Stainless Steel Voltammetric Sensor to Monitor Variations in Oxygen and Humidity Availability in Reinforcement Concrete Structures
Abstract
:1. Introduction
2. Materials and Methods
- Phase (1) Study in solution. In this preliminary phase, the processes that took place on the SS electrode surface when immersed in solution were studied, which represent the different conditions that could come into play in concrete. The obtained results were compared with those found in the literature.
- Phase (2) Studies performed with the SS sensor embedded in conventional concrete. SS voltammetric sensors were embedded in conventional concrete samples at different water/cement ratios (w/c). Samples were studied under distinct O2 availability conditions.
2.1. Studies in Solution
- In the first situation, O2 availability in solution was reduced by argon bubbling for 30 min before testing
- In the second situation, KOH solution was O2-saturated by bubbling synthetic air for 30 min before testing. Synthetic air was used to prevent an atmospheric CO2 solution and to avoid the employed solution’s carbonation.
- Solutions 0.1 m KOH (pH = 13) and 0.1 m NaOH (pH = 12.7) to simulate non-carbonated concrete [32]
- 0.1 m Na2CO3 solution (pH = 11.45) to simulate the initial degree of carbonation [32]
- 0.1 m NaHCO3 solution (pH = 8.35) to simulate carbonated concrete [32]
- Solution 0.1 m NaOH +Na2CO3 Ci m (pH = 12.7), where Ci came in these concentrations: 0.02 m, 0.05 m and 0.1 m. The purpose was to evaluate how the presence of carbonates influenced the obtained results.
- Solution 0.1 m NaOH (pH = 12.7) by adding NaCl at 0.5 m
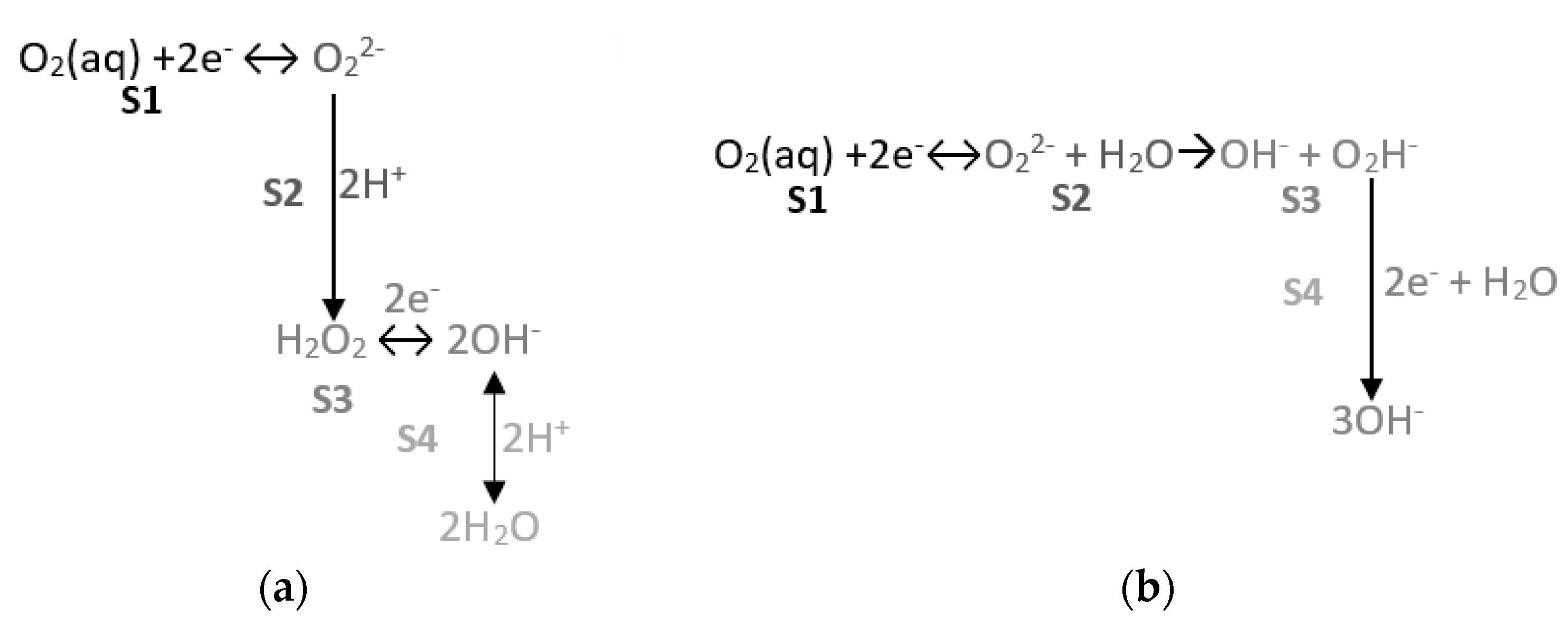
- Solution 0.1 m NaOH (pH = 13) to simulate non-carbonated concrete by adding H2O2 at 0.02 m, and to assess the possible appearance or stabilization of this intermediate product in reducing O2, as suggested in the literature
The Applied Electro-Analytical Techniques
2.2. Studies Performed with the Sensor Embedded in Concrete
- Concrete type. Samples made of three different concrete types and, therefore, with distinct porosity, were used, which would imply differences in O2 availability in the vicinity of the electrode when samples were under similar environmental conditions
- O2 availability. Samples were left under four different environmental O2 availability conditions:
- ∘
- Atmospheric conditions (ATM), O2 molar fraction (xO2) 0.21
- ∘
- Partial air pressure condition, 500 mbar (500 mbar), O2 molar fraction (xO2) 0.105
- ∘
- Vacuum conditions (VAC), the O2 molar fraction (xO2) came very close to 0
- ∘
- H2O saturation conditions (SAT), the available O2 in the concrete matrix was limited by the gas solution capacity in the concrete pore solution.
