Evaluation of Electrochromic Properties of Polypyrrole/Poly(Methylene Blue) Layer Doped by Polysaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Pretreatment of ITO-Coated Glass Slide
2.3. Co-Deposition of Ppy-PMB Layer on ITO Glass Electrode
2.4. Evaluation of Dominant Features by Atomic Force Microscopy and Scanning Electron Microscopy
2.5. Evaluation of Electrochromic Properties of Ppy-PMB Layer at Different PH of the Solution
2.6. Application of (Ppy-PMB)Hep Layer for Sensing Xanthine Derivatives
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolfart, F.; Hryniewicz, B.M.; Góes, M.S.; Corrêa, C.M.; Torresi, R.; Minadeo, M.A.; de Torresi, S.I.C.; Oliveira, R.D.; Marchesi, L.F.; Vidotti, M. Conducting polymers revisited: Applications in energy, electrochromism and molecular recognition. J. Solid State Electrochem. 2017, 21, 2489–2515. [Google Scholar] [CrossRef]
- Leonavicius, K.; Ramanaviciene, A.; Ramanavicius, A. Polymerization Model for Hydrogen Peroxide Initiated Synthesis of Polypyrrole Nanoparticles. Langmuir 2011, 27, 10970–10976. [Google Scholar] [CrossRef] [PubMed]
- Ratautaite, V.; Nesladek, M.; Ramanaviciene, A.; Baleviciute, I.; Ramanavicius, A. Evaluation of Histamine Imprinted Polypyrrole Deposited on Boron Doped Nanocrystalline Diamond. Electroanalysis 2014, 26, 2458–2464. [Google Scholar] [CrossRef]
- Ratautaite, V.; Janssens, S.D.; Haenen, K.; Nesládek, M.; Ramanaviciene, A.; Baleviciute, I.; Ramanavicius, A. Molecularly Imprinted Polypyrrole Based Impedimentric Sensor for Theophylline Determination. Electrochim. Acta 2014, 130, 361–367. