A Compact Detection Platform Based on Gradient Guided-Mode Resonance for Colorimetric and Fluorescence Liquid Assay Detection
Abstract
:1. Introduction
2. Materials and Methods
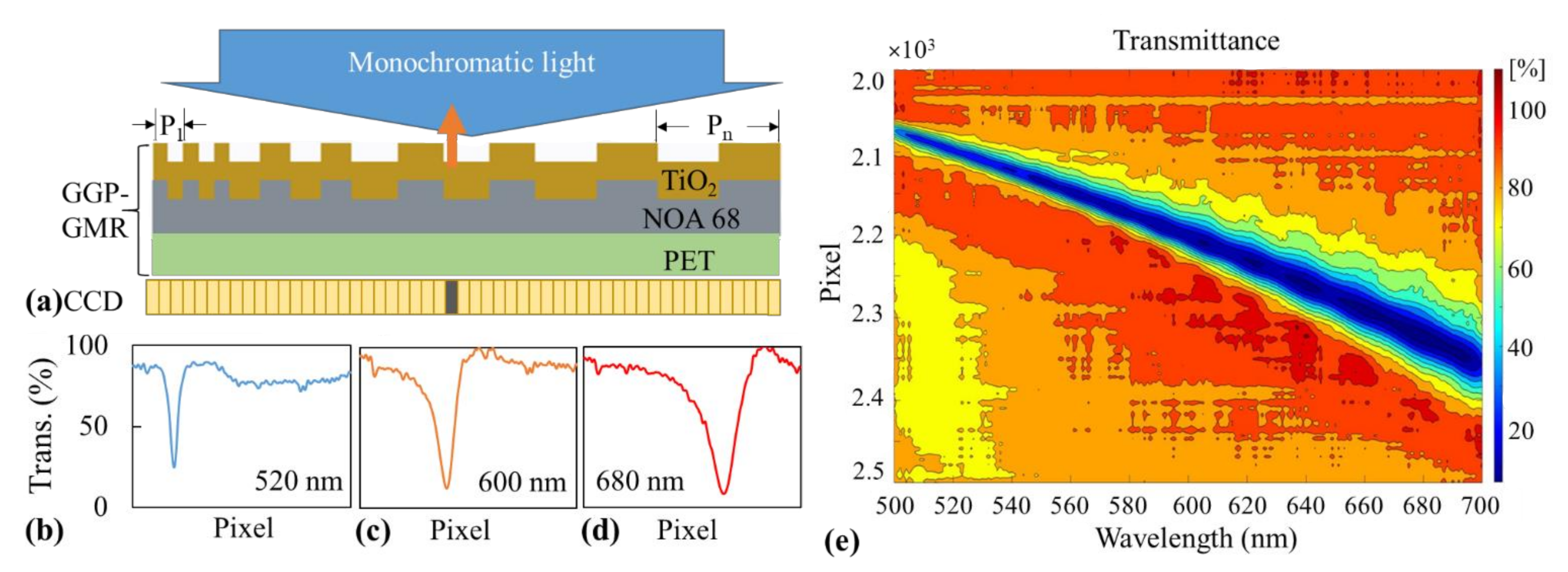
2.1. Sensor Design, Fabrication, and Characterization
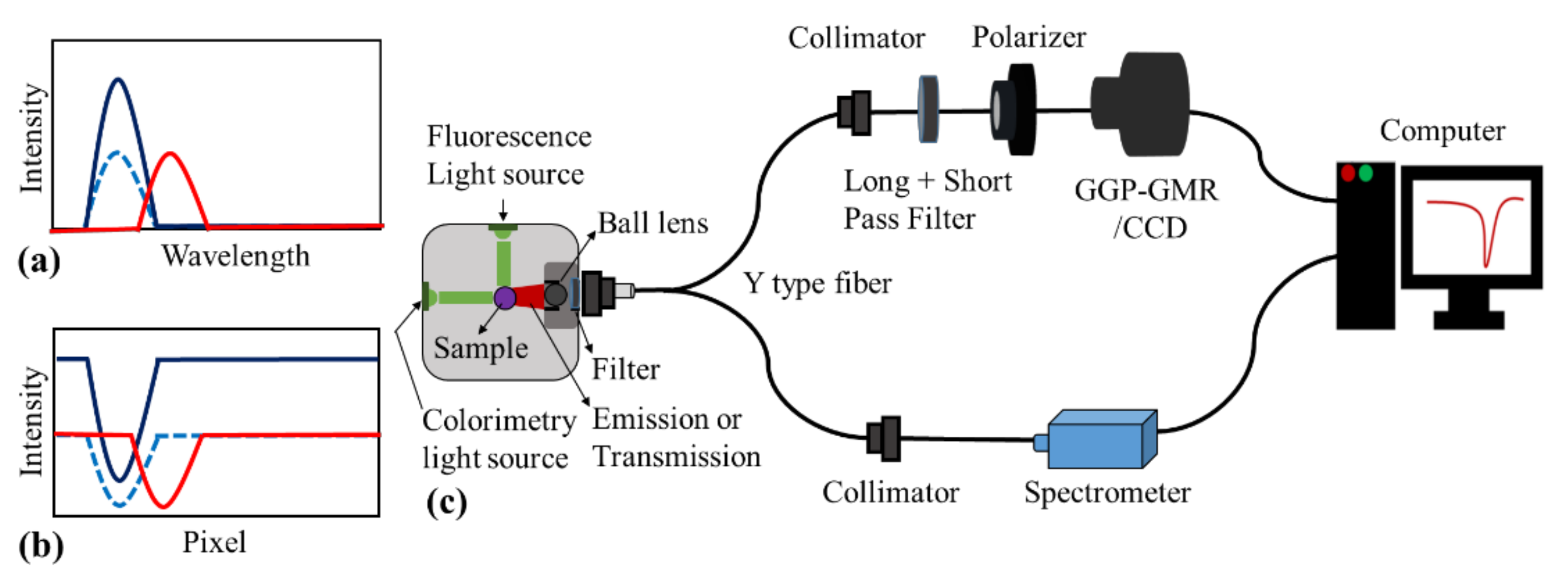
2.2. Detection Mechanism and Experimental Setup
3. Results and Discussion
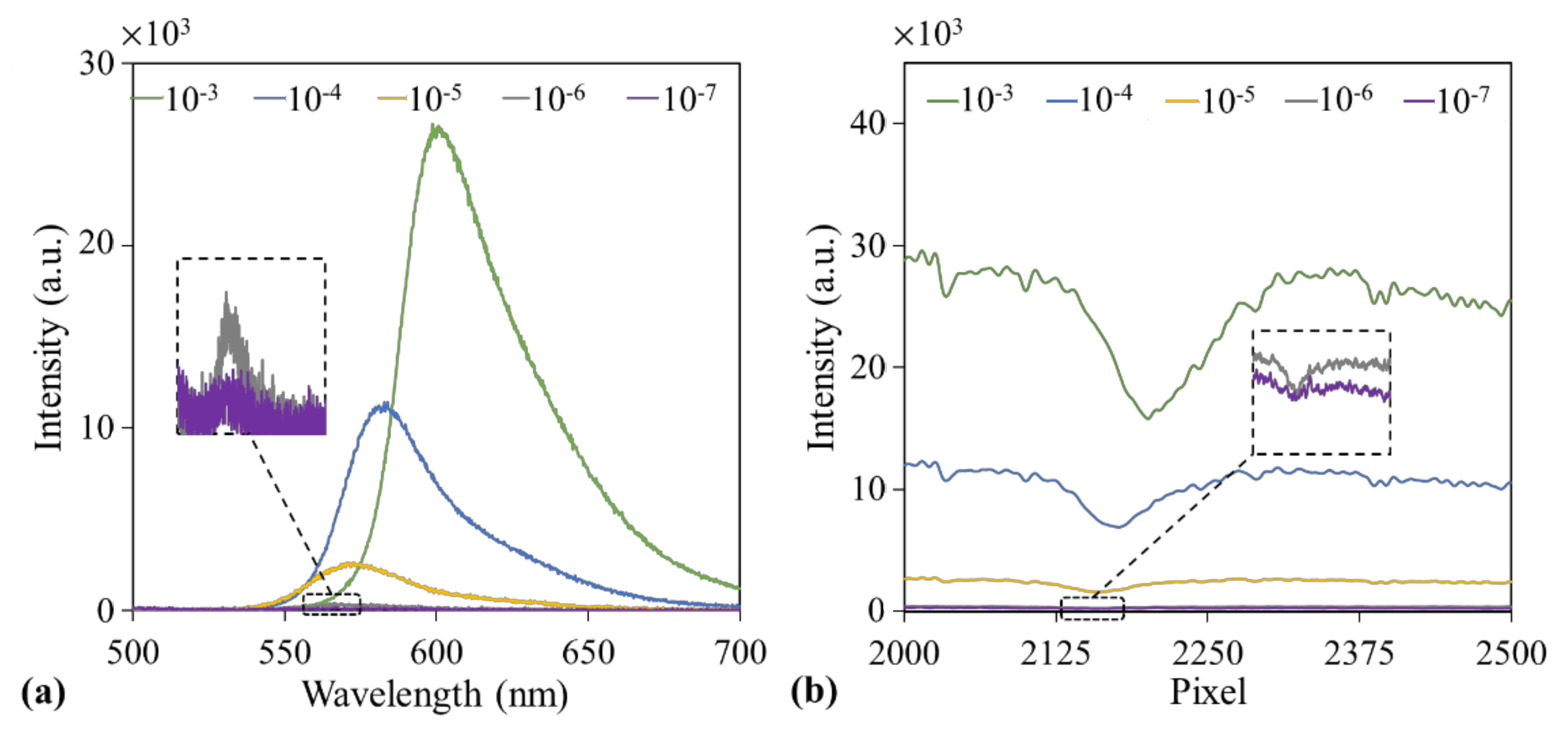
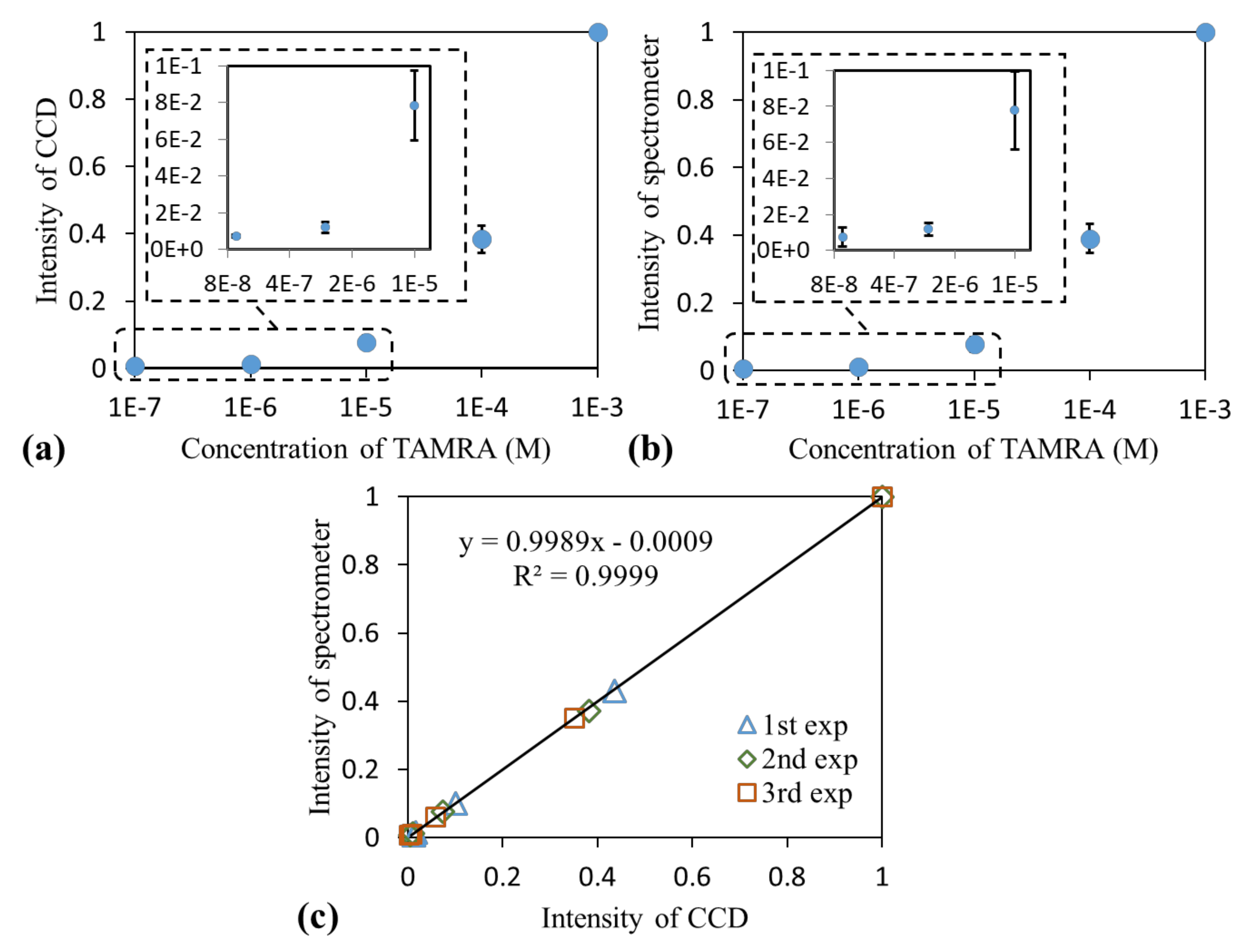
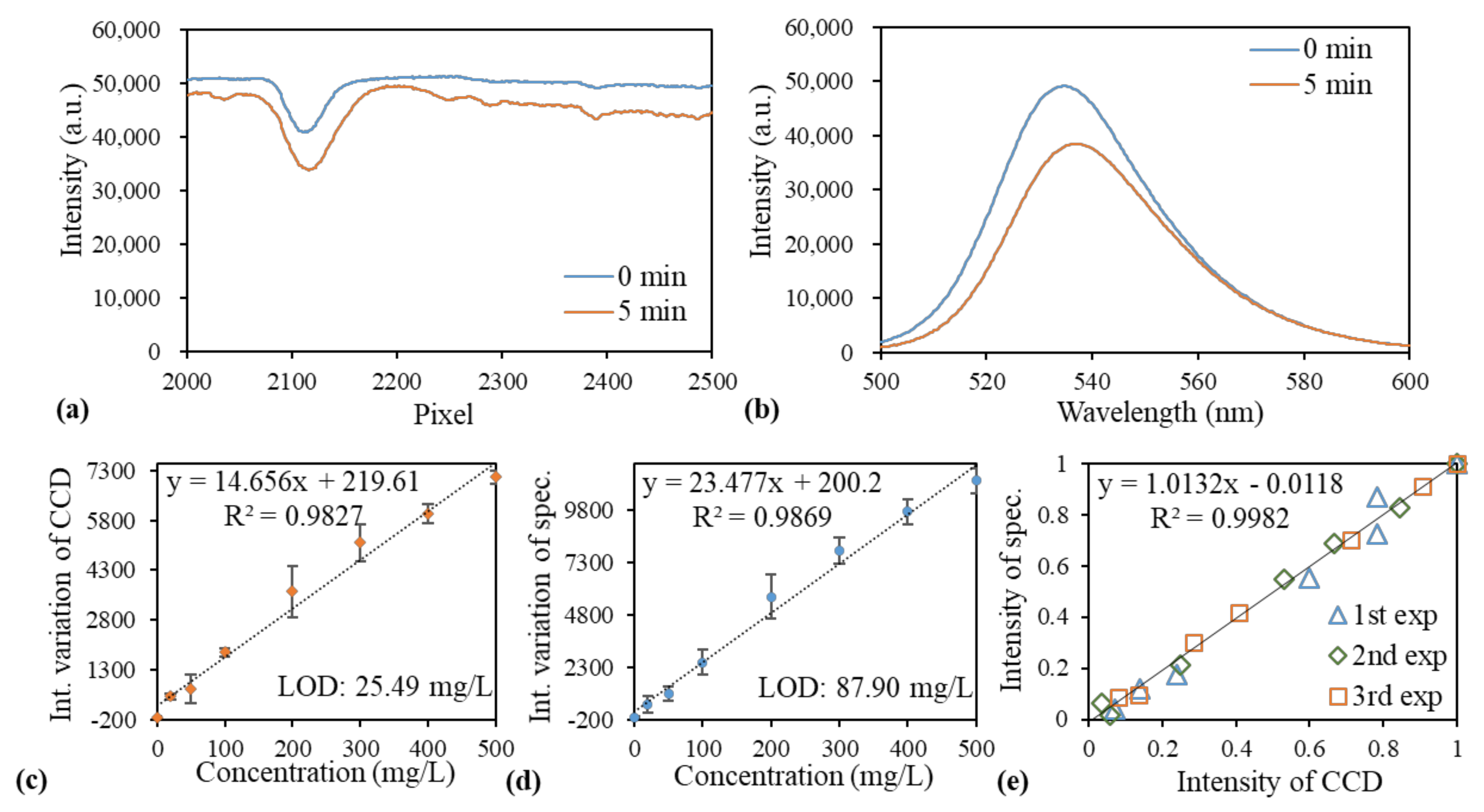
3.1. Measurement of Fluorophore Emission
3.2. Practical Assay Detection
3.2.1. Fluorescence Detection for Albumin Quantification
3.2.2. Colorimetric Measurement for Creatinine and Albumin Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatiboruah, D.; Das, T.; Chamuah, N.; Rabha, D.; Talukdar, B.; Bora, U.; Ahamad, K.U.; Nath, P. Estimation of trace-mercury concentration in water using a smartphone. Measurement 2020, 154, 7. [Google Scholar] [CrossRef]
