Design and Characterization of Electrochemical Sensor for the Determination of Mercury(II) Ion in Real Samples Based upon a New Schiff Base Derivative as an Ionophore
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Chemicals
2.2. Instrumentation
2.3. Synthesis of (4-Bromo-2-[(4-Methoxyphenyl-Imino)Methyl]Phenol, BMPMP
2.4. Construction of Membrane Electrode
2.5. Potentiometric Measurements
2.6. Selectivity Measurements
2.7. Potentiometric Titration
2.8. Preparation of Real Samples
2.8.1. Preparation of Water Samples
2.8.2. Preparation of Compact Fluorescent Lamp
2.8.3. Preparation of a Dental Amalgam Alloy
3. Results and Discussion
3.1. Optimization of PVC Membrane Compositions
3.2. ATR–FTIR Investigation of Hg2+–SE Membrane Based on BMPMP as an Ionophore
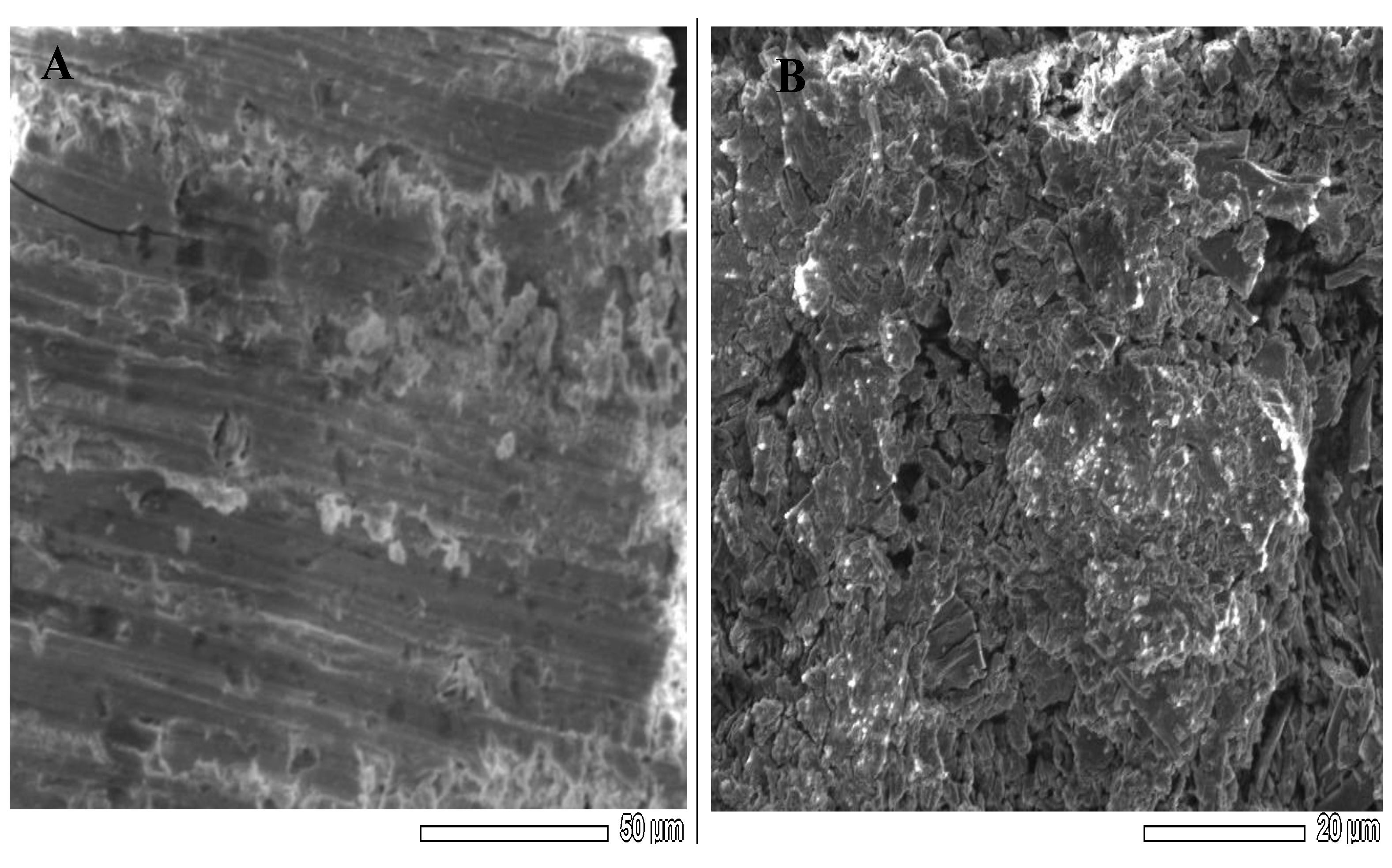
3.3. SEM-EDX Investigations of Hg2+–SE Membrane
3.4. The Influence of the Internal Solution Concentration
3.5. The pH Effect on the Proposed Electrode Response
3.6. Response Time of the Proposed Hg2+–SE
3.7. Life Time of the Proposed Hg2+–SE
3.8. Response of the Proposed Hg2+–SE towards Other Ions (Selectivity)
3.9. Potentiometric Titrations Using Hg(II)-ISEs Based on BMPMP
3.10. Analytical Applications
3.11. Comparison with Previous Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, S.; Chen, B.; He, M.; Huang, T.; Hu, B. Speciation of mercury in water and fish samples by HPLC-ICP-MSafter magnetic solid phase extraction. Talanta 2017, 171, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wu, C.; Liu, J.; Xu, Z. Thiol-functionalized silica microspheres for online preconcentration and determination of mercury species in seawater by high performance liquid chromatography and inductively coupled plasma mass spectrometry. RSC Adv. 2015, 5, 19082–19090. [Google Scholar] [CrossRef]
- Gibb, M.; O’Leary, K.G. Mercury exposure and health impacts among individuals in the artisanal and small-scale goldmining community: A comprehensive review. Environ. Health Perspect. 2014, 122, 667–672. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, M. Comparison of DMA-80 and ICP-MS Combined with Closed-Vessel Microwave Digestion for the Determination of Mercury in Coal. J. Anal. Methods Chem. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Du, J.; Jiang, L.; Shao, Q.; Liu, X.; Marks, R.S.; Ma, J.; Chen, X. Colorimetric Detection of Mercury Ions Based on Plasmonic Nanoparticles. Small 2013, 9, 1467–1481. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.; Bashammakh, A.; Al-Sibaai, A.; Al-Saidi, H.; El-Shahawi, M. Part 1. Spectrophotometric determination of trace mercury (II) in dental-unit wastewater and fertilizer samples using the novel reagent 6-hydroxy-3-(2-oxoindolin-3-ylideneamino)-2-thioxo-2H-1,3-thiazin-4(3H)-one and the dual-wavelength β-correction spectrophotometry. J. Hazard. Mater. 2010, 178, 287–292. [Google Scholar] [CrossRef]
