A Colorimetric Dip Strip Assay for Detection of Low Concentrations of Phosphate in Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solution Preparation
2.2. Reagents
2.3. Device Preparation
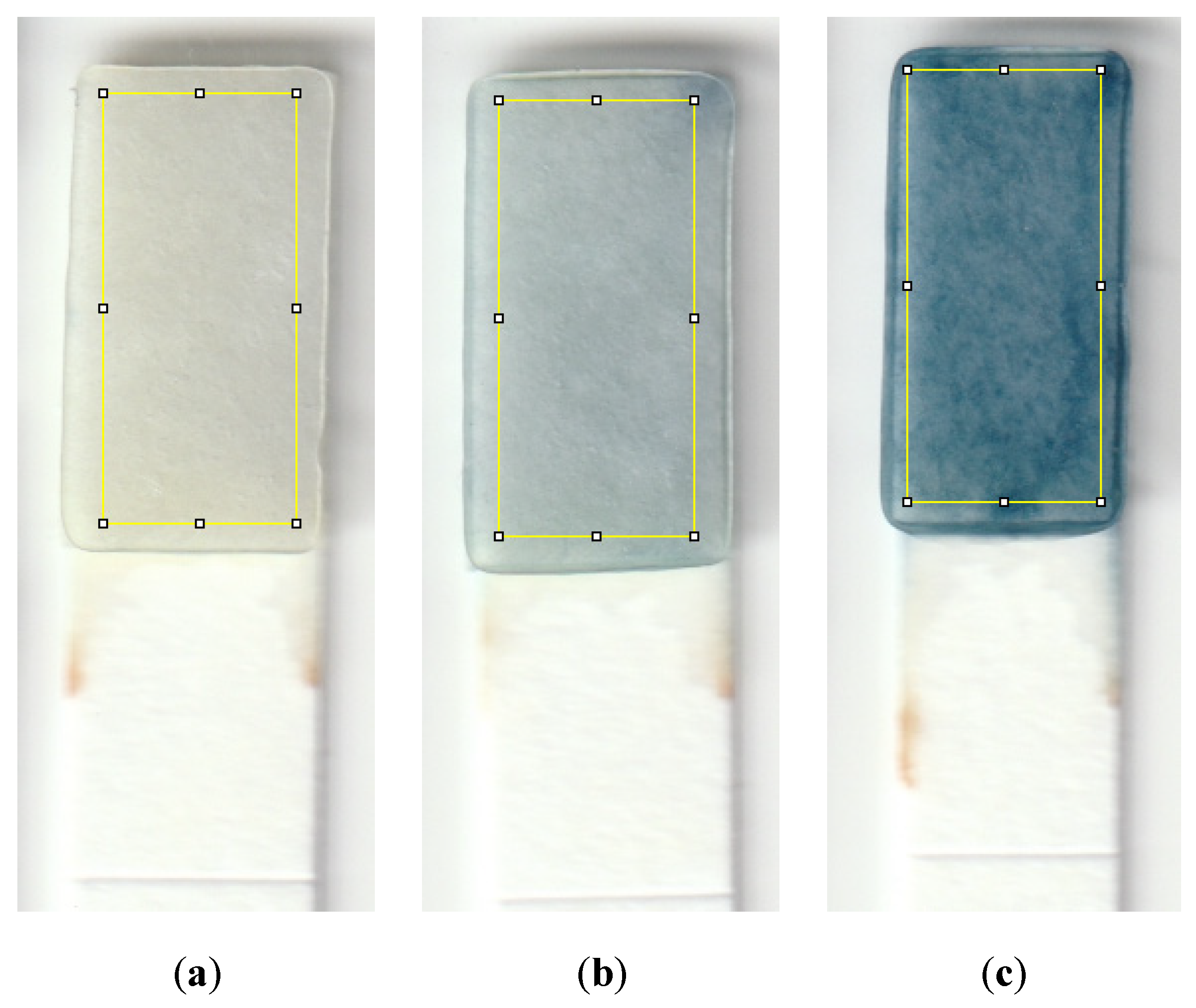
2.4. Device Operation and Analysis Procedure
2.5. Portable Infrared Lightbox
3. Results and Discussion
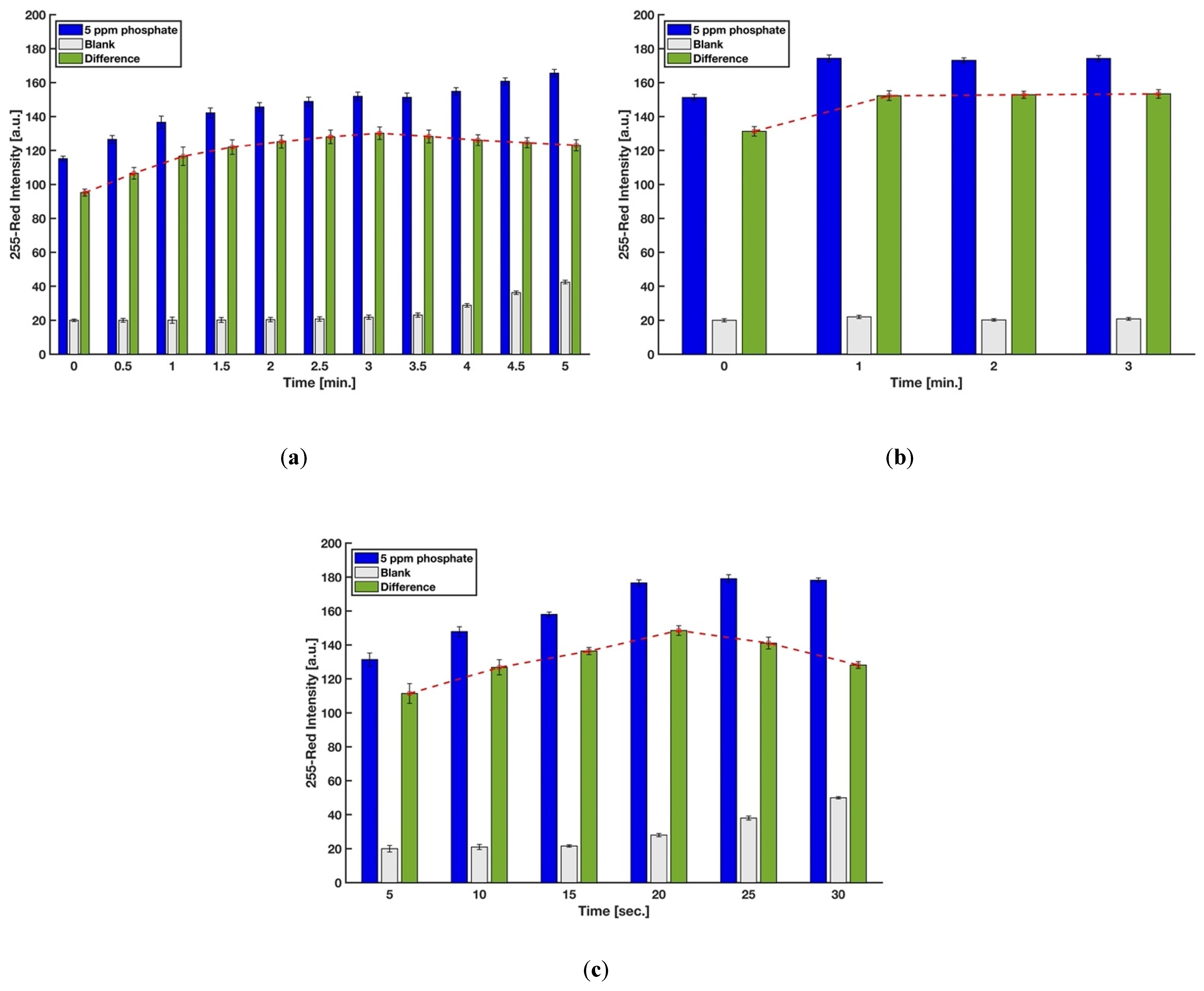
3.1. Timing Optimizations for the Device
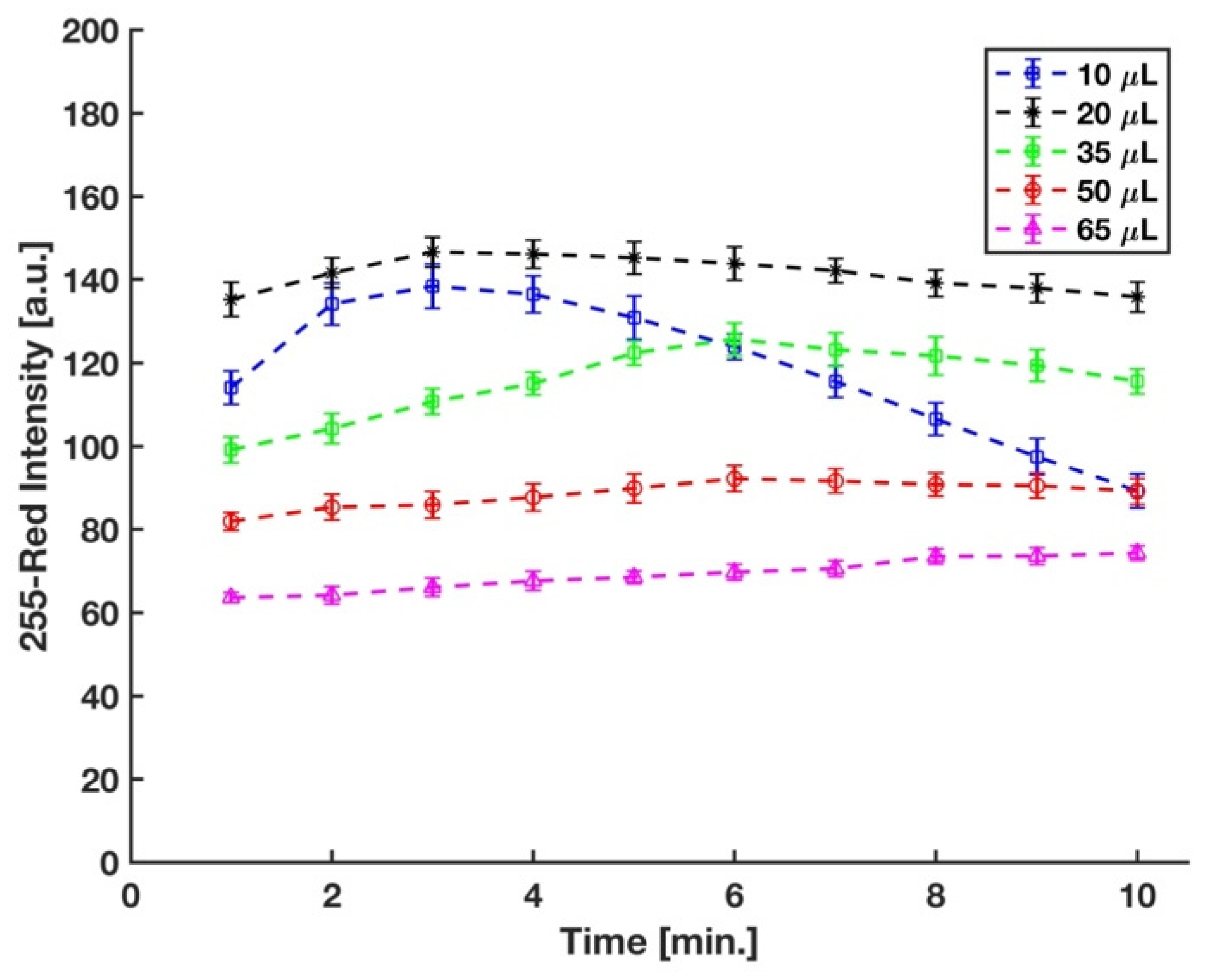
3.2. Optimization of Sulfuric Acid’s Volume
3.3. Role of Antimony in Phosphate Detection
3.4. Calibration Curve and Detection Limit in DI Water
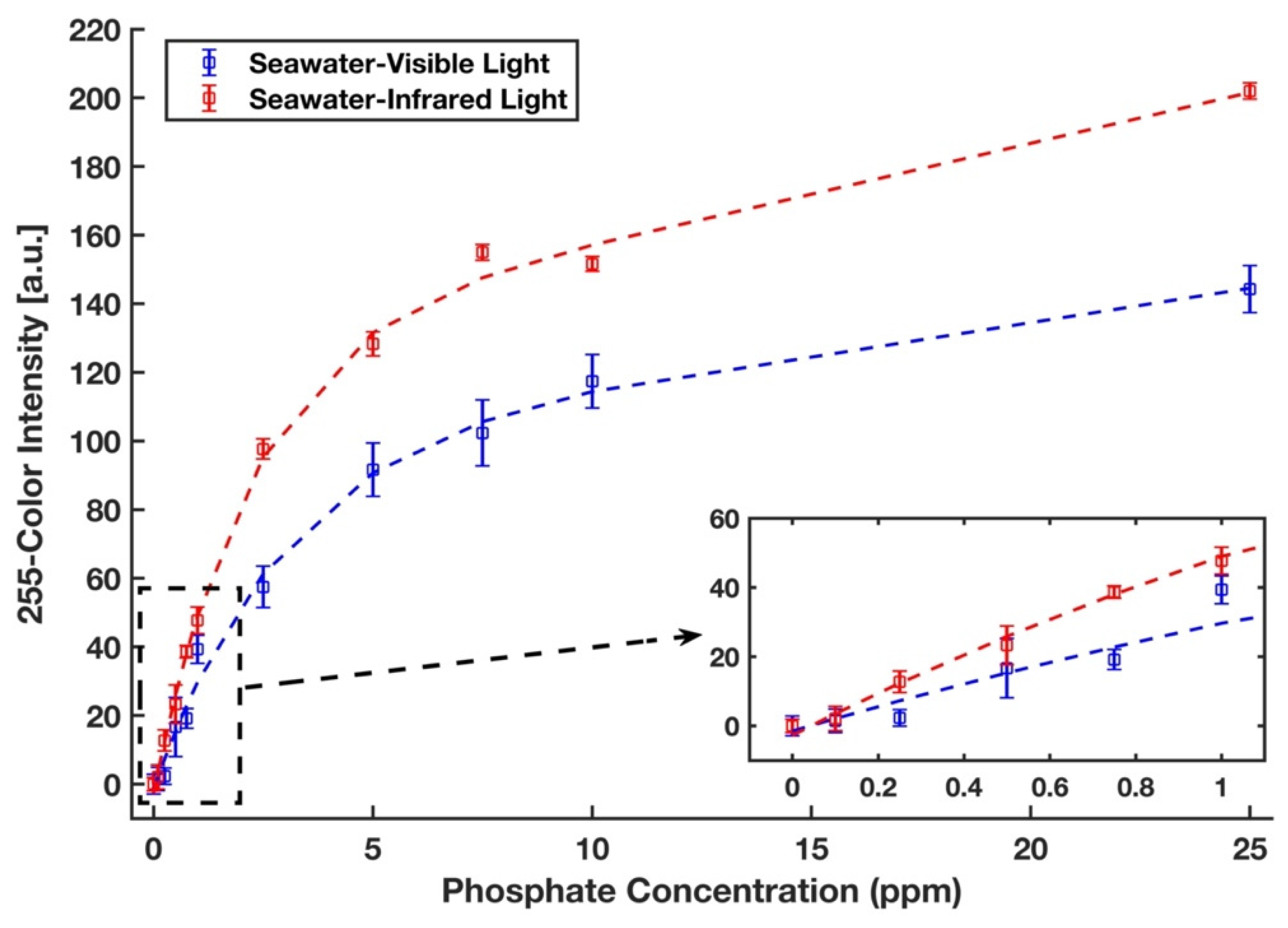
3.5. Calibration Curve and Detection Limit in Seawater with Visible and Infrared Illumination
3.6. Stability of the Device
3.7. Comparison of Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, X.; Ma, J. Recent advances in the determination of phosphate in environmental water samples: Insights from practical perspectives. TrAC Trends Anal. Chem. 2020, 127, 115908. [Google Scholar] [CrossRef]
- Sateanchok, S.; Pankratova, N.; Cuartero, M.; Cherubini, T.; Grudpan, K.; Bakker, E. In-Line Seawater Phosphate Detection with Ion-Exchange Membrane Reagent Delivery. ACS Sens. 2018, 3, 2455–2462. [Google Scholar] [CrossRef]
- Yaroshenko, I.; Kirsanov, D.; Marjanovic, M.; Lieberzeit, P.A.; Korostynska, O.; Mason, A.; Frau, I.; Legin, A. Real-Time Water Quality Monitoring with Chemical Sensors. Sensors 2020, 20, 3432. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.; Sambito, M.; Feo, R.; Freni, G.; Puleo, V. Optimal positioning of water quality sensors in water distribution networks: Comparison of numerical and experimental results. In Proceedings of the CCWI 2017—Computing and Control for the Water Industry, Sheffield, UK, 5–7 September 2017. [Google Scholar]