2.2.1. Study in the Non-Saturated State
- Vacuum conditions: To homogenize the initial behavior of the sensors embedded in the different samples, the studied samples were subjected to a vacuum for 12 h. After finishing the adaptation period, the aforementioned electro-chemical studies were carried out.
- Atmospheric conditions: Once the tests under the vacuum conditions ended, synthetic air was allowed to enter the desiccator. When a 1-bar pressure was achieved inside, 60 min were allowed for the system to stabilize before testing began.
- Partial 500-mbar air pressure: when the tests under atmospheric conditions ended, the pressure inside the desiccator was lowered until vacuum conditions were achieved. At this point, 30 min were allowed before letting air in until a sTable 500-mbar pressure was accomplished. After a 15-min wait, argon was injected until the 1-bar pressure was recovered. In this way, the O2 molar fraction inside the desiccator diminished to half in relation to the working atmospheric conditions. Measurements were taken on sensors after 1 h under 500 mbar conditions.
2.2.2. Study in the Saturated State
2.2.3. Applied Electro-Analytical Techniques
2.2.4. Concrete Characterization Tests
- Hardened concrete tests, Part 3: determining sample strength to compression (UNE 12390-3:2009). Resistance to compression was determined at 28 days (fck). One cylindrical sample (10 cm diameter, 20 cm high) was prepared for each mass per dosage, with nine samples in all. The equipment used was Ibertest MEH-3000.
- Determining H2O absorption, density, and H2O accessible porosity (UNE 83980:2014). One cylindrical sample (10 cm diameter, 5 cm high) was prepared for each mass per dosage. Nine samples in all.
- Determining H2O penetration depth under pressure (UNE 83-309-90). One sample (15 cm diameter, 30 cm high) was produced for this test for each mass per dosage. Nine samples in all.
- Determining air permeability (UNE 83981:2008). One cylindrical sample (15 cm diameter, 5 cm high) was prepared for each mass per dosage. To perform the test, the sides of the samples were covered with sealing paint. The air permeability coefficient was obtained (k). Nine samples in all.
