Fabrication of AuNPs/MWCNTS/Chitosan Nanocomposite for the Electrochemical Aptasensing of Cadmium in Water
Abstract
:1. Introduction
2. Experimental
2.1. Chemical and Reagents
2.2. Instrumentation
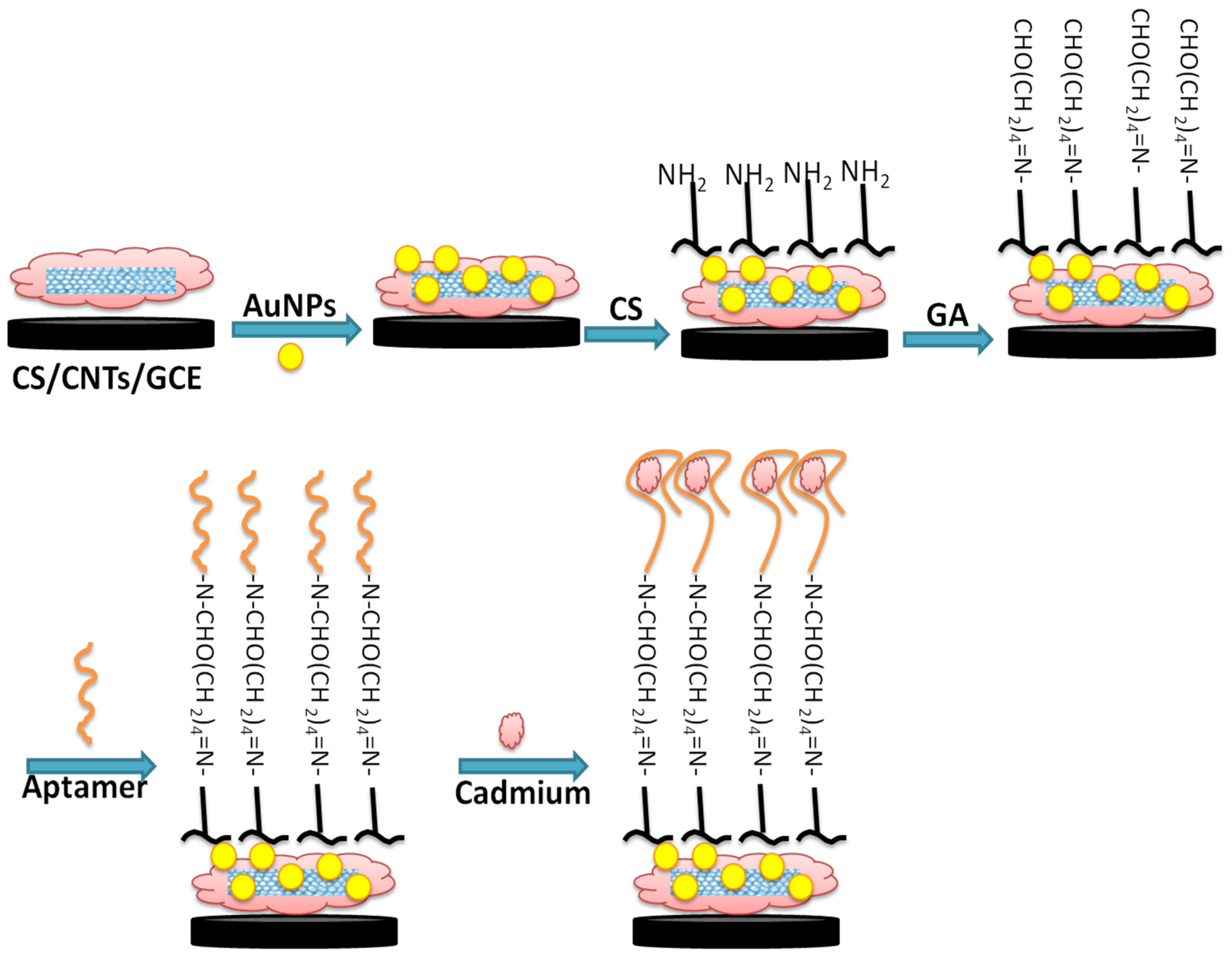
2.3. Fabrication of the Aptasensor
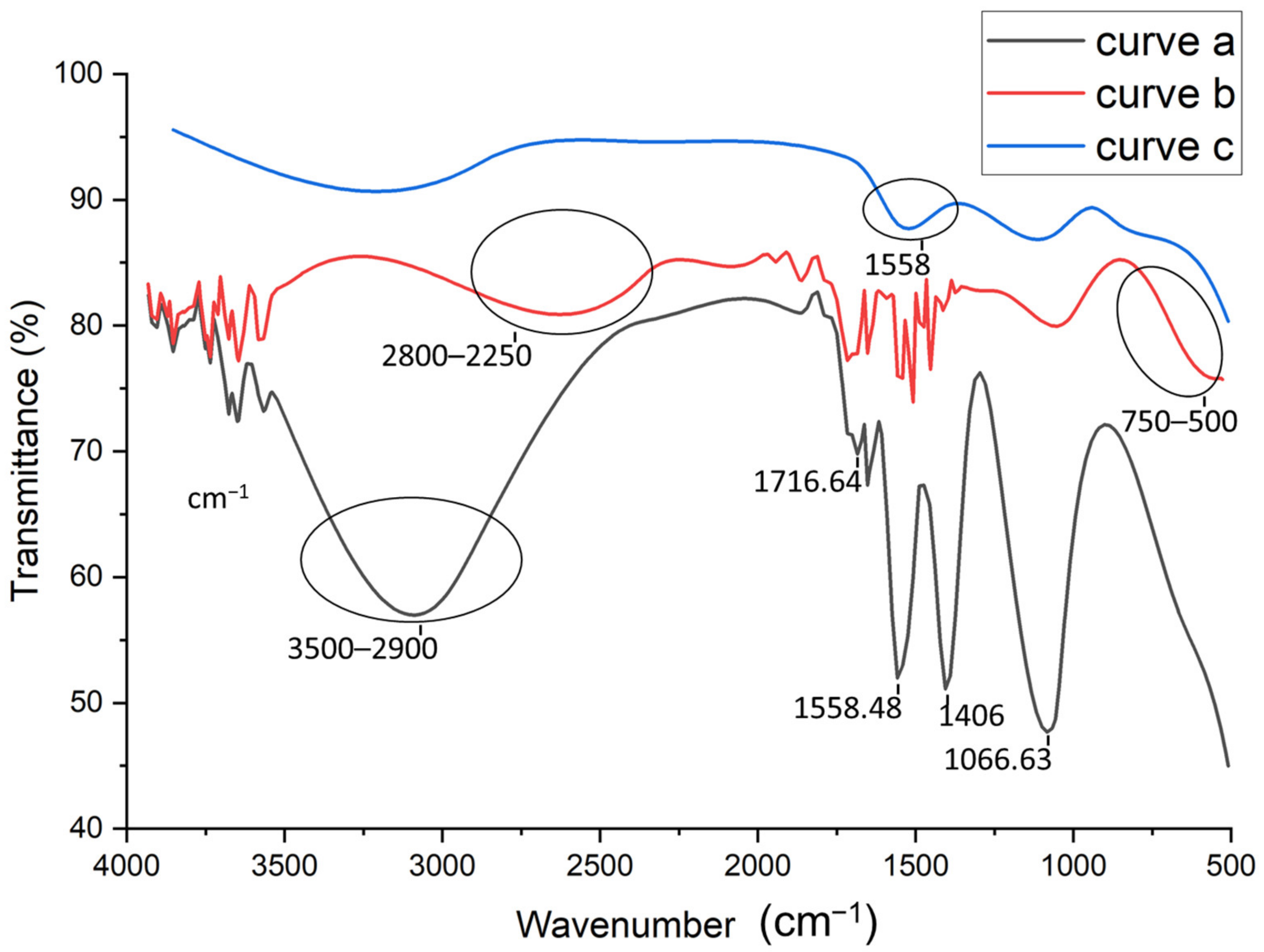
2.4. FTIR Characterization
2.5. Analytical Performance of the Label-Free Aptasensor
2.6. Real Sample Analysis
3. Results and Discussion
3.1. FT-IR Characterization of the Fabrication Steps
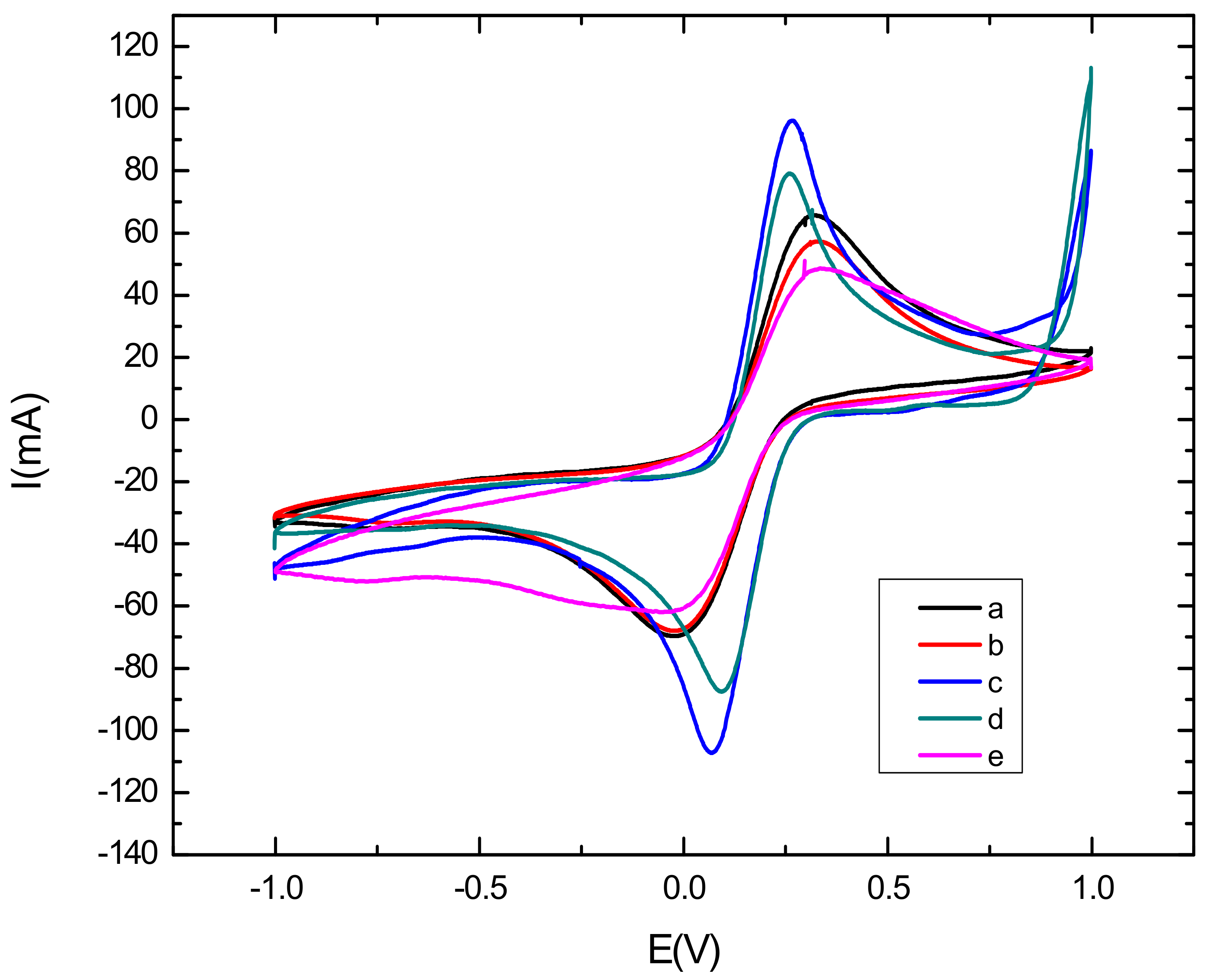
3.2. Electrochemical Characterization of the Aptamer-Modified Surface
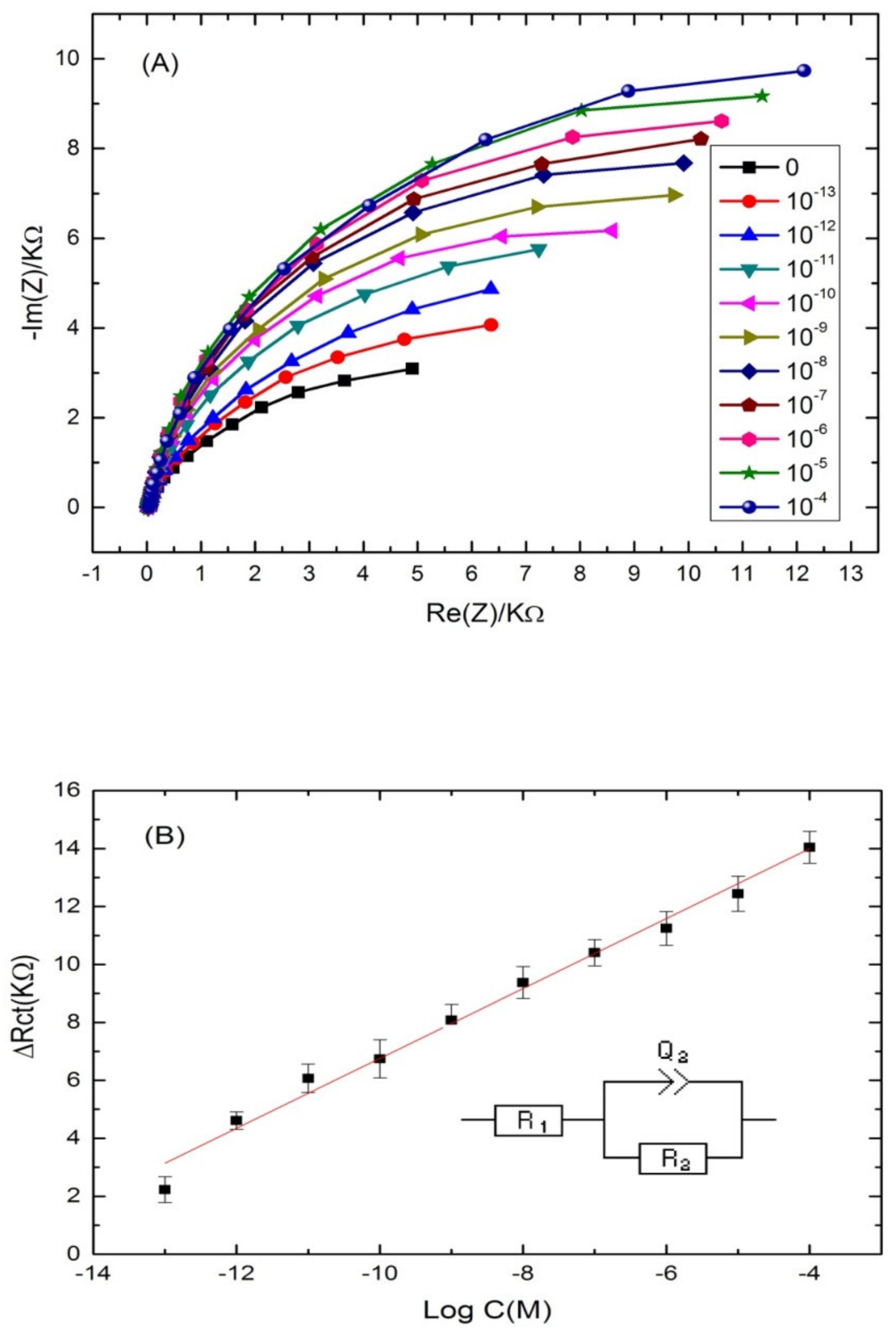
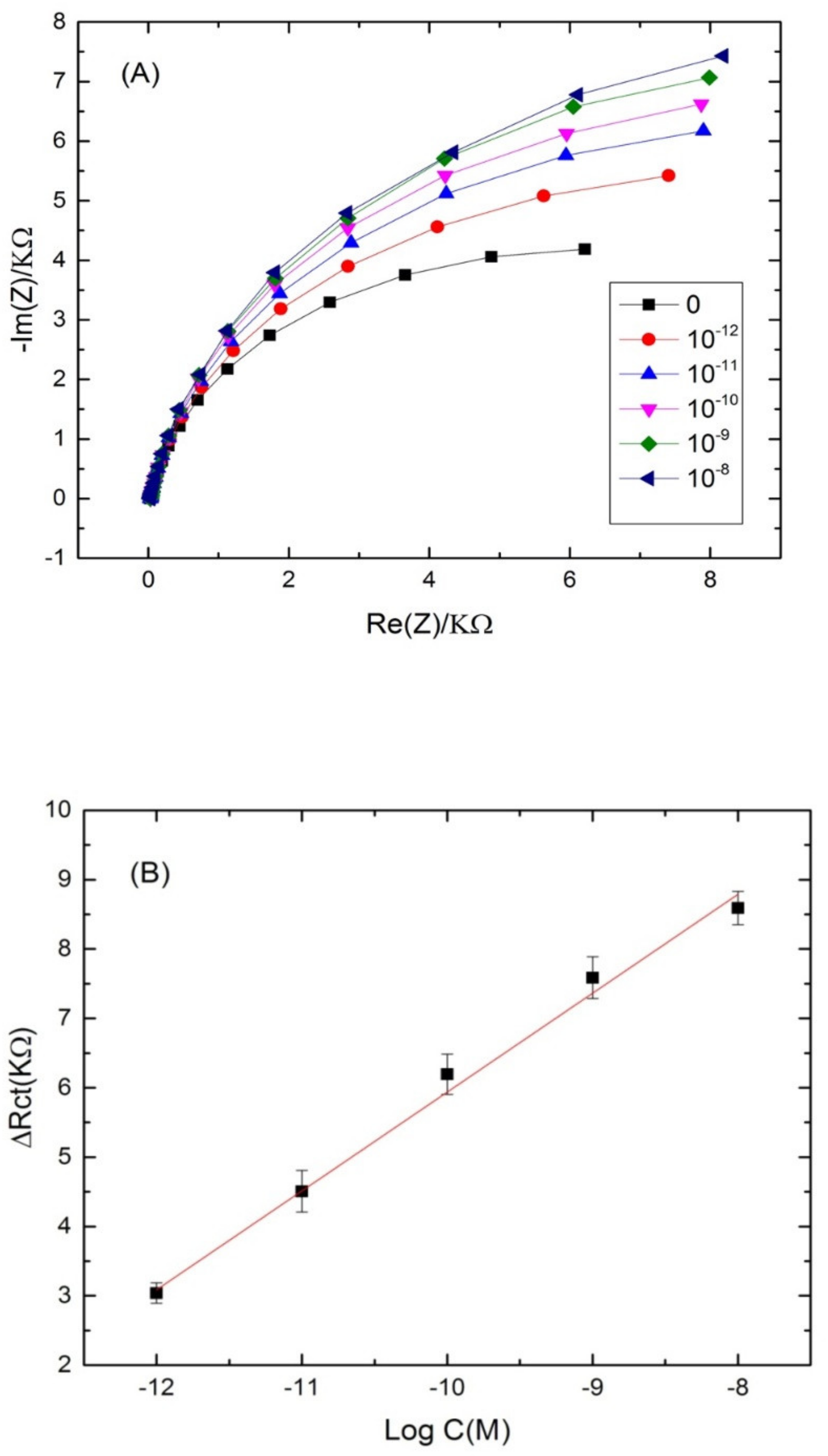
3.3. Application of the Aptasensing Platform in the Impedimetric Detection of Cadmium
3.4. Specificity and Reproducibility
3.5. Applicability in Real Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Li, F.; Shen, G.; Huang, J.; Wang, X. Aptasensor based on the synergistic contributions of chitosan–gold nanoparticles, graphene–gold nanoparticles and multi-walled carbon nanotubes-cobalt phthalocyanine nanocomposites for kanamycin detection. Analyst 2014, 139, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.-H.; Zhang, T.; Luo, H.; Yen, T.M.; Chen, P.-W.; Han, Y.; Lo, Y.-H. Nucleic acid aptamers: An emerging tool for biotechnology and biomedical sensing. Sensors 2015, 15, 16281–16313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar Kulabhusan, P.; Hussain, B.; Yüce, M. Current perspectives on aptamers as diagnostic tools and therapeutic agents. Pharmaceutics 2020, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Bognár, Z.; Gyurcsányi, R.E. Aptamers against immunoglobulins: Design, selection and bioanalytical applications. Int. J. Mol. Sci. 2020, 21, 5748. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, Q.; Chang, Y.; Zhang, Q.; Brennan, J.D.; Li, Y. In vitro selection of circular DNA aptamers for biosensing applications. Angew. Chem. Int. Ed. 2019, 58, 8013–8017. [Google Scholar] [CrossRef]
- Zhang, X.; Yadavalli, V.K. Surface immobilization of DNA aptamers for biosensing and protein interaction analysis. Biosens. Bioelectron. 2011, 26, 3142–3147. [Google Scholar] [CrossRef]
- Wehbe, M.; Labib, M.; Muharemagic, D.; Zamay, A.S.; Berezovski, M.V. Switchable aptamers for biosensing and bioseparation of viruses (swaps-v). Biosens. Bioelectron. 2015, 67, 280–286. [Google Scholar] [CrossRef]
- Urmann, K.; Modrejewski, J.; Scheper, T.; Walter, J.-G. Aptamer-modified nanomaterials: Principles and applications. BioNanoMaterials 2017, 18, 1–18. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. Applications of nanotechnology in daily life. Interface Sci. Technol. 2019, 28, 113–143. [Google Scholar]
- Altintas, Z. Biosensors and Nanotechnology: Applications in Health Care Diagnostics; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Lei, J.; Ju, H. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134. [Google Scholar] [CrossRef]
- Putzbach, W.; Ronkainen, N.J. Immobilization techniques in the fabrication of nanomaterial-based electrochemical biosensors: A review. Sensors 2013, 13, 4811–4840. [Google Scholar] [CrossRef]