- Kent, R.; Johnson, T.D.; Rosen, M.R. Status and trends of orthophosphate concentrations in groundwater used for public supply in California. Environ. Monit. Assess. 2020, 192, 550. [Google Scholar] [CrossRef]
- Sambito, M.; Freni, G. Strategies for Improving Optimal Positioning of Quality Sensors in Urban Drainage Systems for Non-Conservative Contaminants. Water 2021, 13, 934. [Google Scholar] [CrossRef]
- Sarwar, M.; Leichner, J.; Naja, G.M.; Li, C.Z. Smart-phone, paper-based fluorescent sensor for ultra-low inorganic phosphate detection in environmental samples. Microsyst. Nanoeng. 2019, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Heidari-Bafroui, H.; Ribeiro, B.; Charbaji, A.; Anagnostopoulos, C.; Faghri, M. Infrared Lightbox and iPhone App for Improving Detection Limit of Phosphate Detecting Dip Strips. Int. J. Chem. Mol. Eng. 2020, 14, 179–185. [Google Scholar]
- Charbaji, A.; Smith, W.; Anagnostopoulos, C.; Faghri, M. Zinculose: A new fibrous material with embedded zinc particles. Eng. Sci. Technol. Int. J. 2021, 24, 571–578. [Google Scholar] [CrossRef]
- Jońca, J.; Fernández, V.L.; Thouron, D.; Paulmier, A.; Graco, M.; Garçon, V. Phosphate determination in seawater: Toward an autonomous electrochemical method. Talanta 2011, 87, 161–167. [Google Scholar] [CrossRef]
- Sun, S.; Chen, Q.; Sheth, S.; Ran, G.; Song, Q. Direct Electrochemical Sensing of Phosphate in Aqueous Solutions Based on Phase Transition of Calcium Phosphate. ACS Sens. 2020, 5, 541–548. [Google Scholar] [CrossRef]
- Gache, S.A.M.; Angelini, A.A.R.; Sabeckis, M.L.; Flecha, F.L.G. Improving the stability of the malachite green method for the determination of phosphate using Pluronic F68. Anal. Biochem. 2020, 597, 113681. [Google Scholar] [CrossRef]