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation of polyaniline and polypyrrole nanocomposites with embedded glucose oxidase and gold nanoparticles. Polymers 2019, 11, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanaviciene, A.; Kausaite-Minkstimiene, A.; Oztekin, Y.; Carac, G.; Voronovic, J.; German, N.; Ramanavicius, A. Visualization of red-ox proteins on the gold surface using enzymatic polypyrrole formation. Microchim. Acta 2011, 175, 79–86. [Google Scholar] [CrossRef]
- Plausinaitis, D.; Sinkevicius, L.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Evaluation of Electrochemical Quartz Crystal Microbalance Based Sensor Modified by Uric Acid-imprinted Polypyrrole. Talanta 2020, 220, 121414. [Google Scholar] [CrossRef]
- Ratautaite, V.; Plausinaitis, D.; Baleviciute, I.; Mikoliunaite, L.; Ramanaviciene, A.; Ramanavicius, A. Characterization of Caffeine-Imprinted Polypyrrole by a Quartz Crystal Microbalance and Electrochemical Impedance Spectroscopy. Sens. Actuators B 2015, 212, 63–71. [Google Scholar] [CrossRef]
- Plausinaitis, D.; Ratautaite, V.; Mikoliunaite, L.; Sinkevicius, L.; Ramanaviciene, A.; Ramanavicius, A. Quartz Crystal Microbalance-Based Evaluation of the Electrochemical Formation of an Aggregated Polypyrrole Particle-Based Layer. Langmuir 2015, 31, 3186–3193. [Google Scholar] [CrossRef]
- Diaz, A.F.; Kanazawa, K.K.; Gardini, G.P. Electrochemical polymerization of pyrrole. J. Chem. Soc. Chem. Commun. 1979, 14, 635–636. [Google Scholar] [CrossRef]
- Pauliukaite, R.; Ghica, M.E.; Barsan, M.M.; Brett, C.M.A. Phenazines and Polyphenazines in Electrochemical Sensors and Biosensors. Anal. Lett. 2010, 43, 1588–1608. [Google Scholar] [CrossRef]