- Determining electrical resistivity (ρ): direct method (reference method) (UNE 83988-1:2008). A prismatic sample (4 × 4 × 16 cm3) was prepared for each mass per dosage. Nine samples in all.
- Mercury injection porosity (MIP) test (ASTM-D4404-10). This test allows information to be acquired about the volume of interconnected pores and their size distribution. These tests were performed by the ITC of the Universitat Jaume I (Spain). The MIP method consists of injecting mercury at different pressures. The volume of absorbed mercury at each pressure is recorded so that the volume of absorbed mercury within a certain range of pressures is associated with a given access size range.
3. Results
3.1. Phase 1: Studies Performed in Solution
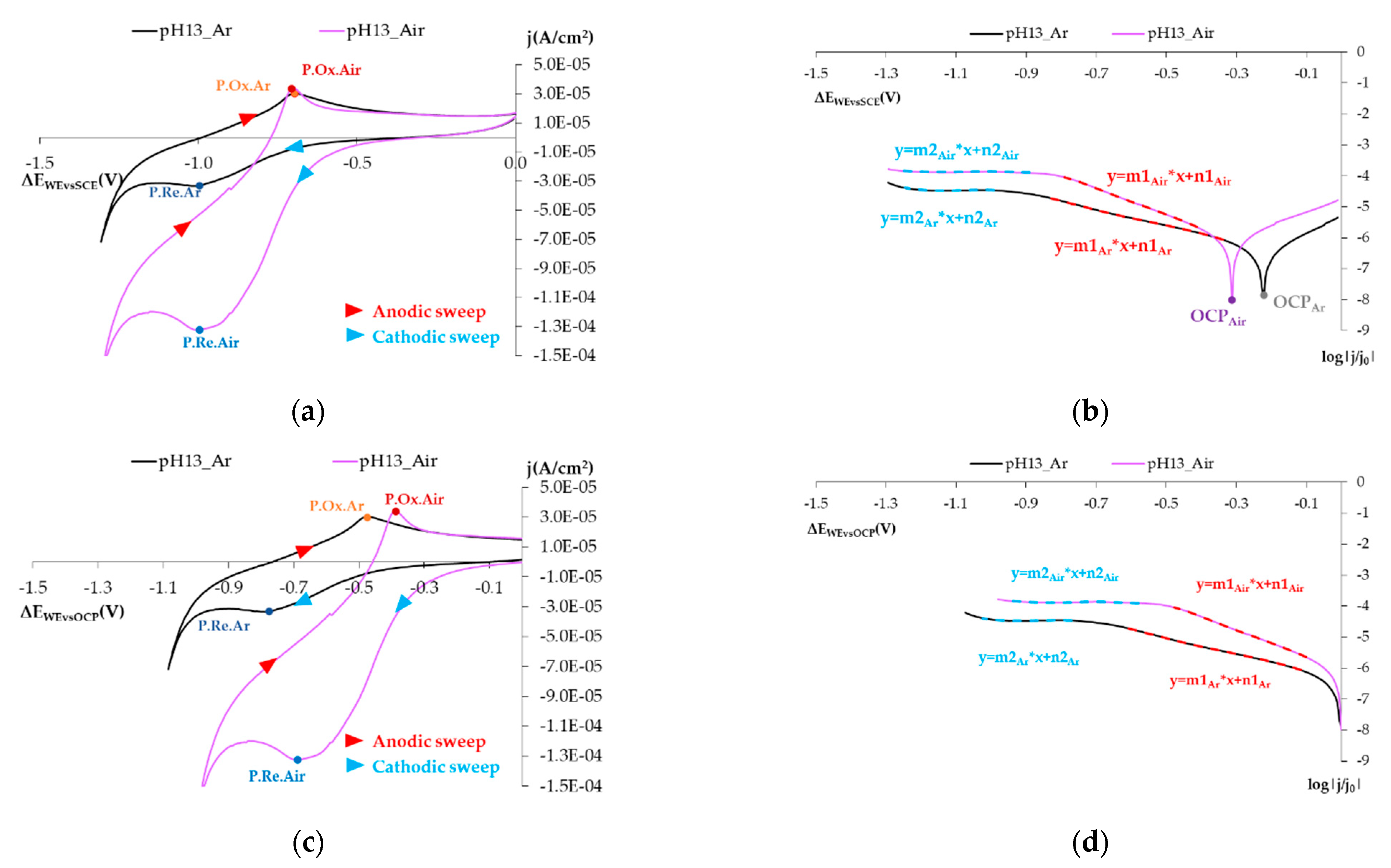
3.1.1. Reducing O2 on the SS Electrode’s Surface
- The open circuit potential (OCP) shifts to the left if the atmosphere is aired.
- In this representation, two straight-lined sections appear. These lines are associated with O2, H2O2, and H2O reduction processes. The following can be deduced from the study:
- ∘
- The first straight line is that which varies the most with changes in O2 availability, and the value of its slope in the airless atmosphere goes from −2.630 V−1 to −4.272 V−1 (Table 5). The variation in the intercept is slight. This variation in the representation of potentials versus the SCE is 6.8% and 4.5% versus the OCP (Table 5). These minor variations do not enable us to establish significant differences between both states.
- ∘
- The second line is practically horizontal. The value of this line’s slope seems to be independent of the solution’s airing (Table 5). This plateau aspect indicates that it is due to a limitation in the speed of the material’s transport process.
3.1.2. Influence of pH on the SS Electrode
- Normal conditions (pH 13 and pH 12.72).
- The initial carbonation state (pH of 11.45).
- Carbonated concrete (pH 8.35).
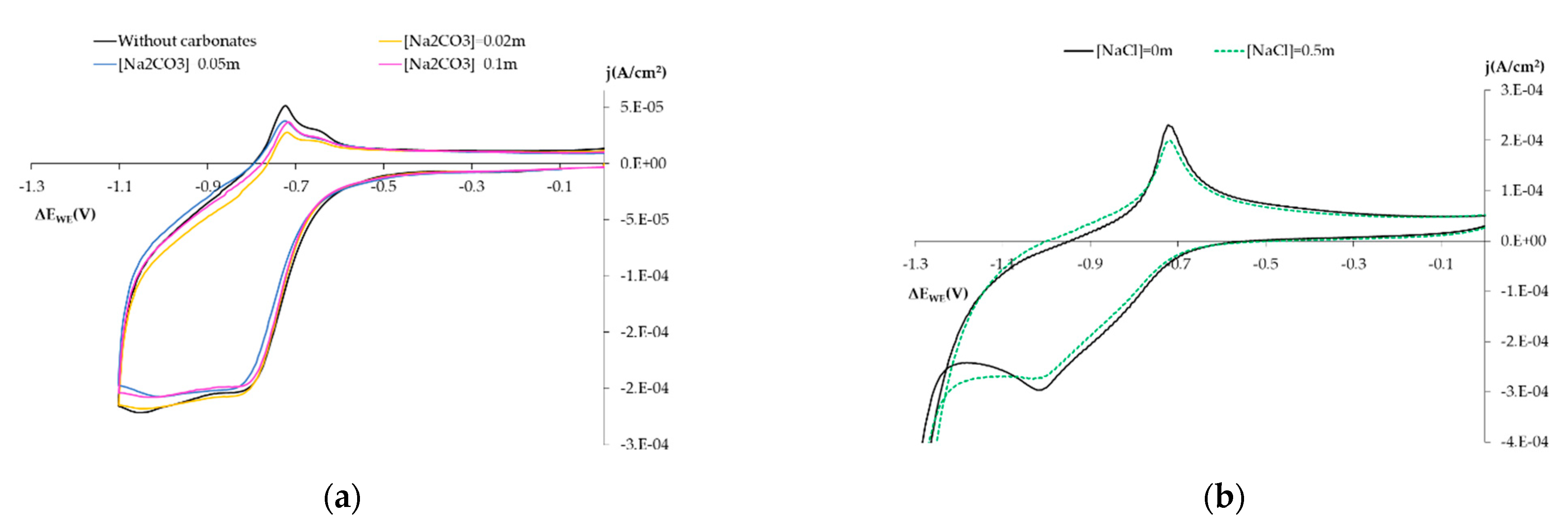
3.1.3. Influence on the Electro-Chemical Behavior of Other Chemical Species
- Influence of carbonates: Solution 0.1 m NaOH + Na2CO3 Ci m (pH = 12.7), with Ci concentrations of 0.02 m, 0.05 m and 0.1 m.