- Yang, L.-L.; Zhang, D.-Q.; Zhou, Q.-X. Determination of Mercury in Biological Tissues by Graphite-furnace Atomic Absorption Spectrometry with an in-situ Concentration Technique. Anal. Sci. 2002, 18, 811–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shekhar, R. Improvement of sensitivity of electrolyte cathode discharge atomic emission spectrometry (ELCAD-AES) for mercury using acetic acid medium. Talanta 2012, 93, 32–36. [Google Scholar] [CrossRef]
- Ai, X.; Wang, Y.; Hou, X.D.; Yang, L.; Zheng, C.B.; Wu, L. Advanced oxidation using Fe3O4 magnetic nanoparticles and its application in mercury speciation analysis by high performance liquid chromatography–cold vapor generation atomic fluorescence spectrometry. Analyst 2013, 138, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Fong, B.M.W.; Siu, T.S.; Lee, J.S.K.; Tam, S. Determination of Mercury in Whole Blood and Urine by Inductively Coupled Plasma Mass Spectrometry. J. Anal. Toxicol. 2007, 31, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Aranda, P.R.; Colombo, L.; Perino, E.; De Vito, I.E.; Raba, J. Solid-phase preconcentration and determination of mercury(II) using activated carbon in drinking water by X-ray fluorescence spectrometry. X-ray Spectrom. 2013, 42, 100–104. [Google Scholar] [CrossRef]
- Liu, Q. Determination of mercury and methylmercury in seafood by ion chromatography using photo-induced chemical vapor generation atomic fluorescence spectrometric detection. Microchem. J. 2010, 95, 255–258. [Google Scholar] [CrossRef]
- de Souza, S.S.; Campiglia, A.D.; Junior, F.B. A simple method for methylmercury, inorganic mercury and ethylmercury determination in plasma samples by high performance liquid chromatography-cold-vapor-inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2013, 761, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Peng, D.; Song, D.; Gong, J. Layered titanate nanosheets as an enhanced sensing platform for ultrasensitive stripping voltammetric detection of mercury(II). Sens. Actuators B Chem. 2013, 181, 432–438. [Google Scholar] [CrossRef]
- Silva, M.F.; Tóth, I.V.; Rangel, A.O. Determination of mercury in fish by cold vapor atomic absorption spectrophotometry using a multicommuted flow injection analysis system. Anal. Sci. 2006, 22, 861–864. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Lisak, G.; Strzalkowska, S.; Bobacka, J. Potentiometric sensing utilizing paper-based microfluidic sampling. Analyst 2014, 139, 2133–2136. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Ganjali, M.R.; Norouzi, P.; Khani, H.; Nayak, A.; Agarwal, S. Electrochemical Analysis of Some Toxic Metals by Ion–Selective Electrodes. Crit. Rev. Anal. Chem. 2011, 41, 282–313. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Ju, H. Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens. Bioelectron. 2014, 63, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Jain, S.; Khurana, U. A PVC-based pentathia-15-crown-5 membrane potentiometric sensor for mercury(II). Electroanalysis 1997, 9, 478–480. [Google Scholar] [CrossRef]
- Javanbakht, M.; Ganjali, M.R.; Eshghi, H.; Sharghi, H.; Shamsipur, M. Mercury(II) ion-selective electrode based on dibenzodiazathia-18-crown-6-dione. Electroanalysis 1999, 11, 81–84. [Google Scholar] [CrossRef]