- Minas, G.; Wolffenbuttel, R.F.; Correia, J.H. A lab-on-a-chip for spectrophotometric analysis of biological fluids. Lab Chip 2005, 5, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Zeinhom, M.M.A.; Wang, Y.J.; Song, Y.; Zhu, M.J.; Lin, Y.H.; Du, D. A portable smart -phone device for rapid and sensitive detection of E. coli O157:H7 in Yoghurt and Egg. Biosens. Bioelectron. 2018, 99, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum Dots in Bioanalysis: A review of applications across various platforms for fluorescence spectroscopy and imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.H.; Carlson, J.A.; Kesler, B.A.; Peng, W.; Su, P.; Al-Mulla, S.A.; Lim, S.J.; Smith, A.M.; Dallesasse, J.M.; Cunningham, B.T. Compact characterization of liquid absorption and emission spectra using linear variable filters integrated with a CMOS imaging camera. Sci. Rep. 2016, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Schena, M.; Shalon, D.; Davis, R.W.; Brown, P.O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 1995, 270, 467–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodbury, R.L.; Varnum, S.M.; Zangar, R.C. Elevated HGF levels in sera from breast cancer patients detected using a protein microarray ELISA. J. Proteome Res. 2002, 1, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.A.; Meinitzer, A.; Wolfbeis, O.S. Albumin blue 580 fluorescence assay for albumin. Anal. Biochem. 1997, 248, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Hennion, M.C.; Barcelo, D. Strengths and limitations of immunoassays for effective and efficient use for pesticide analysis in water samples: A review. Anal. Chim. Acta 1998, 362, 3–34. [Google Scholar] [CrossRef]
- Hall, G.H.; Glerum, D.M.; Backhouse, C.J. Light emitting diode, photodiode-based fluorescence detection system for DNA analysis with microchip electrophoresis. Electrophoresis 2016, 37, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Magnusson, R. Theory and applications of guided-mode resonance