- Guinovart, T.; Parrilla, M.; Crespo, G.A.; Rius, F.X.; Andrade, F.J. Potentiometric sensors using cotton yarns, carbon nanotubes and polymeric membranes. Analyst 2013, 138, 5208–5215. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Chai, C. A novel amperometric sensor based on screen-printed electrode modified with multi-walled carbon nanotubes and molecularly imprinted membrane for rapid determination of ractopamine in pig urine. Sens. Actuators B Chem. 2012, 168, 103–110. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon nanomaterials and their application to electrochemical sensors: A review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Soldano, C. Hybrid metal-based carbon nanotubes: Novel platform for multifunctional applications. Prog. Mater. Sci. 2015, 69, 183–212. [Google Scholar] [CrossRef]
- Muhammad, A.; Yusof, N.A.; Hajian, R.; Abdullah, J. Construction of an electrochemical sensor based on carbon nanotubes/gold nanoparticles for trace determination of amoxicillin in bovine milk. Sensors 2016, 16, 56. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, W.; Lv, Q.; Xi, G.; Bai, H.; Zhang, Q. Disposable paper-based electrochemical sensor based on stacked gold nanoparticles supported carbon nanotubes for the determination of bisphenol a. Electrochem. Commun. 2016, 68, 104–107. [Google Scholar] [CrossRef]
- Hamdy, M.E.; Del Carlo, M.; Hussein, H.A.; Salah, T.A.; El-Deeb, A.H.; Emara, M.M.; Pezzoni, G.; Compagnone, D. Development of gold nanoparticles biosensor for ultrasensitive diagnosis of foot and mouth disease virus. J. Nanobiotechnol. 