- Bakhtiarzadeh, F.; Ghani, S.A. An ion selective electrode for mercury (II) based on mercury (II) complex of poly (4-vinyl pyridine). J. Electroanal. Chem. 2008, 624, 139–143. [Google Scholar] [CrossRef]
- De Namor, A.F.D.; El Gamouz, A.; Alharthi, S.; Al Hakawati, N.; Varcoe, J.R. A ditopic calix[4]pyrrole amide derivative: Highlighting the importance of fundamental studies and the use of NaPh4B as additive in the design and applications of mercury(ii) ion selective electrodes. J. Mater. Chem. A 2015, 3, 13016–13030. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Tong, X.; He, X. A mercury ion-selective electrode based on a calixarene derivative containing the thiazole azo group. J. Electroanal. Chem. 2003, 540, 111–117. [Google Scholar] [CrossRef]
- De Namor, A.F.D.; Alharthi, S.; El Gamouz, A.; Al Hakawati, N.; Cox, B.G. Calix[4] based Hg(II) ion selective electrodes: A thermodynamic protocol to address the selectivity versus the hosting capacity paradigm in the selection of the carrier. Electrochim. Acta 2018, 290, 686–694. [Google Scholar] [CrossRef]
- Perez-Marin, L.; Otazo-Sanchez, E.; Macedo-Miranda, G.; Avila-Perez, P.; Chamaro, J.A.; Lopez-Valdivia, H.L. Mercury (II) ion-selective electrode. Study of 1,3-diphenylthiourea as ionophore. Analyst 2000, 125, 1787–1790. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sethi, B.; Sharma, R.; Agarwal, S.; Bharti, A. Mercury selective potentiometric sensor based on low rim functionalized thiacalix [4]-arene as a cationic receptor. J. Mol. Liq. 2013, 177, 114–118. [Google Scholar] [CrossRef]
- Mahajan, R.K.; Kaur, R.; Kaur, I.; Sharma, V.; Kumar, M. Mercury(II) ion-selective electrodes based on p-tert-butyl calix[4]crowns with imine units. Anal. Sci. 2004, 20, 811–814. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Kim, J.; Kim, K.N.; Chang, S.-K.; Chung, T.D. Potentiometric Response of a Neutral-carrier-based Membrane to Aqueous Mercury in Cl--rich Media. Anal. Sci. 2009, 25, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Wang, X.; Fan, Y.; Li, J.; Zhang, L.; Hu, G.; He, C.; Jin, C. Determination of total mercury in seafood by ion-selective electrodes based on a thiol functionalized ionic liquid. J. Food Drug Anal. 2018, 26, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Chandra, S.; Lang, H. A highly selective mercury electrode based on a diamine donor ligand. Talanta 2005, 66, 575–580. [Google Scholar] [CrossRef]
- Abu-Shawish, H.M. A mercury(II) selective sensor based on N,N′-bis(salicylaldehyde)-phenylenediamine as neutral carrier for potentiometric analysis in water samples. J. Hazard. Mater. 2009, 167, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Jumal, J.; Yamin, B.M.; Ahmad, M.A.; Heng, L.Y. Mercury Ion-Selective Electrode with Self-plasticizing Poly(n–buthylacrylate) Membrane Based On 1,2-Bis-(N’–benzoyl-thioureido) cyclohexane As Ionophore. Apcbee Procedia 2012, 3, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Júarez-Gómez, J.; Ramírez-Silva, M.T.; Romero-Romo, M.; Rodríguez-Sevilla, E.; Pérez-García, F.; Palomar-Pardavé, M. Ion-Selective Electrodes for Mercury Determination at Low Concentrations: Construction, Optimization and Application. J. Electrochem. Soc. 2016, 163, B90–B96. [Google Scholar] [CrossRef]
