Sens. Actuators B Chem. 2012, 161, 1184–1190. [Google Scholar] [CrossRef]
- Guler, M.T.; Isiksacan, Z.; Serhatlioglu, M.; Elbuken, C. Self-powered disposable prothrombin time measurement device with an integrated effervescent pump. Sens. Actuators B Chem. 2018, 273, 350–357. [Google Scholar] [CrossRef]
- D-Dimer. Available online: https://labtestsonline.org/tests/d-dimer#:~:text=D-dimer (accessed on 10 December 2020).
- Franchini, M.; Liumbruno, G.M.; Bonfanti, C.; Lippi, G. The evolution of anticoagulant therapy. Blood Transfus. 2016, 14, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of Coronavirus Disease 2019. Crit. Care Med. 2020, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.C.; Weitz, J.I. COVID-19 coagulopathy, thrombosis, and bleeding. Blood 2020, 136, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Duan, Y.; Bao, T.; Gu, J.; Chen, Y.; Li, Y.; Mao, S.; Chen, Y.; Xie, W. The values of coagulation function in COVID-19 patients. PLoS ONE 2020, 15, 12. [Google Scholar] [CrossRef]
- Parker, B.M.; Hart, V.J.; Rattan, R. Coagulopathy in Covid-19: Review and Recommendations. Available online: https://www.facs.org/-/media/files/covid19/umiami_study_uses_of_coagulopathy.ashx#:~:text=COAGULOPATHY (accessed on 3 December 2020).
- Qari, M. High Throughput Coagulation Analyzers Review. Comb. Chem. High Throughput Screen. 2005, 8, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Favaloro, E.J.; Franchini, M.; Guidi, G.C. Milestones and perspectives in coagulation and hemostasis. Semin. Thromb. Hemost. 2009, 35, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Heins, M.; Reinauer, H. Automation in coagulation testing. J. Int. Fed. Clin. Chem. 1996, 8, 117–122. [Google Scholar]
- McGlasson, D.L. Coagulation Instrumentation | Oncohema Key. Available online: https://oncohemakey.com/coagulation-instrumentation/#s0035 (accessed on 10 October 2020).
- Destiny Plus—Tcoag. Available online: https://www.tcoag.com/instruments/destiny-plus/ (accessed on 3 November 2020).
- Diagnostica Stago, Inc., Product Catalog, STA ® Family of Hemostasis Analyzers. Available online: https://www.stago.com/fileadmin/user_upload/40-Stago_US/catalogue_us/2015/imagespageflip/FEE3A378427744A6B1F323C8567953F6/2015_Stago_US_Product_Catalog-DEF-eb.pdf (accessed on 3 November 2020).
- Helena Laboratories, Pont of Care, Cascade ® POC Analyzer. Available online: https://www.helena.com/Operator%20Manuals/Cascade%20POC%20Op%20Man%20D6500099D.pdf (accessed on 3 November 2020).
- Yang, C.L.; Huang, S.J.; Chou, C.W.; Chiou, Y.C.; Lin, K.P.; Tsai, M.S.; Young, K.C. Design and evaluation of a portable optical-based biosensor for testing whole blood prothrombin time. Talanta 2013, 116, 704–711. [Google Scholar] [CrossRef]
- Coag-SenseTM Prothrombin Time (PT)/INR Monitoring System, Professional User’s Manual. Available online: jantdx.com/wp-content/uploads/2018/01/PRO_User_Manual_CoagSense__CSI_PN100220_Rev_AE_07072012.pdf (accessed on 3 November 2020).
- Yang, C.L.; Chiou, Y.C.; Chou, C.W.; Young, K.C.; Huang, S.J.; Liu, C.Y. Point-of-care testing of portable blood coagulation detectors using optical sensors. J. Med. Biol. Eng. 2013, 33, 319–324. [Google Scholar] [CrossRef]
- Lin, Y.C.; Yen, S.C.; Cheng, S.; Huang, T. Effectiveness of holographic optical element module sensor in measuring blood prothrombin time. Meas. Sci. Technol. 2014, 25. [Google Scholar] [CrossRef][Green Version]
- Isiksacan, Z.; Hastar, N.; Erel, O.; Elbuken, C. An optofluidic point-of-care device for quantitative investigation of erythrocyte aggregation during coagulation. Sens. Actuators A Phys. 2018, 281, 24–30. [Google Scholar] [CrossRef]
- Lin, C.; Liu, C.; Shih, C.; Lu, C. A sample-to-result system for blood coagulation tests on a microfluidic disk analyzer. Biomicrofluidics 2014, 8, 052105. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lin, K.; Yen, D.; Shih, C.; Lu, C.; Wang, J.; Lin, C. A Point-of-Care Prothrombin Time Test on a Microfluidic Disk Analyzer Using Alternate Spinning. J. Nanosci. Nanotechnol. 2015, 15, 1401–1407. [Google Scholar] [CrossRef]
- Faivre, M.; Peltié, P.; Planat-Chrétien, A.; Cosnier, M.-L.; Cubizolles, M.; Nougier, C.; Négrier, C.; Pouteau, P. Coagulation dynamics of a blood sample by multiple scattering analysis. J. Biomed. Opt. 2011, 16, 057001. [Google Scholar] [CrossRef]
- Nadkarni, S.K. Comprehensive Coagulation Profiling at the Point-of-Care Using a Novel Laser-based Approach. Semin. Thromb. Hemost. 2019, 45, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Inoue, S.; Iwasaki, Y.; Seyama, M.; Horiuchi, T.; Tamechika, E. Blood coagulation testing method based on flow velocity measurement using a surface plasmon resonance (SPR)-based microfluidic device. In Proceedings of the 16th International Conference on Miniaturized Systems for Chemistry and Life Sciences MicroTAS 2012, Okinawa, Japan, 28 October–1 November 2012; pp. 1318–1320. [Google Scholar]
- microINR: Instructions for Use. Available online: https://www.ilinemicrosystems.com/nw/docs/microINR%20Instructions%20for%20use.pdf (accessed on 3 November 2020).