filters. Appl. Optics 1993, 32, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, D.; Sharon, A.; Friesem, A.A. Resonant grating waveguide structures. IEEE J. Quantum Electron. 1997, 33, 2038–2059. [Google Scholar] [CrossRef]
- Magnusson, R.; Ding, Y.; Lee, K.J.; Shin, D.; Priambodo, P.S.; Young, P.P.; Maldonado, T.A. Photonic devices enabled by waveguide-mode resonance effects in periodically modulated films. In Proceedings of the Optical Science and Technology, SPIE’s 48th Annual Meeting, San Diego, CA, USA, 3–8 August 2003. [Google Scholar]
- Block, I.D.; Ganesh, N.; Lu, M.; Cunningham, B.T. Sensitivity model for predicting photonic crystal biosensor performance. IEEE Sens. J. 2008, 8, 274–280. [Google Scholar] [CrossRef]
- Lin, H.A.; Huang, C.S. Linear variable filter based on a gradient grating period guided-mode resonance filter. IEEE Photonics Technol. Lett. 2016, 28, 1042–1045. [Google Scholar] [CrossRef]
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 Update. Am. J. Kidney Dis. 2016, 67, 534. [Google Scholar]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; de Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laiwattanapaisal, W.; Saythanu, P.; Nubtueboon, P.; Tencomnao, T.; Santiyanont, R. A high-throughput nonimmunological method for determination of microalbuminuria based on utilization of albumin blue 580. Labmedicine 2008, 39, 727–729. [Google Scholar] [CrossRef]
- Hofmann, O.; Wang, X.H.; deMello, J.C.; Bradley, D.D.C.; deMello, A.J. Towards microalbuminuria determination on a disposable diagnostic microchip with integrated fluorescence detection based on thin-film organic light emitting diodes. Lab Chip 2005, 5, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of Feces and Urine: A review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonsnes, R.W.; Taussky, H.H. On the colorimetric determination of creatinine by the jaffe reaction. J. Biol. Chem. 1945, 158, 11. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.-J.; Chiu, C.-W.; Wen, K.-H.; Huang, C.-S. A Compact Detection Platform Based on Gradient Guided-Mode Resonance for Colorimetric and Fluorescence Liquid Assay Detection. Sensors 2021, 21, 2797. https://doi.org/10.3390/s21082797
Gao J-J, Chiu C-W, Wen K-H, Huang C-S. A Compact Detection Platform Based on Gradient Guided-Mode Resonance for Colorimetric and Fluorescence Liquid Assay Detection. Sensors. 2021; 21(8):2797. https://doi.org/10.3390/s21082797
Chicago/Turabian StyleGao, Jing-Jhong, Ching-Wei Chiu, Kuo-Hsing Wen, and Cheng-Sheng Huang. 2021. "A Compact Detection Platform Based on Gradient Guided-Mode Resonance for Colorimetric and Fluorescence Liquid Assay Detection" Sensors 21, no. 8: 2797. https://doi.org/10.3390/s21082797
APA StyleGao, J. -J., Chiu, C. -W., Wen, K. -H., & Huang, C. -S. (2021). A Compact Detection Platform Based on Gradient Guided-Mode Resonance for Colorimetric and Fluorescence Liquid Assay Detection. Sensors, 21(8), 2797. https://doi.org/10.3390/s21082797