- Patel, B.; Kumar, A.; Menon, S.K. Mercury selective membrane electrode based on dithio derivatized macrotricyclic compound. J. Incl. Phenom. Macrocycl. Chem. 2009, 64, 101–108. [Google Scholar] [CrossRef]
- Khani, H.; Rofouei, M.K.; Arab, P.; Gupta, V.K.; Vafaei, Z. Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: Application to potentiometric monitoring of mercury ion(II). J. Hazard. Mater. 2010, 183, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Mai, P.T.N.; Hoa, P.T. Fabrication of solid contact ion selective electrode for mercury (II) using conductive polymer membrane (polypyrrole-PPy). Mater. Trans. 2015, 56, 1428–1430. [Google Scholar] [CrossRef] [Green Version]
- Juarez-Gomez, J.; Perez-Garcia, F.; Ramirez-Silva, M.; Rojas-Hernandez, A.; Galan-Vidal, C.; Paez-Hernandez, M. Solid-contact Hg(II)-selective electrode based on a carbon-epoxy composite containing a new dithiophosphate-based ionophore. Talanta 2013, 114, 235–242. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Aboul-Enein, H.Y. New Simple Ion-Selective Membrane Electrode for Serious Environmental Pollutant, Mercury (II), Analysis in Aqueous Solution, Fluorescent Mercury Lamp White Dust, Mercurochrome and Dental Alloy. Curr. Anal. Chem. 2018, 14, 36–42. [Google Scholar] [CrossRef]
- Han, W.-S.; Wi, K.-C.; Park, W.-S.; Hong, T.-K. Mercury ion selective poly(aniline) solid contact electrode based on 2-mercaptobenzimidazol ionophore. Russ. J. Electrochem. 2012, 48, 525–531. [Google Scholar] [CrossRef]
- Salib, K.A.R.; Stefan, S.L.; Abu El-Wafa, S.M.; El-Shafiy, H.F. Metal complexes of novel symmetrical schiff base ligands. Synth. React. Inorg. Met. Chem. 2001, 31, 895–915. [Google Scholar] [CrossRef]
- Mishra, P.; Soni, M. Synthesis, Structural, and biological studies of some Schiff bases and their metal complexes. Met. Based Drugs 2008, 7, 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashhadizadeh, M.H.; Sheikhshoaie, I. Mercury (II) ion-selective polymeric membrane sensor based on a recently synthesized Schiff base. Talanta 2003, 60, 73–80. [Google Scholar] [CrossRef]
- Mahajan, R.K.; Kaur, I.; Lobana, T.S. A mercury (II) ion-selective electrode based on neutral salicylaldehyde thiosemicarbazone. Talanta 2003, 59, 101–105. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, R.; Chai, Y.-Q. Mercury(II) ion Potentiometric Sensor Based on a Sulfur Schiff’s Base 1-(2-Hydroxy-1,2-diphenylethylidene)thiosemicarbazide as Ionophore. Chem. Lett. 2005, 34, 440–441. [Google Scholar] [CrossRef]
- Gupta, V.; Singh, A.; Al Khayat, M.; Gupta, B. Neutral carriers based polymeric membrane electrodes for selective determination of mercury (II). Anal. Chim. Acta 2007, 590, 81–90. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, X.; Chai, Y.Q.; Yuan, R.; Ye, G.R. A Novel Ion-Selective Electrode for Determination of the Mercury(II) Ion Based on Schiff Base as a Carrier. J. Chin. Chem. Soc. 2008, 55, 1345–1350. [Google Scholar] [CrossRef]