- Andrew, M.; Ansell, J.L.; Becker, D.M.; Becker, R.C.; Triplett, D.A. Point-of-care prothrombin time measurement for professional and patient self-testing use: A multicenter clinical experience. Am. J. Clin. Pathol. 2001, 115, 288–296. [Google Scholar] [CrossRef]
- Hemochron Signature Elite Operator’s Manual. Available online: http://site.aaawholesalecompany.com/Files/Hemochron_Signature_Elite_Manual.pdf (accessed on 19 October 2020).
- Müller, L.; Sinn, S.; Dreehsel, H.; Ziegler, C.; Wendel, H.P.; Northoff, H.; Gehring, F.K. Erratum: Investigation of prothrombin time in human whole-blood samples with a quartz crystal biosensor (Analytical Chemistry (2010) 82 (658–663)). Anal. Chem. 2010, 82, 2164. [Google Scholar] [CrossRef]
- Hussain, M.; Northoff, H.; Gehring, F.K. QCM-D providing new horizon in the domain of sensitivity range and information for haemostasis of human plasma. Biosens. Bioelectron. 2015, 66, 579–584. [Google Scholar] [CrossRef]
- Yao, J.; Feng, B.; Zhang, Z.; Li, C.; Zhang, W.; Guo, Z.; Zhao, H.; Zhou, L. Blood coagulation testing smartphone platform using quartz crystal microbalance dissipation method. Sensors 2018, 18, 3073. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Song, S.; Ma, J.; Zhang, Z.; Wang, P.; Liu, W.; Guo, Q. Micro-electromechanical film bulk acoustic sensor for plasma and whole blood coagulation monitoring. Biosens. Bioelectron. 2017, 91, 465–471. [Google Scholar] [CrossRef]
- Meyer dos Santos, S.; Zorn, A.; Guttenberg, Z.; Picard-Willems, B.; Kläffling, C.; Nelson, K.; Klinkhardt, U.; Harder, S. A novel μ-fluidic whole blood coagulation assay based on Rayleigh surface-acoustic waves as a point-of-care method to detect anticoagulants. Biomicrofluidics 2013, 7, 1–12. [Google Scholar] [CrossRef]
- Nam, J.; Choi, H.; Kim, J.Y.; Jang, W.; Lim, C.S. Lamb wave-based blood coagulation test. Sens. Actuators B Chem. 2018, 263, 190–195. [Google Scholar] [CrossRef]
- CoaguChek® XS Pro system, Operator’s Manual. Available online: https://diagnostics.roche.com/us/en/products/instruments/coaguchek-xs-pro.html#documents (accessed on 25 November 2020).
- 510(k) Substantial Equivalence Determinaion Decision Summary (for Xprecia Stride). Available online: https://www.accessdata.fda.gov/cdrh_docs/reviews/K151964.pdf (accessed on 3 December 2020).