2018, 16, 48. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Wang, Y.; Zhao, L.; Ji, C.; Chen, D.; Nie, L. Applications of gold nanoparticles in non-optical biosensors. Nanomaterials 2018, 8, 977. [Google Scholar] [CrossRef] [Green Version]
- Yuan, D.; Chen, S.; Yuan, R.; Zhang, J.; Liu, X. An ecl sensor for dopamine using reduced graphene oxide/multiwall carbon nanotubes/gold nanoparticles. Sens. Actuators B Chem. 2014, 191, 415–420. [Google Scholar] [CrossRef]
- Cai, X.; Gao, X.; Wang, L.; Wu, Q.; Lin, X. A layer-by-layer assembled and carbon nanotubes/gold nanoparticles-based bienzyme biosensor for cholesterol detection. Sens. Actuators B Chem. 2013, 181, 575–583. [Google Scholar] [CrossRef]
- Alim, S.; Vejayan, J.; Yusoff, M.M.; Kafi, A. Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review. Biosens. Bioelectron. 2018, 121, 125–136. [Google Scholar] [PubMed]
- He, B.; Wang, L.; Dong, X.; Yan, X.; Li, M.; Yan, S.; Yan, D. Aptamer-based thin film gold electrode modified with gold nanoparticles and carboxylated multi-walled carbon nanotubes for detecting oxytetracycline in chicken samples. Food Chem. 2019, 300, 125179. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Chen, S.-Y.; Liaw, H.-W. Immobilization of lactate dehydrogenase within multiwalled carbon nanotube-chitosan nanocomposite for application to lactate biosensors. Sens. Actuators B Chem. 2007, 125, 474–481. [Google Scholar] [CrossRef]
- Kang, X.; Mai, Z.; Zou, X.; Cai, P.; Mo, J. A novel glucose biosensor based on immobilization of glucose oxidase in chitosan on a glassy carbon electrode modified with gold–platinum alloy nanoparticles/multiwall carbon nanotubes. Anal. Biochem. 2007, 369, 71–79. [Google Scholar] [CrossRef]