- Refat, M.S.; El-Sayed, M.Y.; Adam, A.M.A. Cu(II), Co(II) and Ni(II) complexes of new Schiff base ligand: Synthesis, thermal and spectroscopic characterizations. J. Mol. Struct. 2013, 1038, 62–72. [Google Scholar] [CrossRef]
- Debye, P.; Huckel, E. The theory of electrolytes. I. Freezing point depression and related phenomena. Phys. Z. 1923, 24, 185–206. [Google Scholar]
- Buck, R.P.; Lindner, E.; Kutner, W.; Inzelt, G. Piezoelectric chemical sensors (IUPAC Technical Report). Pure Appl. Chem. 2004, 76, 1139–1160. [Google Scholar] [CrossRef]
- Afkhami, A.; Bagheri, H.; Khoshsafar, H.; Saber-Tehrani, M.; Tabatabaee, M.; Shirzadmehr, A. Simultaneous trace-levels determination of Hg(II) and Pb(II) ions in various samples using a modified carbon paste electrode based on multi-walled carbon nanotubes and a new synthesized Schiff base. Anal. Chim. Acta 2012, 746, 98–106. [Google Scholar] [CrossRef]
- Kaushal, S.; Badru, R.; Kumar, S.; Mittal, S.K.; Singh, P. Fabrication of a mercury(II) ion selective electrodebased on poly-o-toluidine–zirconiumphosphoborate. RSC Adv. 2016, 6, 3150–3158. [Google Scholar] [CrossRef]
- Soleymanpour, A.; Shafaatian, B.; Hanifi, A.; Jarrahpour, A.A. Construction of a Solid Contact Polymeric Membrane Electrode for pH Measurements in Acidic Media. J. Electrochem. Soc. 2013, 161, B14–B18. [Google Scholar] [CrossRef]
- Gurtova, O.; Ye, L.; Chmilenko, F. Potentiometric propranolol-selective sensor based on molecularly imprinted polymer. Anal. Bioanal. Chem. 2012, 405, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Demiral, A.; Dogan, A.; Cancel, E.; Memon, S.; Yilmaz, M.; Kilic, E. Hydrogen ion-selective poly(vinyl chloride) membrane electrode based on a p-tert-butylcalix[4]arene-oxacrown-4. Talanta 2004, 62, 123–129. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 3rd ed.; Wiley: New York, NY, USA, 1978; p. 305. [Google Scholar]
- Socrstes, G. Infrared Characteristics Group Frequencies, 3rd ed.; John Wiley & Sons: Chichester, UK, 1980; p. 145. [Google Scholar]
- Li, X.-Q.; Liang, H.-Q.; Cao, Z.; Xiao, Q.; Xiao, Z.-L.; Song, L.-B.; Chen, D.; Wang, F.-L. Simple and rapid mercury ion selective electrode based on 1-undecanethiol assembled Au substrate and its recognition mechanism. Mater. Sci. Eng. C 2017, 72, 26–33. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G. Quimica Inorganica Avanzada, 4th ed.; Editorial Limusa: Mexico City, Mexico, 1996; pp. 731–732. [Google Scholar]
- Gupta, V.; Jain, A.; Kumar, P.; Agarwal, S.; Maheshwari, G. Chromium(III)-selective sensor based on tri-o-thymotide in PVC matrix. Sens. Actuators B Chem. 2006, 113, 182–186. [Google Scholar] [CrossRef]
- Abu Ismaiel, A.; Aroua, M.K.; Yusoff, R. A New Electrochemical Sensor Based on Task-Specific Ionic Liquids-Modified Palm Shell Activated Carbon for the Determination of Mercury in Water Samples. Sensors 2014, 14, 13102–13113. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Ghafarloo, A.; Faridbod, F.; Norouzi, P. Copper-Selective PVC Membrane Sensor. Int. J. Electrochem. Sci. 2012, 7, 3706–3716. [Google Scholar]