- Martin, C.L. i-STAT—Combining Chemistry and Haematology in PoCT. Clin. Biochem. Rev. 2010, 31, 81–84. [Google Scholar]
- Thuerlemann, C.; Haeberli, A.; Alberio, L. Monitoring thrombin generation by electrochemistry: Development of an amperometric biosensor screening test for plasma and whole blood. Clin. Chem. 2009, 55, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.M.; Hodges, A.M. Apparatus and Method for Electrochemical Detection. U.S. Patent WO2009053834A1, 30 April 2009. [Google Scholar]
- Zhao, T.X. Electrical impedance and haematocrit of human blood with various anticoagulants. Physiol. Meas. 1993, 14, 299–307. [Google Scholar] [CrossRef]
- Fricke, H.; Morse, S. The electric resistance and capacity of blood for frequencies between 800 and 41/2 million cycles. J. Gen. Physiol. 1925, 9, 153–167. [Google Scholar] [CrossRef]
- Lei, K.F.; Chen, K.H.; Tsui, P.H.; Tsang, N.M. Real-Time Electrical Impedimetric Monitoring of Blood Coagulation Process under Temperature and Hematocrit Variations Conducted in a Microfluidic Chip. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Berney, H.; O’Riordan, J.J. Impedance Measurement Monitors Blood Coagulation | Analog Devices. Analog Dialogue 2008, 42, 2–4. [Google Scholar]
- Farnam, W.E.; Navarro, M.C. Device and Method for Measuring Properties of a Sample. U.S. Patent 7,674,616, 9 March 2010. [Google Scholar]
- Chen, S.-H.; Lu, Y.-C.; Yen, C.-Y. Electrochemical Blood Test Strips and Diagnosis Systems using the Same. U.S. Patent 9,000,770, 7 April 2015. [Google Scholar]
- Zhang, L. Method and System for Assembling a Microfluidic Sensor. U.S. Patent US20150140671A1, 21 May 2015. [Google Scholar]
- Chen, S.-H.; Tw, H.; Chu, C.-Y.; Cheng, C.; Tw, H.; Lu, Y.; Shen, T.Y.S. Device and Method for Measuring Prothrombin Time and Hematocrit by Analyzing change in Reactance in a Sample. U.S. Patent US8828322B2, 9 September 2014. [Google Scholar]
- Prothrombin Time: HemoSenseTM INRatio. Available online: http://scscls.les3z.org/current_CCC_stuff/handouts/site-inspections-a-survival-kit-roberta-bartholdi-3.pdf (accessed on 27 November 2020).