- Snigur, D.; Chebotarev, A.; Bulat, K.; Duboviy, V. Fast room temperature cloud point extraction procedure for spectrophotometric determination of phosphate in water samples. Anal. Biochem. 2020, 597, 113671. [Google Scholar] [CrossRef]
- Salem, J.K.; Draz, M.A. Selective colorimetric nano-sensing solution for the determination of phosphate ion in drinking water samples. Int. J. Environ. Anal. Chem. 2020, 1–10. [Google Scholar] [CrossRef]
- Moonrungsee, N.; Pencharee, S.; Jakmunee, J. Colorimetric analyzer based on mobile phone camera for determination of available phosphorus in soil. Talanta 2015, 136, 204–209. [Google Scholar] [CrossRef]
- Zhao, H.X.; Liu, L.Q.; De Liu, Z.; Wang, Y.; Zhao, X.J.; Huang, C.Z. Highly selective detection of phosphate in very complicated matrixes with an off–on fluorescent probe of europium-adjusted carbon dots. Chem. Commun. 2011, 47, 2604–2606. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, X.; Cao, H.; Huang, Y. Polydopamine coated copper nanoclusters with aggregation-induced emission for fluorometric determination of phosphate ion and acid phosphatase activity. Microchim. Acta 2020, 187, 1–9. [Google Scholar] [CrossRef]
- Rahman, M.A.; Park, D.S.; Chang, S.C.; McNeil, C.J.; Shim, Y.B. The biosensor based on the pyruvate oxidase modified conducting polymer for phosphate ions determinations. Biosens. Bioelectron. 2006, 21, 1116–1124. [Google Scholar] [CrossRef]
- Franz, P.; Gassl, V.; Topf, A.; Eckelmann, L.; Iorga, B.; Tsiavaliaris, G. A thermophoresis–based biosensor for real–time detection of inorganic phosphate during enzymatic reactions. Biosens. Bioelectron. 2020, 169, 112616. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. Determrnation Single Solution Method for the in Natural. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Going, J.E.; Eisenreich, S.J. Spectrophotometric studies of reduced molybdoantimonylphosphoric acid. Anal. Chim. Acta 1974, 70, 95–106. [Google Scholar] [CrossRef]