- Sadeghi, S.; Dashti, G.R.; Shamsipur, M. Lead-selective poly(vinyl cholride) membrane electrode based on piroxicam as a neutral carrier. Sens. Actuators B Chem. 2002, 81, 223–228. [Google Scholar] [CrossRef]
- Xing, Y.; Han, J.; Wu, X.; Pierce, D.T.; Zhao, J.X. Aggregation-based determination of mercury(II) using DNA-modified single gold nanoparticle, T-Hg(II)-T interaction, and single-particle ICP-MS. Microchim. Acta 2019, 187, 56. [Google Scholar] [CrossRef]
- Achadu, O.J.; Revaprasadu, N. Microwave-assisted synthesis of thymine-functionalized graphitic carbon nitride quantum dots as a fluorescent nanoprobe for mercury(II). Microchim. Acta 2018, 185, 461. [Google Scholar] [CrossRef]
- Liang, P.; Yu, J.; Yang, E.; Mo, Y. Determination of Mercury in Food and Water Samples by Displacement-Dispersive Liquid-Liquid Microextraction Coupled with Graphite Furnace Atomic Absorption Spectrometry. Food Anal. Methods 2014, 8, 236–242. [Google Scholar] [CrossRef]
- Xie, Y. Colorimetric determination of Hg(II) via the gold amalgam induced deaggregation of gold nanoparticles. Microchim. Acta 2018, 185, 351. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.A.; Nobre, N.F.; Lopes, G.S. Rapid and Low Cost Determination of Total Mercury in Cat Foods by Photochemical Vapor Generation Coupled to Atomic Absorption Spectrometry. Biol. Trace Elem. Res. 2021, 199, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Verma, A.K.; Yoshida, M.; Naik, R.M.; Prasad, S. A novel catalytic kinetic method for the determination of mercury(ii) in water samples. RSC Adv. 2020, 10, 25100–25106. [Google Scholar] [CrossRef]
- Duval, B.; Gredilla, A.; de Vallejuelo, S.F.-O.; Tessier, E.; Amouroux, D.; de Diego, A. A simple determination of trace mercury concentrations in natural waters using dispersive Micro-Solid phase extraction preconcentration based on functionalized graphene nanosheets. Microchem. J. 2020, 154, 104549. [Google Scholar] [CrossRef]
- Hosseini, M.; Rahimi, M.; Sadeghi, H.B.; Taghvaei-Ganjali, S.; Abkenar, S.D.; Ganjali, M.R. Determination of Hg(II) ions in water samples by a novel Hg(II) sensor, based on calix[4]arene derivative. Int. J. Environ. Anal. Chem. 2009, 89, 407–422. [Google Scholar] [CrossRef]
- Khan, A.A.; Shaheen, S.; Habiba, U. Synthesis and characterization of poly-o-anisidine Sn(IV) tungstate: A new and novel ‘organic–inorganic’ nano-composite material and its electro-analytical applications as Hg(II) ion-selective membrane electrode. J. Adv. Res. 2012, 3, 269–278. [Google Scholar] [CrossRef] [Green Version]












| ISE No. | Membrane Composition (%) | Slope (mV/Decade) a | D.L. b (mol.L−1) | L.R. b (mol.L−1) | R.T. b (s) | |||
|---|---|---|---|---|---|---|---|---|
| BMPMP | PVC | Plasticizer o-NPOE | Additive NaTPB | |||||
| 1 | 0 | 32 | 68 | 0 | 0.61 ± 0.2 | - | - | - |
| 2 | 0 | 32 | 67 | 1 | 4.30 ± 0.2 | - | - | - |
| 3 | 1 | 32 | 67 | 0 | 17.07 ± 0.2 | 1.78 × 10−6 | 3.16 × 10−6–5.62 × 10−4 | 20 |
| 4 | 1 | 32 | 66 | 1 | 22.62 ± 0.2 | 3.16 × 10−5 | 4.47 × 10−5–3.16 × 10−3 | 10–15 |
| 5 | 1.5 | 32 | 66 | 0.5 | 25.92 ± 0.2 | 1.58 × 10−6 | 2.51 × 10−6–1.0 × 10−3 | 10–15 |
| 6 | 1.5 | 32 | 65 | 1.5 | 27.78 ± 0.2 | 1.58 × 10−7 | 6.31 × 10−7–7.08 × 10−3 | >10 |