- Turner, A. Cadmium pigments in consumer products and their health risks. Sci. Total Environ. 2019, 657, 1409–1418. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for drinking-water quality. WHO Chron. 2011, 38, 104–108. [Google Scholar]
- Rabai, S.; Benounis, M.; Catanante, G.; Baraket, A.; Errachid, A.; Renault, N.J.; Marty, J.-L.; Rhouati, A. Development of a label-free electrochemical aptasensor based on diazonium electrodeposition: Application to cadmium detection in water. Anal. Biochem. 2021, 612, 113956. [Google Scholar] [CrossRef]
- Wu, Y.; Zhan, S.; Wang, L.; Zhou, P. Selection of a DNA aptamer for cadmium detection based on cationic polymer mediated aggregation of gold nanoparticles. Analyst 2014, 139, 1550–1561. [Google Scholar] [CrossRef]
- Araar, H.; Benounis, M.; Direm, A.; Touati, A.; Atailia, S.; Barhoumi, H.; Jaffrezic-Renault, N. A new thin film modified glassy carbon electrode based on melaminium chloride pentachlorocuprate (ii) for selective determination of nitrate in water. Mon. Chem.-Chem. Mon. 2019, 150, 1737–1744. [Google Scholar] [CrossRef]
- He, B.-S.; Yan, S.-s. Electrochemical aptasensor based on aptamer-complimentary strand conjugate and thionine for sensitive detection of tetracycline with multi-walled carbon nanotubes and gold nanoparticles amplification. Anal. Methods 2018, 10, 783–790. [Google Scholar] [CrossRef]