- Influence of chlorides: Solution 0.1 m NaOH (pH = 12.7) by adding NaCl at 0.5 m.
- Influence of H2O2: Solution 0.1 m NaOH (pH = 12.7) by adding H2O2 at 0.02 m.
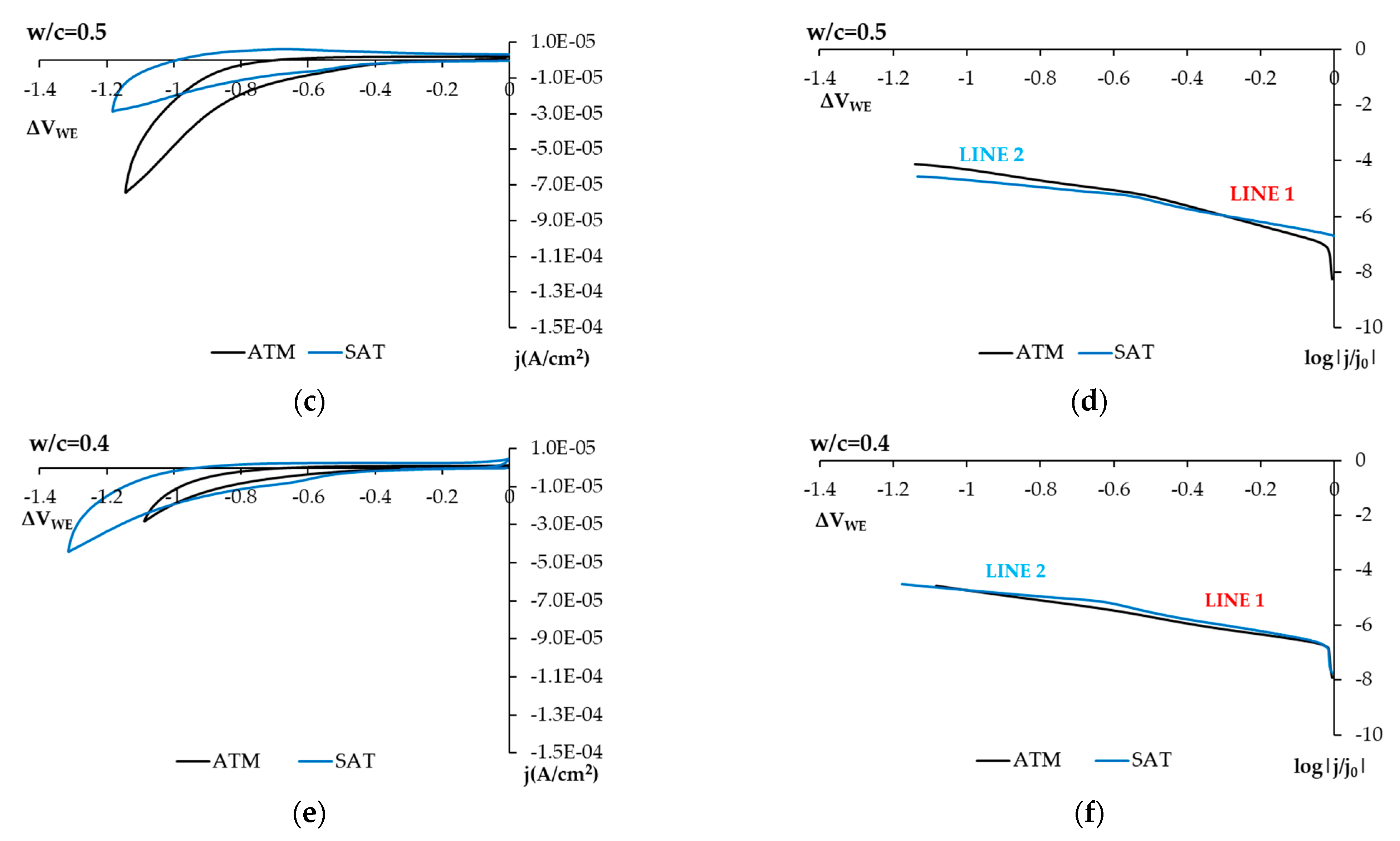
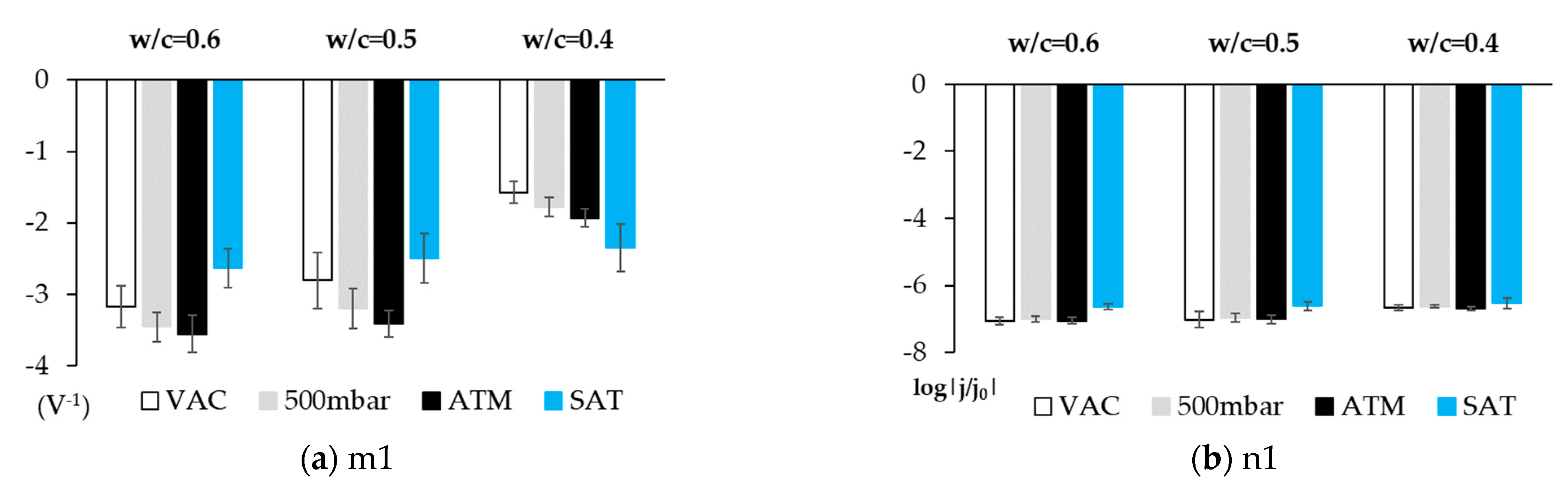
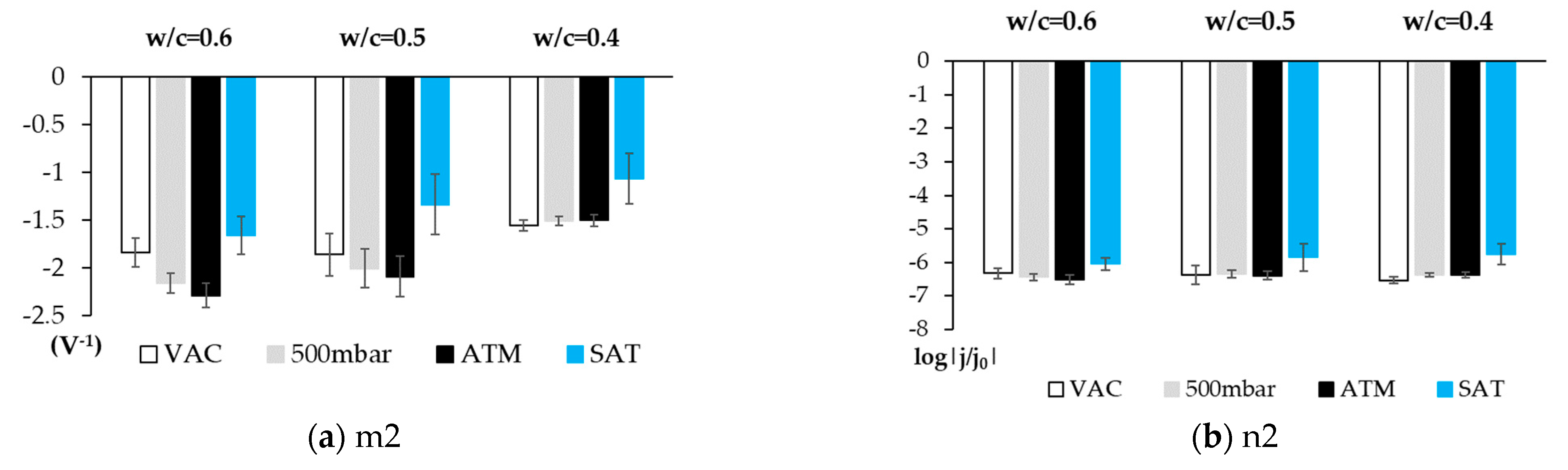
3.2. Phase 2: Studies Performed with the Sensor Embedded in Concrete
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Kumar Verma, S.; Singh Bhadauria, S.; Akhtar, S. Estimating Residual Service Life of Deteriorated Reinforced Concrete Structures. Am. J. Civ. Eng. Archit. 2013, 1, 92–96. [Google Scholar] [CrossRef]
- Bertolini, L.; Elsener, B.; Pedeferri, P. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair; Wiley: Weinheim, Germany, 2004; Available online: https://books.google.es/books?id=cEmq232h1zcC (accessed on 20 December 2020).