- Dick, W.A.; Tabatabai, M. Kinetic parameters of phopsphates in soils and organic waste materials. Soil Sci. 1984, 137, 7–15. [Google Scholar] [CrossRef]
- Drummond, L.; Maher, W. Determination of phosphorus in aqueous solution via formation of the phosphoantimonylmolybdenum blue complex. Re-examination of optimum conditions for the analysis of phosphate. Anal. Chim. Acta 1995, 302, 69–74. [Google Scholar] [CrossRef]
- Nagul, E.A.; McKelvie, I.D.; Worsfold, P.; Kolev, S.D. The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box. Anal. Chim. Acta 2015, 890, 60–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibnul, N.K.; Tripp, C.P. A solventless method for detecting trace level phosphate and arsenate in water using a transparent membrane and visible spectroscopy. Talanta 2021, 225, 122023. [Google Scholar] [CrossRef] [PubMed]
- Jayawardane, B.M.; Wongwilai, W.; Grudpan, K.; Kolev, S.D.; Heaven, M.W.; Nash, D.M.; McKelvie, I.D. Evaluation and Application of a Paper-Based Device for the Determination of Reactive Phosphate in Soil Solution. J. Environ. Qual. 2014, 43, 1081–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, A.Y.; Hussein, Y.Z.; Mohammed, M.A. Simultaneous determination of phosphate and silicate in detergents and waters by first-derivative spectrophotometry. Analyst 2001, 126, 1810–1815. [Google Scholar] [CrossRef]
- Charbaji, A.; Heidari-Bafroui, H.; Anagnostopoulos, C.; Faghri, M. A New Paper-Based Microfluidic Device for Improved Detection of Nitrate in Water. Sensors 2021, 21, 102. [Google Scholar] [CrossRef] [PubMed]
- Jayawardane, B.M.; McKelvie, I.D.; Kolev, S.D. A paper-based device for measurement of reactive phosphate in water. Talanta 2012, 100, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B. Development of a Paper-Based Microfluidic Device for the Detection of Orthophosphate in Water; University of Rhode Island: Kingston, RI, USA, 2019. [Google Scholar]