- Azadbakht, A.; Roushani, M.; Abbasi, A.R.; Derikvand, Z. A novel impedimetric aptasensor, based on functionalized carbon nanotubes and prussian blue as labels. Anal. Biochem. 2016, 512, 58–69. [Google Scholar] [CrossRef]
- Saeedfar, K.; Heng, L.Y.; Chiang, C.P. A DNA biosensor based on gold nanoparticle decorated on carboxylated multi-walled carbon nanotubes for gender determination of arowana fish. Bioelectrochemistry 2017, 118, 106–113. [Google Scholar] [CrossRef]
- Wang, Y.; Iqbal, Z.; Mitra, S. Microwave-induced rapid chemical functionalization of single-walled carbon nanotubes. Carbon 2005, 43, 1015–1020. [Google Scholar] [CrossRef]
- Molaei, R.; Sabzi, R.E.; Farhadi, K.; Kheiri, F.; Forough, M. Amperometric biosensor for cholesterol based on novel nanocomposite array gold nanoparticles/acetone-extracted propolis/multiwall carbon nanotubes/gold. Micro Nano Lett. 2014, 9, 100–104. [Google Scholar] [CrossRef]
- Tlili, A.; Abdelghani, A.; Hleli, S.; Maaref, M.A. Electrical characterization of a thiol sam on gold as a first step for the fabrication of immunosensors based on a quartz crystal microbalance. Sensors 2004, 4, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Huerta, F.; Montilla, F.; Morallón, E. Study on electroactive and electrocatalytic surfaces of single walled carbon nanotube-modified electrodes. Electrochim. Acta 2011, 56, 2464–2470. [Google Scholar] [CrossRef]
- Wei, X.; Xu, X.; Qi, W.; Wu, Y.; Wang, L. Molecularly imprinted polymer/graphene oxide modified glassy carbon electrode for selective detection of sulfanilamide. Prog. Nat. Sci. Mater. Int. 2017, 27, 374–379. [Google Scholar] [CrossRef]