- Martínez, I.; Andrade, C. Examples of reinforcement corrosion monitoring by embedded sensors in concrete structures. Cem. Concr. Compos. 2009, 31, 545–554. [Google Scholar] [CrossRef]
- Figueira, R.B. Electrochemical sensors for monitoring the corrosion conditions of reinforced concrete structures: A review. Appl. Sci. 2017, 7, 1157. [Google Scholar] [CrossRef] [Green Version]
- Tutti, K. Corrosion of Steel in Concrete; Swedish Cement and Concrete Institute: Stokholm, Sweden, 1982. [Google Scholar]
- Correia, M.J.; Pereira, E.V.; Salta, M.M.; Fonseca, I.T.E. Sensor for oxygen evaluation in concrete. Cem. Concr. Compos. 2006, 28, 226–232. [Google Scholar] [CrossRef]
- Gulikers, J. Development of a Galvanic Monitoring Probe to Improve Service Life Prediction of Reinforced Concrete Structures with respect to Reinforcement Corrosion. Constr. Build. Mater. 1997, 11, 143–148. [Google Scholar] [CrossRef]
- McCarter, W.J.; Vennesland, Ø. Sensor systems for use in reinforced concrete structures. Constr. Build. Mater. 2004, 18, 351–358. [Google Scholar] [CrossRef]
- Yoo, J.H.; Park, Z.T.; Kim, J.G.; Chung, L. Development of a galvanic sensor system for detecting the corrosion damage of the steel embedded in concrete structures: Part 1. Laboratory tests to correlate galvanic current with actual damage. Cem. Concr. Res. 2003, 33, 2057–2062. [Google Scholar] [CrossRef]
- Gandía-Romero, J.M.; Campos, I.; Valcuende, M.; García-Breijo, E.; Marcos, M.D.; Payá, J.; Soto, J. Potentiometric thick-film sensors for measuring the pH of concrete. Cem. Concr. Compos. 2016, 68, 66–76. [Google Scholar] [CrossRef]
- Jabir, S.A.A.; Gupta, N.K. Thick-film ceramic strain sensors for structural health monitoring. IEEE Trans. Instrum. Meas. 2011, 60, 3669–3676. [Google Scholar] [CrossRef]
- Park, Z.T.; Choi, Y.S.; Kim, J.G.; Chung, L. Development of a galvanic sensor system for detecting the corrosion damage of the steel embedded in concrete structure: Part 2. Laboratory electrochemical testing of sensors in concrete. Cem. Concr. Res. 2005, 35, 1814–1819. [Google Scholar] [CrossRef]
- Pereira, E.V.; Figueira, R.B.; Salta, M.M.L.; da Fonseca, I.T.E. A galvanic sensor for monitoring the corrosion condition of the concrete reinforcing steel: Relationship between the galvanic and the corrosion currents. Sensors 2009, 9, 8391–8398. [Google Scholar] [CrossRef] [Green Version]
- Gandía-Romero, J.M.; Bataller, R.; Monzón, P.; Campos, I.; García-Breijo, E.; Valcuende, M.; Soto, J. Characterization of embeddable potentiometric thick-film sensors for monitoring chloride penetration in concrete. Sens. Actuators B Chem. 2016, 222, 407–418. [Google Scholar] [CrossRef]
- Ramón, J.E.; Gandía-Romero, J.M.; Valcuende, M.; Bataller, R. Integrated Sensor Network for Monitoring Steel Corrosion in Concrete Structures. Int. J. Archit. Technol. Sustain. 2016, 1, 64–79. [Google Scholar] [CrossRef] [Green Version]
- Compagnone, D. Electrochemical Sensors and Biosensors in Italy. Electroanalysis 2012, 24, 717. [Google Scholar] [CrossRef]
- Kovendhan, M.; Kang, H.; Youn, J.S.; Cho, H.; Jeon, K. ScienceDirect Alternative cost-effective electrodes for hydrogen production in saline water condition. Int. J. Hydrogen. Energy 2018, 44, 5090–5098. [Google Scholar] [CrossRef]
- Kovendhan, M.; Kang, H.; Jeong, S.; Youn, J.S.; Oh, I.; Park, Y.K.; Jeon, K.J. Study of stainless steel electrodes after electrochemical analysis in sea water condition. Environ. Res. 2019, 73, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, M.; Pavitra, K.; Jana, V.; Kachwala, T. Manufacturing and critical applications of stainless steel- an overview. Adv. Mater. Res. 2013, 794, 163–173. [Google Scholar] [CrossRef]