| 7 | 2 | 32 | 64.5 | 1.5 | 29.74 ± 0.2 | 3.98 × 10−8 | 9.33 × 10−8–3.98 × 10−3 | <10 |
| 8 | 2 | 33 | 63.5 | 1.5 | 34.41 ± 0.2 | 1.58 × 10−7 | 2.82 × 10−7–1.00 × 10−3 | 10–15 |
| 9 | 2 | 34 | 62.5 | 1.5 | 32.62 ± 0.2 | 1.26 × 10−5 | 3.16 × 10−5–7.94 × 10−3 | 10–15 |
| Concentration of Internal Solution (molL–1) | Slope (mV/Decade) | D.L. (molL–1) | L.R. (molL–1) | R.T. (s) |
|---|---|---|---|---|
| 0.01 | 29.74 ± 0.2 | 3.98 × 10−8 | 9.33 × 10−8–3.98 × 10−3 | 5 |
| 0.001 | 27.16 ± 0.2 | 5.01 × 10−5 | 1.15 × 10−4–1.95 × 10−2 | 10 |
| 0.0001 | 17.63 ± 0.2 | 7.08 × 10−6 | 1.26 × 10−5–3.16 × 10−3 | 7 |
| Interfering Ion, M | Interfering Ion, M | ||
|---|---|---|---|
| Hg2+ | 1.00 | Fe3+ | 1.9 × 10−4 |
| Na+ | 2.81 × 10−5 | Ag+ | 1.5 × 10−3 |
| K+ | 1.94 × 10−5 | Cu2+ | 2.55 × 10−4 |
| Ca2+ | 5.71 × 10−5 | Zn2+ | 1.85 × 10−5 |
| Mg2+ | 3.67 × 10−6 | Co2+ | 7.67 × 10−6 |
| Ba2+ | 1.86 × 10−5 | Ni2+ | 1.98 × 10−5 |
| Al3+ | 1.33 × 10−5 | Fe3+ | 9.59 × 10−5 |
| Cd2+ | 2.65 × 10−5 | Cr3+ | 1.06 × 10−6 |
| Mn2+ | 1.19 × 10−5 | Pb2+ | 1.40 × 10−5 |
| Sample * | Hg2+ Added (µM) | Hg2+ Ions Concentration (µM) | |||
|---|---|---|---|---|---|
| Hg2+–SE | ICP-OES | ||||
| Found | Recovery (%) | Found | Recovery (%) | ||
| Tap water | 0 | ND | - | ND | - |
| 20 | 19.97 ± 0.15 | 99.85 | 20.03 ± 0.15 | 100.15 | |
| Red sea water | 0 | ND | - | 0.040 ± 0.001 | - |
| 26 | 25.77 ±0.25 | 99.11 | 26.1 ± 0.2 | 100.23 | |
| Compact fluorescent lamp (CFL) | 0 | 1.89 ± 0.65 | - | 1.95 ± 0.18 | - |
| 26 | 27.86 ± 0.36 | 99.88 | 28.1 ± 0.22 | 100.58 | |
| Ionophore | Slope (mV/decade) | L.R. (mol.L−1) | D.L. (mol.L−1) | Working pH | R.T. (s) | Significant Interference | Ref. |
|---|---|---|---|---|---|---|---|
| meso-tetramethyl-tetrakis-(4N,N-diethylacetamide) phenoxymethyl] calix[4]pyrrole | 29.0 | 1.81 × 10−7–1.78 × 10−3 | 1 × 10−5 | 4.3–8.5 | 10 | Ag+ (4.2 × 10−2) | [22] |
| calix[4]pyrrole amide derivative | 29.10 | 6.15 × 10−8–2.28 × 10−2 | 6.0 × 10−8 | 3.2–8.8 | 5–8 | - | [24] |
| 1,3-diphenylthiourea | 30.8 | 2.00 × 10−6–2.10 × 10−4 | 1.0 × 10−6 | 4.0 | 30 | Ag+ (3.1 × 10−2) | [25] |
| 1-methyl-2-butylthioimidazolium bis(trifluoromethanesulphonyl)imide | 6.70 | 1.00 × 10−10–1.00 × 10−5 | 4.1 × 10−11 | 2.0–3.0 | 5 | - | [29] |
| 1,2-bis-(N’-benzoylthioureido)cyclohexane | 28.1 | 1.00 × 10−5–1.00 × 10−1 | 2.5 × 10−6 | 7.0 | 50–100 | - | [32] |
| O,O-(2,2_-biphenylene) dithiophosphate alkyl | 29.80 | 1.00 × 10−9–1.00 × 10−1 | 9.1 × 10−10 | 0.0–6.0 | 30 | Fe3+ (1.2 × 10−5) | [33] |
| 1-n-butyl-3-methylimidazolium tetrafluoroborate | 29.3 | 5.00 × 10−9–1.00 × 10−4 | 2.5 × 10−9 | 2.0–4.3 | ~5 | - | [35] |
| polypyrrole-PPy | 23.7 ± 1.4 | 1.0 × 10−9–1.0 × 10−2 | 6.0 × 10−10 | 3.0 | 60 | Ag+ (1.0 × 10−3) | [36] |
| bis [5-((4-nitrophenyl) azo salicylaldehyde)] | 30 ± 1.0 | 7.00 × 10−7–5.00 × 10−2 | 7.0 × 10−8 | 1.0–3.5 | 10 | - | [42] |
| salicylaldehyde thiosemicarbazone | 29 | 1.78 × 10−6–1.00 × 10−1 | 1.0 × 10−6 | 1.0–3.0 | 30 | Ag+ (3.1 × 10−2) | [43] |
| 1-(2-hydroxy-1,2-diphenylethylidene)thiosemicarbazide | 30.5 | 2.00 × 10−6–1.00 × 10−2 | 1.0 × 10−6 | 2.0–5.0 | 30 | Ag+ (2.7 × 10−1) | [44] |