- Professional User Guide INRatio®2 Prothrombin Time (PT) Monitoring System. Available online: http://www.nwprimarycare.com/StaffTrainingInfo/INR-User-Guide.pdf (accessed on 29 November 2020).
- Substantial Equivalence Determination Decision Summary, Alere INRatio®2 PT/INR Monitoring System. Available online: https://www.accessdata.fda.gov/cdrh_docs/reviews/K110212.pdf (accessed on 3 December 2020).
- Lim, J.H.; Hong, S.Y.; Kang, S.J.; Doh, H.J.; Song, J.; Choi, J.R.; Chung, K.H.; Choi, J. Electrochemical determination of whole blood clotting time by using nanodot arrays. Electrochem. Commun. 2009, 11, 2141–2144. [Google Scholar] [CrossRef]
- Zilberman-Rudenko, J.; White, R.M.; Zilberman, D.A.; Lakshmanan, H.H.S.; Rigg, R.A.; Shatzel, J.J.; Maddala, J.; McCarty, O.J.T. Design and Utility of a Point-of-Care Microfluidic Platform to Assess Hematocrit and Blood Coagulation. Cell. Mol. Bioeng. 2018, 11, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Jina, A.N. Method and Device for Measuring Blood Coagulating or Lysis by Viscosity Changes. U.S. Patent EP1482296A1, 1 December 2004. [Google Scholar]
- Ramaswamy, B.; Yeh, Y.T.T.; Zheng, S.Y. Microfluidic device and system for point-of-care blood coagulation measurement based on electrical impedance sensing. Sens. Actuators B Chem. 2013, 180, 21–27. [Google Scholar] [CrossRef]
- Lifei, X.; Hong, L. An Electrochemical Sensor for Prothrombin-Time Test. In Proceedings of the 3rd International Conference on Advances in Materials, Mechatronics and Civil Engineering (ICAMMCE 2018), Hangzhou, China, 13–15 April 2018; Volume 162, pp. 11–17. [Google Scholar]
- Maji, D.; Suster, M.A.; Stavrou, E.; Gurkan, U.A.; Mohseni, P. Monitoring time course of human whole blood coagulation using a microfluidic dielectric sensor with a 3D capacitive structure. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Milano, Italy, 25–29 August 2015; pp. 5904–5907. [Google Scholar]