- Baddoo, N.R. Stainless steel in construction: A review of research, applications, challenges and opportunities. J. Constr. Steel Res. 2008, 64, 1199–1206. [Google Scholar] [CrossRef]
- Gojkovic, S.L.; Zecevic, S.K.; Drazic, D.M. Oxygen reduction on iron-Part VI. Processes in alkaline solutions. Electrochim. Acta 1994, 39, 975–982. [Google Scholar] [CrossRef]
- Pérez-Ràfols, C.; Serrano, N.; Ariño, C.; Esteban, M.; Díaz-Cruz, J.M. Voltammetric electronic tongues in food analysis. Sensors 2019, 19, 4261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Ibernón, A.; Ramon, J.E.; Gandía-Romero, J.M.; Gasch, I.; Valcuende, M.; Alcaniz, M.; Soto, J. Electrochimica Acta Characterization of electrochemical systems using potential step voltammetry. Part II: Modeling of reversible systems. Electrochim. Acta 2019, 328, 135111. [Google Scholar] [CrossRef]
- Xu, Y.; Li, K.; Liu, L.; Yang, L.; Wang, X.; Huang, Y. Experimental study on rebar corrosion using the galvanic sensor combined with the electronic resistance technique. Sensors 2016, 16, 1451. [Google Scholar] [CrossRef]
- Tabatabai, H.; Aljuboori, M. A novel concrete-based sensor for detection of ice and water on roads and bridges. Sensors 2017, 17, 2912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babic, R.; Metikos-Hukovic, M. Oxygen reduction on stainless steel. J. Appl. Electrochem. 1993, 23, 352–357. [Google Scholar] [CrossRef]
- Calvo, E.J.; Schiffrin, D.J. The electrochemical reduction of oxygen on passive iron in alkaline solutions. J. Electroanal. Chem. 1988, 243, 171–185. [Google Scholar] [CrossRef]
- Zečević, S.; Dražić, D.M.; Gojković, S. Oxygen reduction on iron. Part III. An analysis of the rotating disk-ring electrode measurements in near neutral solutions. J. Electroanal. Chem. 1989, 265, 179–193. [Google Scholar] [CrossRef]
- Alonso, C.; Garcia, A.M. Oxygen availability in the corrosion of reinforcements. Adv. Cem. Res. 1990, 3, 127–132. [Google Scholar]
- Duffó, G.S.; Farina, S.B.; Giordano, C.M. Characterization of solid embeddable reference electrodes for corrosion monitoring in reinforced concrete structures. Electrochim. Acta 2009, 54, 1010–1020. [Google Scholar] [CrossRef]
- Martínez-Ibernón, A.; Roig-Flores, M.; Lliso-Ferrando, J.; Mezquida-Alcaraz, E.J.; Valcuende, M.; Serna, P. Influence of cracking on oxygen transport in UHPFRC using stainless steel sensors. Appl. Sci. 2020, 10, 239. [Google Scholar] [CrossRef] [Green Version]
- Gandía Romero, J.M. Sensores Electroquímicos Aplicados al Estudio de la Corrosión en Estructuras de Hormigón Armado; Universitat Politècnica de València: Valencia, Spain, 2014; Available online: https://riunet.upv.es/handle/10251/48516 (accessed on 26 March 2018).
- Ait Albrimi, Y.; Eddib, A.; Douch, J.; Berghoute, Y.; Hamdani, M.; Souto, R.M. Electrochemical behaviour of AISI 316 austenitic stainless steel in acidic media containing chloride ions. Int. J. Electrochem. Sci. 2011, 6, 4614–4627. [Google Scholar]
- Ramón Zamora, J.E. Sistema de Sensores Embebidos para Monitorizar la Corrosión en Estructuras de Hormigón Armado. Fundamentos, Metodología y Aplicaciones. Ph.D. Thesis, University Politècnica València, València, Spain, 2018. [Google Scholar]
- ASTM E104-20a. Standard Practice for Maintaining Constant Relative Humidity by Means of Aqueous Solutions; ASTM International: West Conshohocken, PA, USA, 2020; Available online: www.astm.org (accessed on 1 January 2021).