- Feng, Y.; Wei, Z.; Zhang, J. Determination of ursolic acid in extracts from ligustri lucidum fruit using an electrochemical method. Front. Chem. 2020, 8, 444. [Google Scholar] [CrossRef]
- Anupriya, J.; Babulal, S.M.; Chen, T.-W.; Chen, S.-M.; Kumar, J.V.; Lee, J.-W.; Rwei, S.-P.; Yu, J.; Yu, R.; Hong, C.-Y. Facile hydrothermal synthesis of cubic zinc ferrite nanoparticles for electrochemical detection of anti-inflammatory drug nimesulide in biological and pharmaceutical sample. Int. J. Electrochem. Sci. 2021, 16, 1–19. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Raoof, J.B.; Azimi, R.; Ojani, R. Label-free and sensitive aptasensor based on dendritic gold nanostructures on functionalized sba-15 for determination of chloramphenicol. Anal. Bioanal. Chem. 2016, 408, 2557–2565. [Google Scholar] [CrossRef]
- Luan, Y.; Lu, A.; Chen, J.; Fu, H.; Xu, L. A label-free aptamer-based fluorescent assay for cadmium detection. Appl. Sci. 2016, 6, 432. [Google Scholar] [CrossRef] [Green Version]
- Bagheryan, Z.; Raoof, J.-B.; Golabi, M.; Turner, A.P.; Beni, V. Diazonium-based impedimetric aptasensor for the rapid label-free detection of salmonella typhimurium in food sample. Biosens. Bioelectron. 2016, 80, 566–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, C.M.; Rosanske, T.W. Development and Validation of Analytical Methods; Elsevier: New York, NY, USA, 1996. [Google Scholar]