- Chiang, C.K.; Kurniawan, A.; Kao, C.Y.; Wang, M.J. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta 2019, 194, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.; Giri Nandagopal, M.S.; Peralam Yegneswaran, P.; Singhal, H.R.; Mani, N.K. Inkjet printing of paraffin on paper allows low-cost point-of-care diagnostics for pathogenic fungi. Cellulose 2020, 27, 7691–7701. [Google Scholar] [CrossRef]
- Chen, F.; Varghese, D.; McDermott, S.T.; George, I.; Geng, L.; Adamson, D.H. Interface-exfoliated graphene-based conductive screen-printing inks: Low-loading, low-cost, and additive-free. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, D.; Pauletti, G.M.; Steckl, A.J. Blood coagulation screening using a paper-based microfluidic lateral flow device. Lab Chip 2014, 14, 4035–4041. [Google Scholar] [CrossRef]
- Li, H.; Han, D.; Hegener, M.A.; Pauletti, G.M.; Steckl, A.J. Flow reproducibility of whole blood and other bodily fluids in simplified no reaction lateral flow assay devices. Biomicrofluidics 2017, 11, 1–15. [Google Scholar] [CrossRef]
- Li, H.; Han, D.; Pauletti, G.M.; Hegener, M.A.; Steckl, A.J. Correcting the effect of hematocrit in whole blood coagulation analysis on paper-based lateral flow device. Anal. Methods 2018, 10, 2869–2874. [Google Scholar] [CrossRef]
- Hegener, M.A.; Li, H.; Han, D.; Steckl, A.J.; Pauletti, G.M. Point-of-care coagulation monitoring: First clinical experience using a paper-based lateral flow diagnostic device. Biomed. Microdevices 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.J.; Fitzmaurice, D.A.; Kitchen, S.; Mackie, I.J.; Mallett, S. Point-of-care testing in haemostasis. Br. J. Haematol. 2010, 150, 501–514. [Google Scholar] [CrossRef]
- Johnson, S.A. Point-of-Care or Clinical Lab INR for Anticoagulation Monitoring: Which to Believe? Clin. Lab. News Am. Assoc. Clin. Chem. 2017, 1–7. [Google Scholar]
- Shikdar, S.; Vashisht, R.; Bhattacharya, P.T.; Catholic, M.; Clinic, C. International Normalized Ratio (INR); StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Ansell, J.; Jacobson, A.; Levy, J.; Vo, H.; Hasenkam, J.M. Guidelines for implementation of patient self-testing and patient self-management of oral anticoagulation. International consensus guidelines prepared by International Self-Monitoring Association for Oral Anticoagulation. Int. J. Cardiol. 2005, 99, 37–45. [Google Scholar] [CrossRef]