- Manasa, G.; Mascarenhas, R.J.; Satpati, A.K.; D’Souza, O.J.; Dhason, A. Facile preparation of poly(methylene blue) modified carbon paste electrode for the detection and quantification of catechin. Mater. Sci. Eng. C 2017, 73, 552–561. [Google Scholar] [CrossRef]
- Liu, B.; Cang, H.; Cui, L.; Zhang, H. Electrochemical polymerization of methylene blue on glassy carbon electrode. Int. J. Electrochem. Sci. 2017, 12, 9907–9913. [Google Scholar] [CrossRef]
- Liu, J.; Mu, S. The electrochemical polymerization of methylene blue and properties of polymethylene blue. Synth. Met. 1999, 107, 159–165. [Google Scholar] [CrossRef]
- Damos, F.S.; Luz, R.C.S.; Kubota, L.T. Study of poly(methylene blue) ultrathin films and its properties by electrochemical surface plasmon resonance. J. Electroanal. Chem. 2005, 581, 231–240. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Inzelt, G.; Kertesz, V. Poly(methylene blue) modified electrode sensor for haemoglobin. Anal. Chim. Acta 1999, 385, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Samukaite-Bubniene, U.; Valiūnienė, A.; Bucinskas, V.; Genys, P.; Ratautaite, V.; Ramanaviciene, A.; Aksun, E.; Tereshchenko, A.; Zeybek, B.; Ramanavicius, A. Towards supercapacitors: Cyclic voltammetry and fast Fourier transform electrochemical impedance spectroscopy based evaluation of polypyrrole electrochemically deposited on the pencil graphite electrode. Colloids Surf. A Physicochem. Eng. Asp. 2020, 610, 125750. [Google Scholar] [CrossRef]
- Kuwabata, S.; Nakamura, J.; Yoneyama, H. The effect of basicity of dopant anions on the conductivity of polypyrrole films. J. Chem. Soc. Chem. Commun. 1988, 12, 779–780. [Google Scholar] [CrossRef]