- Bösing, I.; Thöming, J.; Baune, M. Electrolyte Composition for Distinguishing Corrosion Mechanisms in Steel Alloy Screening. Int. J. Corros. 2017, 2017, 9425864. [Google Scholar] [CrossRef] [Green Version]
- Le Bozec, N.; Compère, C.; L’Her, M.; Laouenan, A.; Costa, D.; Marcus, P. Influence of stainless steel surface treatment on the oxygen reduction reaction in seawater. Corros. Sci. 2001, 43, 765–786. [Google Scholar] [CrossRef]
- Kim, Y.P.; Fregonese, M.; Mazille, H.; Feron, D.; Santarini, G. Study of oxygen reduction on stainless steel surfaces and its contribution to acoustic emission recorded during corrosion processes. Corros. Sci. 2006, 48, 3945–3959. [Google Scholar] [CrossRef]








| Materials | kg/m3concrete | ||
|---|---|---|---|
| w/c = 0.6 | w/c = 0.5 | w/c = 0.4 | |
| Cement I 42.5 R-SR5 Water Superplastificiser | 315 | 385 | 490 |
| 189 | 193 | 196 | |
| 2.2 | 2.7 | 34 | |
| Silica sand Coarse aggregate | 1212 | 1179 | 1115 |
| 653 | 635 | 601 | |
| w/c | Number of Masses | Number of Samples/Mass | Total No. Samples |
|---|---|---|---|
| 0.6 | 3 | 2 | 6 |
| 0.5 | 3 | 2 | 6 |
| 0.4 | 3 | 2 | 6 |
| TOTAL | 18 |
| NON-SATURATED STATE | SEQUENCE 1: samples dosage w/c = 0.6 | Samples were tested per dosage under the ATM, 500 mbar, and VAC conditions. Each sample was tested 3 times in each state. |
| SEQUENCE 2: samples dosage w/c = 0.5 | ||
| SEQUENCE 3: samples dosage w/c = 0.4 | ||
| SATURATED STATE | SEQUENCE 4: samples dosage w/c = 0.6, w/c = 0.5 and w/c = 0.4 | All the samples were tested under SAT conditions. Each sample was tested 3 times in this state |
| fcK28days (MPa) | CV | %W.A.P | CV | %Abs. H2O | CV | W.P.D (mm) | CV | ||
| w/c = 0.6 | 34.5 | 6.03% | 19.19% | 13.79% | 7.59% | 6.81% | 47 | 17.71% | |
| w/c = 0.5 | 42.9 | 5.93% | 17.21% | 6.04% | 7.47% | 7.32% | 26 | 3.10% | |
| w/c = 0.4 | 56.8 | 4.50% | 14.85% | 2.59% | 6.65% | 4.68% | 10 | 17.63% | |
| (a) | |||||||||
| fcK28days (MPa) | k (×10−18 m2) | CV | ρ (Ωm) | CV | %Total Pores MIP | ||||
| w/c = 0.6 | 34.5 | 667.76 | 18.82% | 50.68 | 12.88% | 13.2% | |||
| w/c = 0.5 | 42.9 | 521.37 | 3.45% | 52.38 | 11.37% | 12.9% | |||
| w/c = 0.4 | 56.8 | 413.56 | 12.64% | 62.56 | 9.88% | 10.6% | |||
| (b) | |||||||||
| Line 1 | Line 2 | |||||
|---|---|---|---|---|---|---|
| Line Range | m1 | n1 | Line Range | m2 | n2 | |
| Airless atmosphere (Ref.SCE) | −0.34 V to −0.870 V | −2.630 V−1 | −6.920 | −0.940 V to −1.220 V | −0.006 V−1 | −4.468 |
| Airless atmosphere (Ref.OCP) | −0.110 V to −0.61 V | −2.630 V−1 | −6.336 | −0.770 V to −0.880 V | −0.006 V−1 | −4.467 |
| Aired atmosphere (Ref. SCE) | −0.400 V to −0.800 V | −4.272 V−1 | −7.388 | −1.210 V to −0.880 V | −0.010 V−1 | −3.879 |
| Aired atmosphere (Ref.OCP) | −0.100 V to −0.460 V | −4.272 V−1 | −6.052 | −0.630 V to −0.920 V | −0.010 V−1 | −3.876 |
| pH | LINE 1 | LINE 2 | ||||
|---|---|---|---|---|---|---|
| Range | m1 (V−1) | n1 (log|A/cm2|) | Range | m2 (V−1) | n2 (log|A/cm2|) | |
| 13 | −0.46 V a −0.79 V | −6.0452 | −8.6277 | −0.82 V a −1.1 V | −0.2934 | −3.9496 |
| 12.72 | −0.38 V a −0.78 V | −5.4329 | −8.07202 | −0.82 V a −1 V | −0.5868 | −4.1606 |
| 11.35 | −0.43 V a −0.68 V | −7.0652 | −8.5235 | −0.74 V a −1.12 V | 0.1537 | −3.5363 |
| 8.35 | −0.24 V a −0.40 V | −7.3822 | −7.1177 | −0.46 V a −0.9 V | −0.1207 | −3.992 |
| σ | - | 0.9 | 0.69 | - | 0.31 | 0.26 |
| Whithout H2O2 | 0.02 m H2O2 | |||
|---|---|---|---|---|
| m | n | m | n | |
| LINE 1 | −5.388 | −8.041 | −7.037 | −8.183 |
| LINE 2 | −0.608 | −4.180 | 0.0149 | −3.412 |
| LINE 3 | −3.438 | −6.471 | ||
| LINE 4 | −2.112 | −5.012 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ibernón, A.; Lliso-Ferrando, J.; Gandía-Romero, J.M.; Soto, J. Stainless Steel Voltammetric Sensor to Monitor Variations in Oxygen and Humidity Availability in Reinforcement Concrete Structures. Sensors 2021, 21, 2851. https://doi.org/10.3390/s21082851
Martínez-Ibernón A, Lliso-Ferrando J, Gandía-Romero JM, Soto J. Stainless Steel Voltammetric Sensor to Monitor Variations in Oxygen and Humidity Availability in Reinforcement Concrete Structures. Sensors. 2021; 21(8):2851. https://doi.org/10.3390/s21082851
Chicago/Turabian StyleMartínez-Ibernón, Ana, Josep Lliso-Ferrando, José M. Gandía-Romero, and Juan Soto. 2021. "Stainless Steel Voltammetric Sensor to Monitor Variations in Oxygen and Humidity Availability in Reinforcement Concrete Structures" Sensors 21, no. 8: 2851. https://doi.org/10.3390/s21082851