- Heneghan, C.; Ward, A.; Perera, R.; Bankhead, C.; Fuller, A.; Stevens, R.; Bradford, K.; Tyndel, S.; Alonso-coello, P.; Ansell, J.; et al. Self-monitoring of oral anticoagulation: Systematic review and meta-analysis of individual patient data. Lancet 2012, 379, 322–334. [Google Scholar] [CrossRef]
- Bernardo, A. Post-conference session: Experience with patient self-management of oral anticoagulation. J. Thromb. Thrombolysis 1996, 2, 321–325. [Google Scholar] [CrossRef]






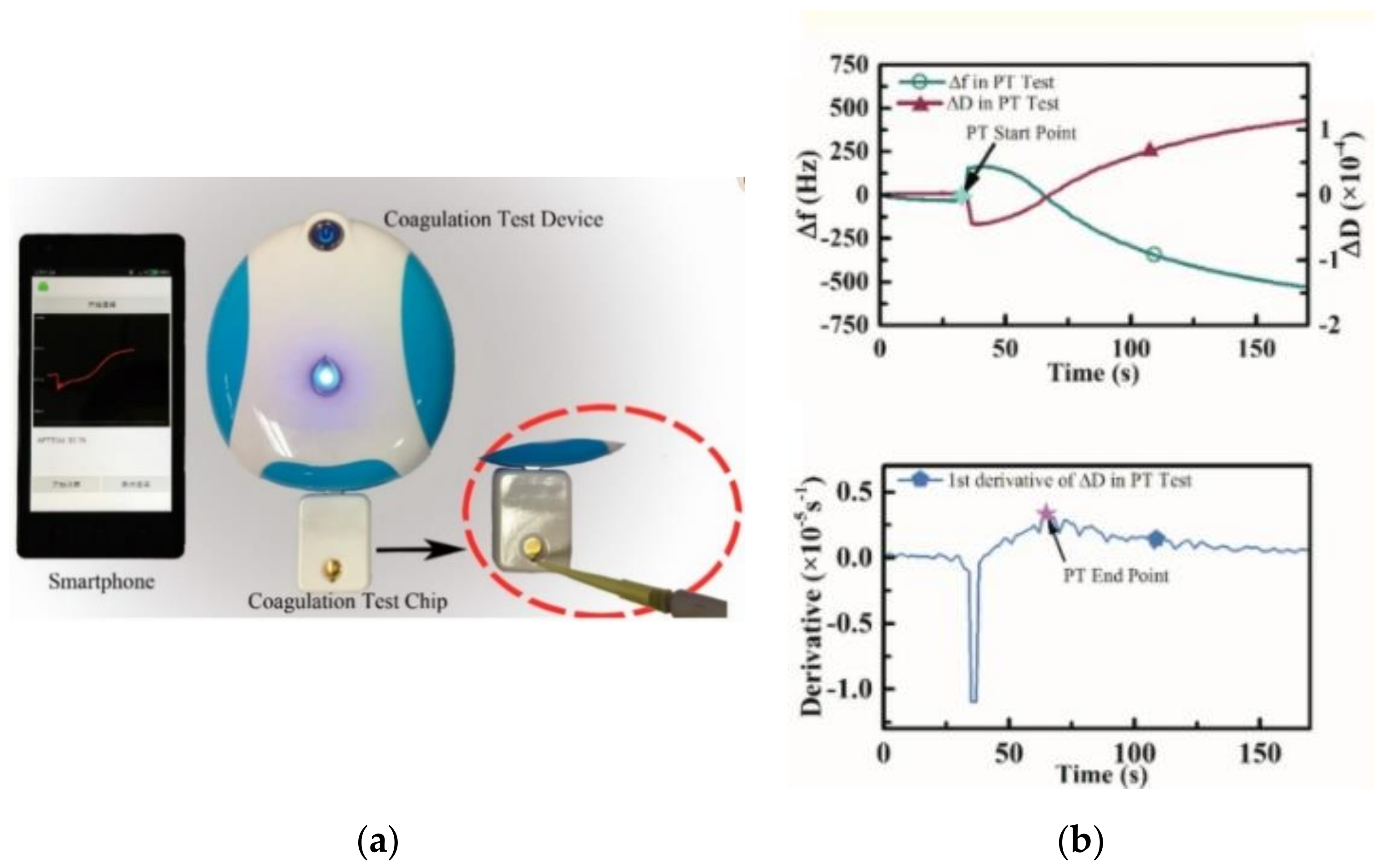
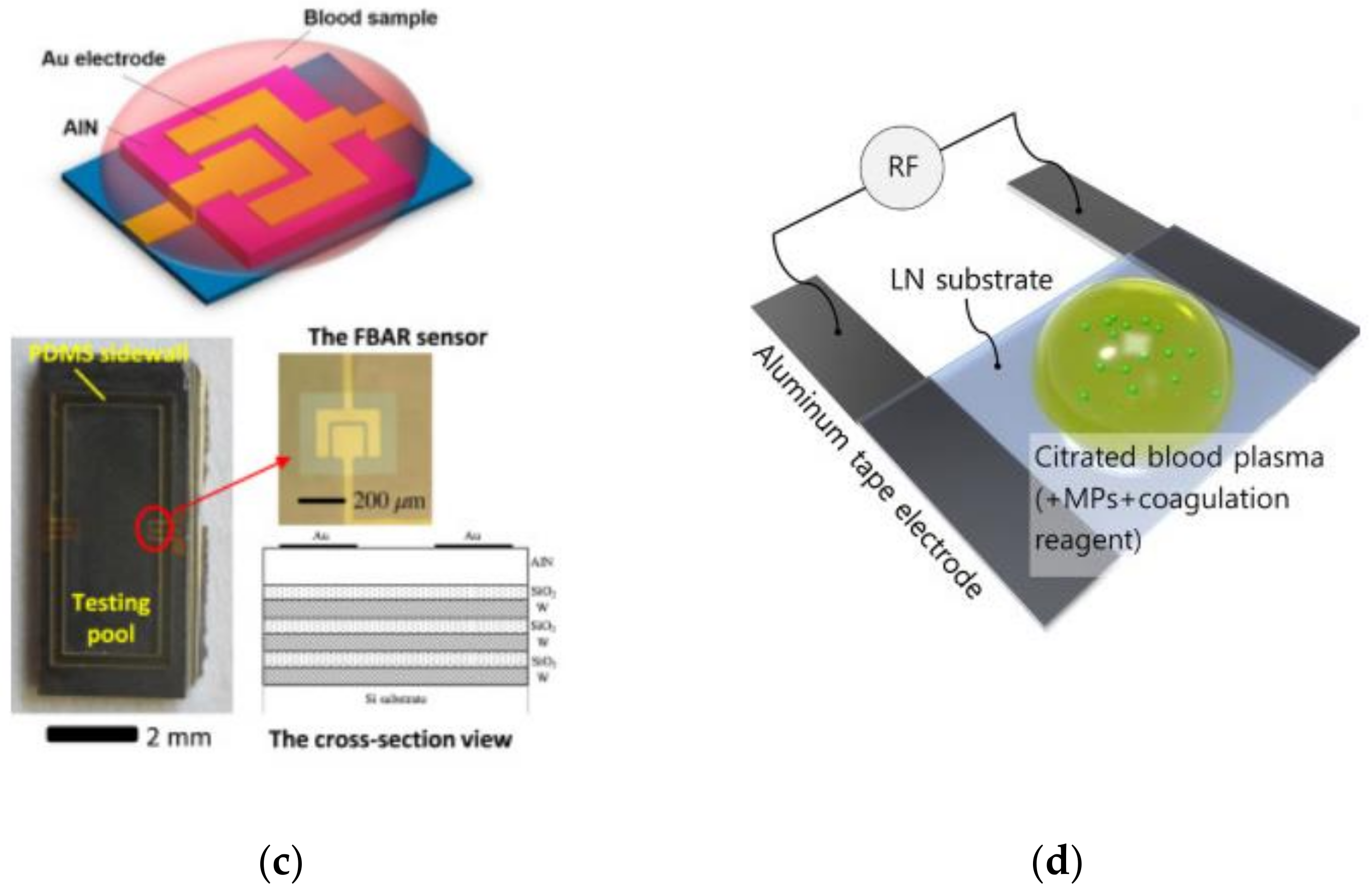

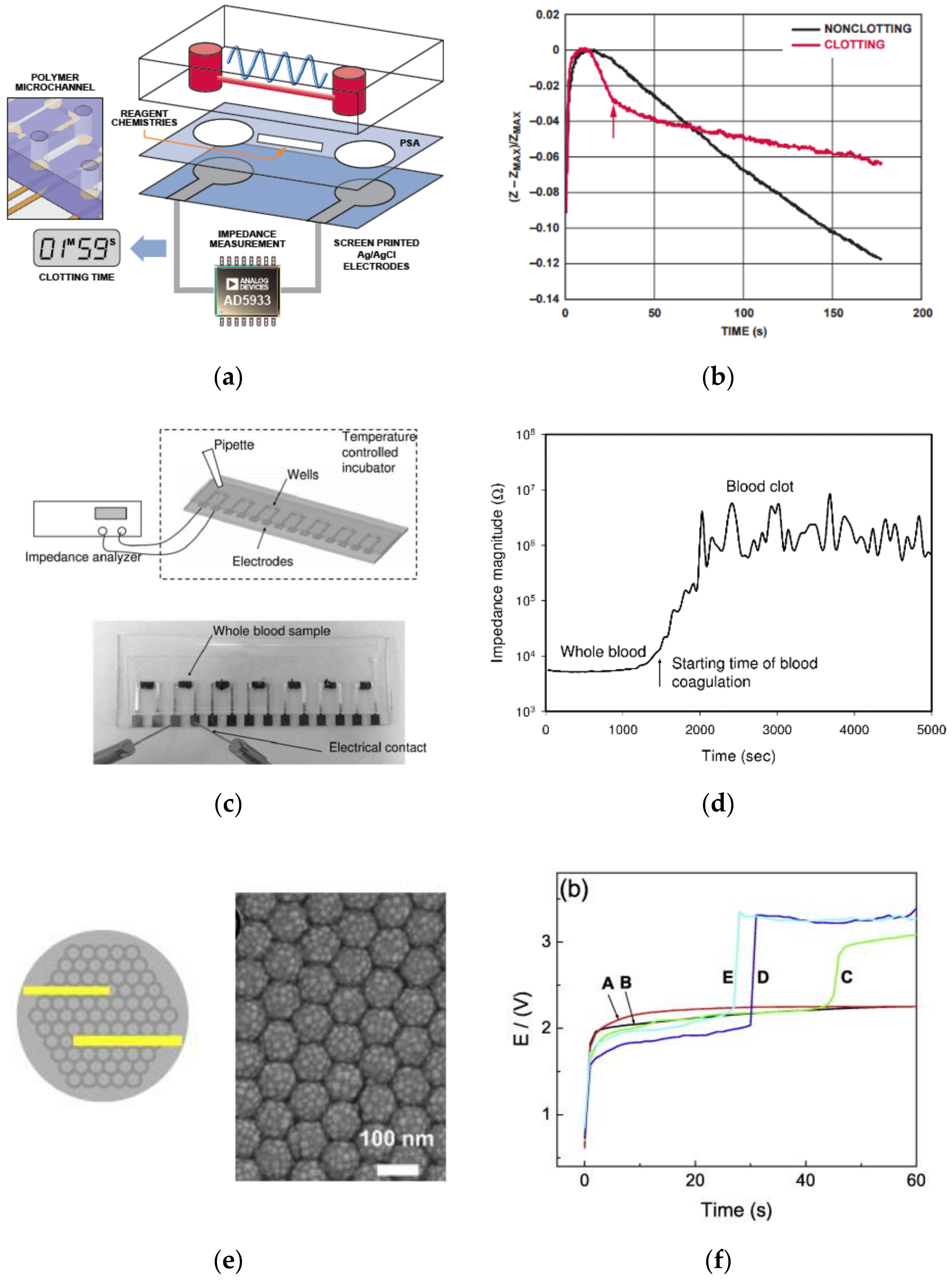
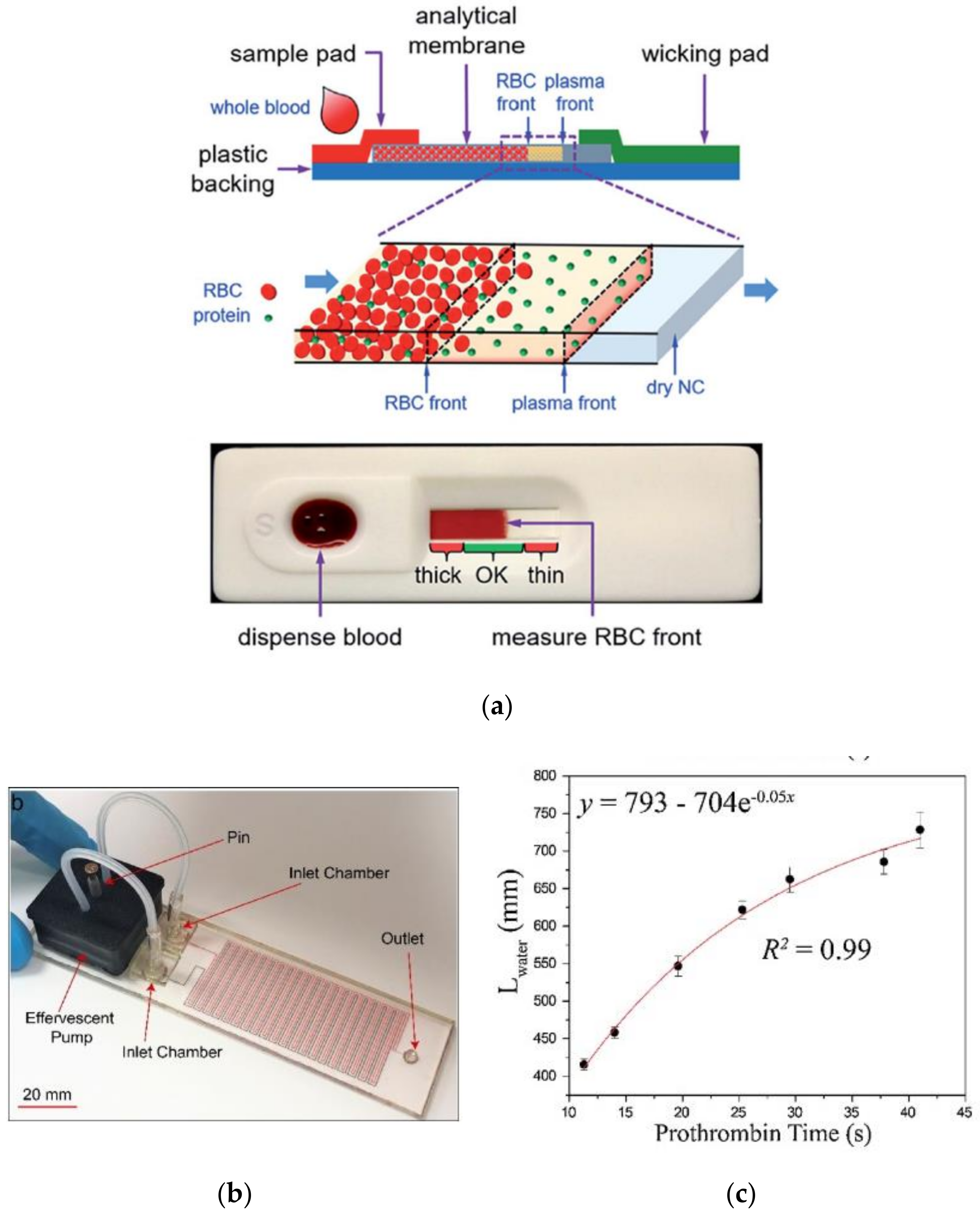
| Coagulation Factor | Common Name | Source |
|---|---|---|
| Factor I | Fibrinogen | Liver |
| Factor II | Prothrombin | Liver |
| Factor III | Tissue factor and thromboplastin | Damaged tissue cells release tissue thromboplastin. Platelets release platelet thromboplastin. |
| Factor IV | Calcium ions | Bone and absorption through the lining of the small intestine |
| Factor V | Proaccelerin and labile factors | Liver and platelets |
| Factor VI | No longer used | N/A |
| Factor VII | Pro-convertin or stable factor | Liver |
| Factor VIII | Anti-hemophilic factor | Platelets and lining of blood vessels |
| Factor IX | Christmas factor | Liver |
| Factor X | Stuart prower factor | Liver |
| Factor XI | Plasma thromboplastin antecedent | Liver |
| Factor XII | Hageman factor | Liver |
| Factor XIII | Fibrin stabilizing factor | Liver |
| Working Mechanism | Sensor or the Physical Phenomenon Sensed | Author/Developer (Year) | Volume and Blood Type | Sample Size (n) | Correlation Coefficient (r)/Coefficient of Determination (R2) | Comparative Gold Standard Device | Turnaround Time |
|---|---|---|---|---|---|---|---|
| Optical | Transmittance | Yang et al. (2013) [48] | ~60 μL whole Blood (WB) | n = 26 relative error: 4.8 + 3.5% (>1 s) | r = 0.997, | Standard Coagulation Analyzer | <5 min |