- Serra Moreno, J.; Panero, S.; Materazzi, S.; Martinelli, A.; Sabbieti, M.G.; Agas, D.; Materazzi, G. Polypyrrole-polysaccharide thin films characteristics: Electrosynthesis and biological properties. J. Biomed. Mater. Res. A 2009, 88A, 832–840. [Google Scholar] [CrossRef]
- Fonner, J.M.; Schmidt, C.E.; Ren, P. A combined molecular dynamics and experimental study of doped polypyrrole. Polymer 2010, 51, 4985–4993. [Google Scholar] [CrossRef] [Green Version]
- Ratautaite, V.; Ramanaviciene, A.; Oztekin, Y.; Voronovic, J.; Balevicius, Z.; Mikoliunaite, L.; Ramanavicius, A. Electrochemical stability and repulsion of polypyrrole film. Colloids Surf. A Physicochem. Eng. Asp. 2013, 418, 16–21. [Google Scholar] [CrossRef]
- Ratautaite, V.; Bagdziunas, G.; Ramanavicius, A.; Ramanaviciene, A. An Application of Conducting Polymer Polypyrrole for the Design of Electrochromic pH and CO2 Sensors. J. Electrochem. Soc. 2019, 166, B297–B303. [Google Scholar] [CrossRef]
- John, J.; Sivaraman, S.; Jayalekshmy, S.; Anantharaman, M.R. Investigations on the mechanism of carrier transport in plasma polymerized pyrrole thin films. J. Phys. Chem. Solids 2010, 71, 935–939. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, N.; Tavoli, F. Enhancing electrochromic contrast and redox stability of nanostructure polypyrrole film doped by heparin as polyanion in different solvents. J. Polym. Sci. A Polym. Chem. 2014, 52, 3365–3371. [Google Scholar] [CrossRef]
- Horikawa, M.; Fujiki, T.; Shirosaki, T.; Ryu, N.; Sakurai, H.; Nagaoka, S.; Ihara, H. The development of a highly conductive PEDOT system by doping with partially crystalline sulfated cellulose and its electric conductivity. J. Mater. Chem. C 2015, 3, 8881–8887. [Google Scholar] [CrossRef]