- Racicot, J.M.; Mako, T.L.; Olivelli, A.; Levine, M. A Paper-Based Device for Ultrasensitive, Colorimetric Phosphate Detection in Seawater. Sensors 2020, 20, 2766. [Google Scholar] [CrossRef]
- Waghwani, B.; Balpande, S.; Kalambe, J. Development of microfluidic paper based analytical device for detection of phosphate in water. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 592–595. [Google Scholar]
- Lawal, A.T.; Adeloju, S.B. Polypyrrole Based Amperometric and Potentiometric Phosphate Biosensors: A Comparative Study. J. Appl. Sci. 2012, 12, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Puri, A. A review of permissible limits of drinking water. Indian, J. Occup. Environ. Med. 2012, 16, 40. [Google Scholar] [CrossRef] [Green Version]
- Fawcett, S.E.; Johnson, K.S.; Riser, S.C.; Van Oostende, N.; Sigman, D.M. Low-nutrient organic matter in the Sargasso Sea thermocline: A hypothesis for its role, identity, and carbon cycle implications. Mar. Chem. 2018, 207, 108–123. [Google Scholar] [CrossRef]
- Tsigdinos, G.A.; Chen, H.Y.; Streusand, B.J. Molybdate Solutions for Catalyst Preparation. Stability, Adsorption Properties, and Characterization. Ind. Eng. Chem. Prod. Res. Dev. 1981, 20, 619–623. [Google Scholar] [CrossRef]
- Weiss, J.; Weis, T. Handbook of Ion. Chromatography, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Kasetsirikul, S.; Shiddiky, M.J.; Nguyen, N.T. Challenges and perspectives in the development of paper-based lateral flow assays. Microfluid. Nanofluid. 2020, 24, 17. [Google Scholar] [CrossRef]
- Heidari-Bafroui, H.; Ribeiro, B.; Charbaji, A.; Anagnostopoulos, C.; Faghri, M. Portable infrared lightbox for improving the detection limits of paper-based phosphate devices. Meas. J. Int. Meas. Confed. 2020. [Google Scholar] [CrossRef]