| 2-amino-6-purinethiol | 29.4 | 7.00 × 10−8–1.00 × 10−1 | 4.4 × 10−8 | 2.0–4.5 | 10 | Ag+ (1.5 × 10−2); Cu2+ (3.1 × 10−2); Pb2+ (5.0 × 10−2) | [45] |
| bis-salicyladehydediaminjodipropylamine | 30.5 ± 0.4 | 9.50 × 10−7–6.40 × 10−2 | 7.0 × 10−7 | 1.5–3.5 | 10–25 | Ag+ (3.1 × 10−2) | [46] |
| poly-o-toluidine into zirconium phosphoborate | 28.0 | 1.0 × 10−7–1.0 × 10−1 | 7.08 × 10−8 | 2.5–10.0 | 6 | K+ (6.4 × 10−2) | [51] |
| 1-undecanethiol assembled Au substrate | 28.83 | 1.00 × 10−8–1.00 × 10−4 | 4.5 × 10−9 | 6.0 | ~30 | - | [54] |
| trioctylmethylammonium thiosalicylate | 44.08 | 1.00 × 10−9–1.00 × 10−2 | 1.0 × 10−10 | 3.0–9.0 | ~5 | Ag+ (8.9 × 10−4); Cu2+ (8.9 × 10−4); Pb2+ (9.6 × 10−4) | [60] |
| dithio derivatized macrotricyclic | 29.4 ± 0.4 | 1.00 × 10−6–1.00 × 10−1 | 4.0 × 10−7 | 1.5–4.0 | <10 | Ag+ (1.3 × 10−2) | [70] |
| poly-o-anisidine Sn(IV) tungstate | 21.0 | 1.00 × 10−7–1.00 × 10−1 | 1.00 × 10−7 | 4.0–8.0 | 30 | _____ | [71] |
| 4-Bromo-2-[(4-methoxyphenylimino)methyl]phenol | 29.74 | 9.33 × 10−8–3.98 × 10−3 | 3.98 × 10−8 | 3.0–7.0 | 10 | _____ | P.W. |
| Materials | Method | Sample | L.R. (molL−1) | D.L. (mgL−1) | pH | Recovery | Ref. |
|---|---|---|---|---|---|---|---|
| AuNPs, DNA | spICP-MS | tap water | 1.0 × 10−7–1.0 × 10−6 | 3.1 × 10−8 | - | 110 ± 10 | [63] |
| T-gCNQDs | Fluorescence | pond water | 1.0 × 10−8–5.0 × 10−7 | 1.5 × 10−10 | 7.2 | 95 | [64] |
| pyrrolidinedithiocarbamate (PDC) | D-DLLME/GFAAS | rice | 0.01–0.3 | 1.8 × 10−7 | 6.0 | 98 | [65] |
| AuNPs, APTES | Colorimetric | river water | 0–9.23 × 10−8 | 1.0 × 10−8 | 7.2 | 96.4 | [66] |
| - | PVG-CVAAS | DORM-1 | 1.75 × 10−7–1.69 × 10−6 | 1.4 × 10−9 | - | 92.7 | [67] |
| - | CKM | wastewater | 1.0–30.0 × 10−6 | 1.5 × 10−7 | 4 | 97.93 | [68] |
| graphene nanosheets | DMSPE | Fresh water | 1.9 × 10−9–5.19 × 10−6 | 1.9 × 10−9 | - | 95.0< | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthi, S.S.; Fallatah, A.M.; Al-Saidi, H.M. Design and Characterization of Electrochemical Sensor for the Determination of Mercury(II) Ion in Real Samples Based upon a New Schiff Base Derivative as an Ionophore. Sensors 2021, 21, 3020. https://doi.org/10.3390/s21093020
Alharthi SS, Fallatah AM, Al-Saidi HM. Design and Characterization of Electrochemical Sensor for the Determination of Mercury(II) Ion in Real Samples Based upon a New Schiff Base Derivative as an Ionophore. Sensors. 2021; 21(9):3020. https://doi.org/10.3390/s21093020
Chicago/Turabian StyleAlharthi, Salman S., Ahmed M. Fallatah, and Hamed M. Al-Saidi. 2021. "Design and Characterization of Electrochemical Sensor for the Determination of Mercury(II) Ion in Real Samples Based upon a New Schiff Base Derivative as an Ionophore" Sensors 21, no. 9: 3020. https://doi.org/10.3390/s21093020
APA StyleAlharthi, S. S., Fallatah, A. M., & Al-Saidi, H. M. (2021). Design and Characterization of Electrochemical Sensor for the Determination of Mercury(II) Ion in Real Samples Based upon a New Schiff Base Derivative as an Ionophore. Sensors, 21(9), 3020. https://doi.org/10.3390/s21093020