- Ion, R.M.; Scarlat, F.; Scarlat, F.; Niculescu, V.I.R. Methylene—Blue modified polypyrrole film electrode for optoelectronic applications. J. Optoelectron. Adv. Mater. 2003, 5, 109–115. [Google Scholar]
- Godwin, J.M.; Evitts, R.W.; Kennell, G.F. Microbial fuel cell with a polypyrrole/poly(methylene blue) composite electrode. Rep. Electrochem. 2012, 2, 3–11. [Google Scholar]
- Godwin, J.M.; Evitts, R. Polypyrrole/Poly(Methylene Blue) Composite Electrode Films on Stainless Steel. ECS Trans. 2019, 33, 181–188. [Google Scholar] [CrossRef]
- Vy Phan, T.T.; Bharathiraja, S.; Nguyen, V.T.; Moorthy, M.S.; Manivasagan, P.; Lee, K.D.; Oh, J. Polypyrrole–methylene blue nanoparticles as a single multifunctional nanoplatform for near-infrared photo-induced therapy and photoacoustic imaging. RSC Adv. 2017, 7, 35027–35037. [Google Scholar] [CrossRef] [Green Version]
- Somani, P.R.; Radhakrishnan, S. Sensitization effect in doped and undoped state of polypyrrole by methylene blue in solid state electrochemical cells. Chem. Phys. Lett. 2003, 379, 401–405. [Google Scholar] [CrossRef]
- Boguzaite, R.; Ratautaite, V.; Mikoliunaite, L.; Pudzaitis, V.; Ramanaviciene, A.; Ramanavicius, A. Towards analytical application of electrochromic polypyrrole layers modified by phenothiazine derivatives. J. Electroanal. Chem. 2021, 886, 115132. [Google Scholar] [CrossRef]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar]
- Britton, H.T.S.; Robinson, R.A. CXCVIII.—Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]
- Ion, R.M.; Blair, D.F.; Radovici, O. Spectral properties of methylene blue modified polypyrrole film. J. Serb. Chem. Soc. 1997, 62, 1063–1068. [Google Scholar]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers—Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef] [PubMed]