- Chiang, C.-K.; Huang, C.-C.; Liu, C.-W.; Chang, H.-T. Oligonucleotide-based fluorescence probe for sensitive and selective detection of mercury (ii) in aqueous solution. Anal. Chem. 2008, 80, 3716–3721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Wang, Q.; Gao, F.; Gao, F. A high-performance aptasensor for mercury (ii) based on the formation of a unique ternary structure of aptamer–hg 2+–neutral red. Chem. Commun. 2014, 50, 9397–9400. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-X.; Zhao, Y.; He, X.-W.; Yin, X.-B. A “turn-on” electrochemiluminescent biosensor for detecting hg2+ at femtomole level based on the intercalation of ru (phen) 32+ into ds-DNA. Chem. Commun. 2010, 46, 9022–9024. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabai, S.; Teniou, A.; Catanante, G.; Benounis, M.; Marty, J.-L.; Rhouati, A. Fabrication of AuNPs/MWCNTS/Chitosan Nanocomposite for the Electrochemical Aptasensing of Cadmium in Water. Sensors 2022, 22, 105. https://doi.org/10.3390/s22010105
Rabai S, Teniou A, Catanante G, Benounis M, Marty J-L, Rhouati A. Fabrication of AuNPs/MWCNTS/Chitosan Nanocomposite for the Electrochemical Aptasensing of Cadmium in Water. Sensors. 2022; 22(1):105. https://doi.org/10.3390/s22010105
Chicago/Turabian StyleRabai, Selma, Ahlem Teniou, Gaëlle Catanante, Messaoud Benounis, Jean-Louis Marty, and Amina Rhouati. 2022. "Fabrication of AuNPs/MWCNTS/Chitosan Nanocomposite for the Electrochemical Aptasensing of Cadmium in Water" Sensors 22, no. 1: 105. https://doi.org/10.3390/s22010105
APA StyleRabai, S., Teniou, A., Catanante, G., Benounis, M., Marty, J.-L., & Rhouati, A. (2022). Fabrication of AuNPs/MWCNTS/Chitosan Nanocomposite for the Electrochemical Aptasensing of Cadmium in Water. Sensors, 22(1), 105. https://doi.org/10.3390/s22010105