| Transmittance | Yang et al. (2013) [46] | ~60 μL Both whole Blood (WB) | n = 167 | whole blood INR r = 0.985, p < 0.001 plasmaINR(r = 0.948, p < 0.001) | conventional manual method and ACL TOP 700 bench-top coagulometer (Beckman Colter) | <5 min | |
| Transmittance | Isiksacan et al. (2018) [53] | 50 μL WB | n = 21 | R2 = 0.97 | Conventional benchtop PT analyzer | <2 min | |
| Scattering | Faivre et al. (2011) [53] | 10 μL whole blood | R2 = 0.94 | Automated analyzerMDA II (Trinity Biotech, Bray, Ireland) | 7 min | ||
| LSR | Tripathi et al. (2017) [27] | 40 μL whole blood | n = 60 | R2 = 0.94 p < 0.001 | laboratory PTLab/INRLab values | <30 s | |
| SPR | Hayashi et al. (2012) [55] | 2 μL plasma | few mins | ||||
| Acoustic | QCM | Muller et al. (2010) [59] | 200 μL | 2 samples n = 4 | p < 0.05 | Coagulometer | |
| QCM | Munawar Hussain (2015) [60] | 2.66 μL of plasma | n = 20 | mechanical coagulometer (tCoag). | |||
| QCM | Yao et al. (2018) [61] | 75 μL plasma | R2 = 0.961 | CS-5100 hemostasis system | 15 min | ||
| FBAR | Chen et al. (2017) [62] | 1 μL Both whole blood and plasma | R2 = 0.99969 | commercial coagulometer | ~12–15 min | ||
| SAW | Santos et al. (2013) [63] | 6 μL citrated whole blood | ~3 min | ||||
| Lamb wave-based | Nam et al. (2018) [64] | PT reagent = 3 μL citrated blood plasma = 1.5 μL | n = 5 | R2 = 0.9432 | commercial instrument (STA-R Evolution) | ||
| LFAs (optical) | Distance | Hegener et al. (2017) [93] | 30 μL Capillary whole blood | n = 25/28 | p < 0.05 | CoaguChek®XS coagulometer | 240 s |
| Distance | Guler et al. (2018) [32] | 50 μL whole blood | 7 samples n = 3 | R2 = 0.99 | conventional benchtop (Sysmex, Siemens) | <2 min |
| Device Name/Manufacturer | Physical Phenomenon Sensed | Sample Type/Volume | Detection Time | Market Price |
|---|---|---|---|---|
| HemoSenseTM INRatio Prothrombin Time/INR | Impedance | Capillary whole blood 15 μL | <1 min | |
| Alere INRatio®2 PT/INR Monitoring System | Impedance | Capillary whole blood 9.5–15 μL | 1 min | |