- Knochen, M.; Rodríguez-Silva, J.C.; Silva-Silva, J. Exploitation of reaction mechanisms for sensitivity enhancement in the determination of phosphorus by sequential injection analysis. Talanta 2020, 209, 120589. [Google Scholar] [CrossRef]
- van Staden, J.F.; Taljaard, R.E. On-line monitoring of phosphate in natural water and effluent streams using sequential injection analysis. Microchim. Acta 1998, 128, 223–228. [Google Scholar] [CrossRef]
- Pinit, S.; Chadchawan, S.; Chaiwanon, J. A simple high-throughput protocol for the extraction and quantification of inorganic phosphate in rice leaves. Appl. Plant. Sci. 2020, 8, e11395. [Google Scholar] [CrossRef]
- Galhardo, C.X.; Masini, J.C. Spectrophotometric determination of phosphate and silicate by sequential injection using molybdenum blue chemistry. Anal. Chim. Acta 2000, 417, 191–200. [Google Scholar] [CrossRef]
- Koronkiewicz, S.; Trifescu, M.; Smoczynski, L.; Ratnaweera, H.; Kalinowski, S. A novel automatic flow method with direct-injection photometric detector for determination of dissolved reactive phosphorus in wastewater and freshwater samples. Environ. Monit. Assess. 2018, 190, 133. [Google Scholar] [CrossRef] [Green Version]
- Divya, S.; Sharmila, P.; Dinakaran, J.; Yamal, G.; Rao, K.S.; Pardha-Saradhi, P. Specific H+ level is crucial for accurate phosphate quantification using ascorbate as a reductant. Protoplasma 2020, 257, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Tanaka, M. Analysis of Complex-formation Reaction in Molybdenum Blue Method by ESI-MS. Bunseki Kagaku 2012, 61, 1049–1054. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, I.; Miras, H.N.; Fujiwara, A.; Fujibayashi, M.; Song, Y.F.; Cronin, L.; Tsunashima, R. Investigating the Formation of ‘Molybdenum Blues’ with Gel Electrophoresis and Mass Spectrometry. J. Am. Chem. Soc. 2015, 137, 6524–6530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Fischer, C.J.; Ortner, P.B. Optimization of performance and minimization of silicate interference in continuous flow phosphate analysis. Talanta 1999, 49, 293–304. [Google Scholar] [CrossRef]
- Pai, S.C.; Wang, T.Y.; Fang, T.H.; Jiann, K.T. Effect of Heating on the Color Formation Reaction in the Murphy and Riley Method for the Determination of Phosphate in Natural Waters. J. Environ. Anal. Chem. 2015, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Motulsky, H.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; GraphPad Software Inc.: San Diego, CA, USA, 2003. [Google Scholar]
- Cao, P.; Zhu, Y.; Zhao, W.; Liu, S.; Gao, H. Chromaticity Measurement Based on the Image Method and Its Application in Water Quality Detection. Water 2019, 11, 2339. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.; Miller, J.C. Statistics, and Chemometrics for Analytical Chemistry, 5th ed.; Pearson Education Limited: Harlow, UK, 2005. [Google Scholar]
- He, Z.; Honeycutt, C.W. A Modified Molybdenum Blue Method for Orthophosphate Determination Suitable for Investigating Enzymatic Hydrolysis of Organic Phosphates. Commun. Soil Sci. Plant. Anal. 2005, 36, 1373–1383. [Google Scholar] [CrossRef]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab. Chip 2020, 20, 9–34. [Google Scholar] [CrossRef]
- Cinti, S.; Talarico, D.; Palleschi, G.; Moscone, D.; Arduini, F. Novel reagentless paper-based screen-printed electrochemical sensor to detect phosphate. Anal. Chim. Acta 2016, 919, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Charbaji, A.; Heidari-Bafroui, H.; Kumar, A.; Rahmani, N.; Anagnostopoulos, C.; Faghri, M. Characterization and Modeling of Paper-based Bi-Material Actuator Cantilever; Application in Phosphate Detection. In Proceedings of the Innovations in Microfluidics, Boston, MA, USA, 18–19 March 2021. [Google Scholar]