| CoaguChek XS Pro Meter (Roche Diagnostics) | Amperometry | Capillary whole blood 8 μL | 1 min | $2014.00 |
| Xprecia Stride® Coagulation Analyser (Siemens Healthcare GmbH) | Amperometry | Capillary whole blood 6 µL | ~$1428.00 | |
| i-STAT PT/INR by Abbott Labortories, USA | Amperometry | Capillary whole blood 20 μL | 5 min | |
| ProTime InRhythm. (International Technidyne Corp, Edison, NJ, USA) | Pressure driven clot detection technology | Capillary whole blood ~13 μL | <1 min | |
| Coumatrak (Biotrack, USA) | ||||
| Cascade POC (Helena Laboratories Point of care, USA) | Photodetection (transmission) | 30 to 35 µL | <1 min | |
| Coag-Sense™ PT/INR Monitoring System (CoaguSense Inc., Fremont, CA, USA) | Optical (transmission) | minimum 10 µL | $56.95–950.00 | |
| MicroINR (iLine microsystems | Optical (blood flow behavior) | 3 μL | $ 442.79 | |
| Hemochron Signature (ITC) | Optical detection (oscillatory flow behavior based) | Few minutes | $495.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, A.; Bajpai, A.; Krishna, V.; Bhattacharya, S. Evolving Paradigm of Prothrombin Time Diagnostics with Its Growing Clinical Relevance towards Cardio-Compromised and COVID-19 Affected Population. Sensors 2021, 21, 2636. https://doi.org/10.3390/s21082636
Saha A, Bajpai A, Krishna V, Bhattacharya S. Evolving Paradigm of Prothrombin Time Diagnostics with Its Growing Clinical Relevance towards Cardio-Compromised and COVID-19 Affected Population. Sensors. 2021; 21(8):2636. https://doi.org/10.3390/s21082636
Chicago/Turabian StyleSaha, Anubhuti, Ashutosh Bajpai, Vinay Krishna, and Shantanu Bhattacharya. 2021. "Evolving Paradigm of Prothrombin Time Diagnostics with Its Growing Clinical Relevance towards Cardio-Compromised and COVID-19 Affected Population" Sensors 21, no. 8: 2636. https://doi.org/10.3390/s21082636
APA StyleSaha, A., Bajpai, A., Krishna, V., & Bhattacharya, S. (2021). Evolving Paradigm of Prothrombin Time Diagnostics with Its Growing Clinical Relevance towards Cardio-Compromised and COVID-19 Affected Population. Sensors, 21(8), 2636. https://doi.org/10.3390/s21082636