| Device | Working Range (ppm) | LOD (ppm) | Repeatability | Reaction Time (min.) | Shelf Life | Ref. |
|---|---|---|---|---|---|---|
| 3D colorimetric paper-based device | 0.6–30 | 0.153 | Less than 2% RSD | 40 | 122 days stored in freezer at <−20 °C | [29] |
| 2D colorimetric paper-based device | 0.1–10 | 0.160 | N/A | 4 | 9 months in refrigerator at <4 °C | [31] |
| Quantofix phosphate test kit | 0.1–50 | 1.352 | 2.1% RSD | 1 | 2 years under room temperature | [39] |
| Electrochemical paper-based device | 1–30 | 0.38 | Less than 6% RSD | 2.5 | 30 days at room temperature | [55] |
| Dip strip with wet chemistry | 0.1–25 | 0.134 | 1.8% RSD | 3 | 4 months and expected to be 2 years under room temperature | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidari-Bafroui, H.; Charbaji, A.; Anagnostopoulos, C.; Faghri, M. A Colorimetric Dip Strip Assay for Detection of Low Concentrations of Phosphate in Seawater. Sensors 2021, 21, 3125. https://doi.org/10.3390/s21093125
Heidari-Bafroui H, Charbaji A, Anagnostopoulos C, Faghri M. A Colorimetric Dip Strip Assay for Detection of Low Concentrations of Phosphate in Seawater. Sensors. 2021; 21(9):3125. https://doi.org/10.3390/s21093125
Chicago/Turabian StyleHeidari-Bafroui, Hojat, Amer Charbaji, Constantine Anagnostopoulos, and Mohammad Faghri. 2021. "A Colorimetric Dip Strip Assay for Detection of Low Concentrations of Phosphate in Seawater" Sensors 21, no. 9: 3125. https://doi.org/10.3390/s21093125
APA StyleHeidari-Bafroui, H., Charbaji, A., Anagnostopoulos, C., & Faghri, M. (2021). A Colorimetric Dip Strip Assay for Detection of Low Concentrations of Phosphate in Seawater. Sensors, 21(9), 3125. https://doi.org/10.3390/s21093125