- Spãtaru, N.; Sarada, B.V.; Tryk, D.A.; Fujishima, A. Anodic Voltammetry of Xanthine, Theophylline, Theobromine and Caffeine at Conductive Diamond Electrodes and Its Analytical Application. Electroanalysis 2002, 14, 721–728. [Google Scholar] [CrossRef]
- Matissek, R. Evaluation of xanthine derivatives in chocolate—Nutritional and chemical aspects. Z. Lebensm Unters Forsch. A 1997, 205, 175–184. [Google Scholar] [CrossRef]
- Ulyanova, Y.V.; Blackwell, A.E.; Minteer, S.D. Poly(methylene green) employed as molecularly imprinted polymer matrix for electrochemical sensing. Analyst 2006, 131, 257–261. [Google Scholar] [CrossRef]
- Bukkitgar, S.D.; Shetti, N.P. Electrochemical behavior of theophylline at methylene blue dye modified electrode and its analytical application. Mater. Today Proc. 2018, 5, 21474–21481. [Google Scholar] [CrossRef]
- Baleviciute, I.; Ratautaite, V.; Ramanaviciene, A.; Balevicius, Z.; Broeders, J.; Croux, D.; McDonald, M.; Vahidpour, F.; Thoelen, R.; Ceuninck, W.D.; et al. Evaluation of theophylline imprinted polypyrrole film. Synth. Met. 2015, 209, 206–211. [Google Scholar] [CrossRef]
- Kim, J.M.; Yang, J.C.; Park, J.Y. Quartz crystal microbalance (QCM) gravimetric sensing of theophylline via molecularly imprinted microporous polypyrrole copolymers. Sens. Actuators B 2015, 206, 50–55. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lee, U.-H.; Chang, S.-M.; Park, J.Y. Gravimetric detection of theophylline on pore-structured molecularly imprinted conducting polymer. Sens. Actuators B 2014, 200, 25–30. [Google Scholar] [CrossRef]
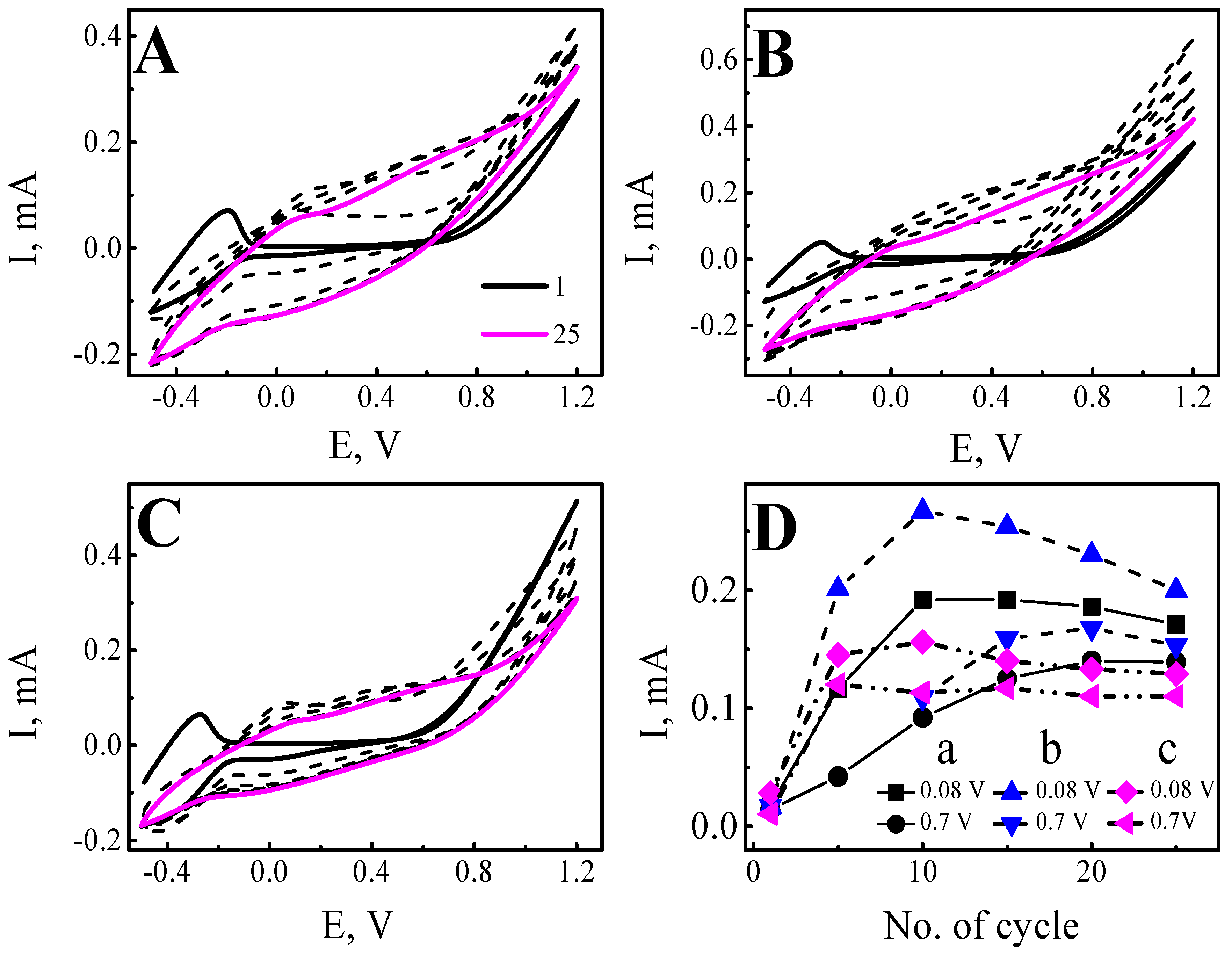
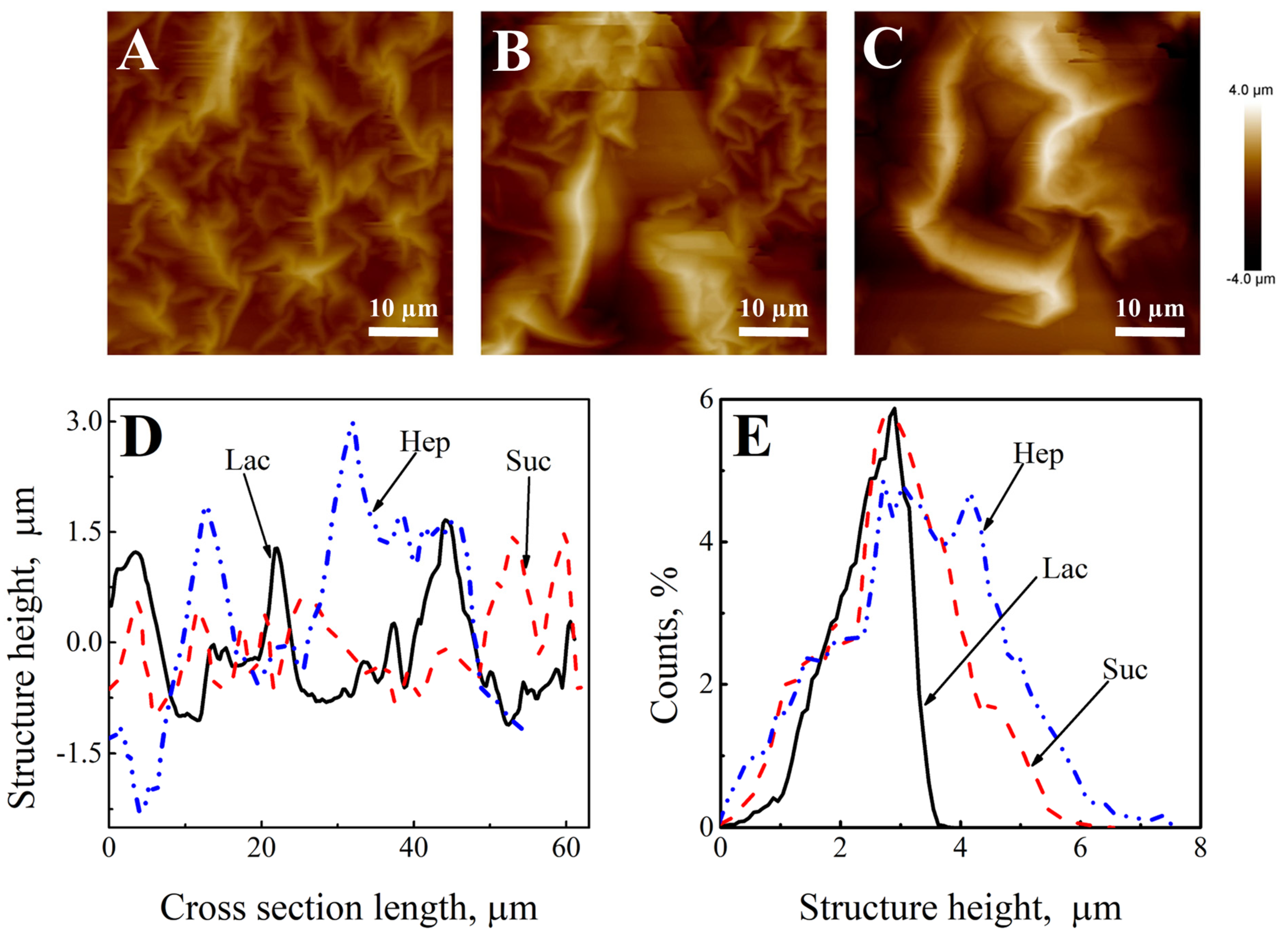
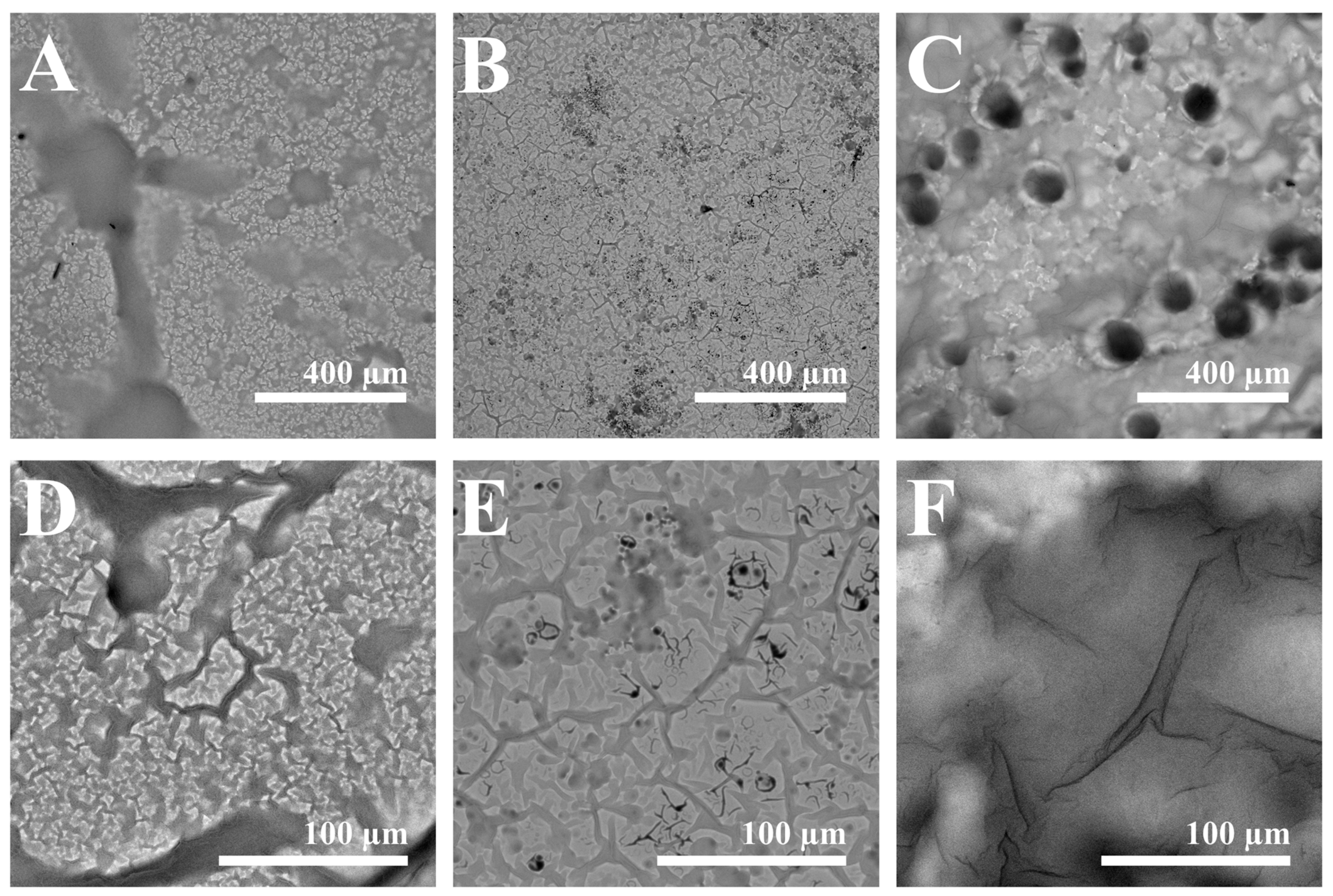

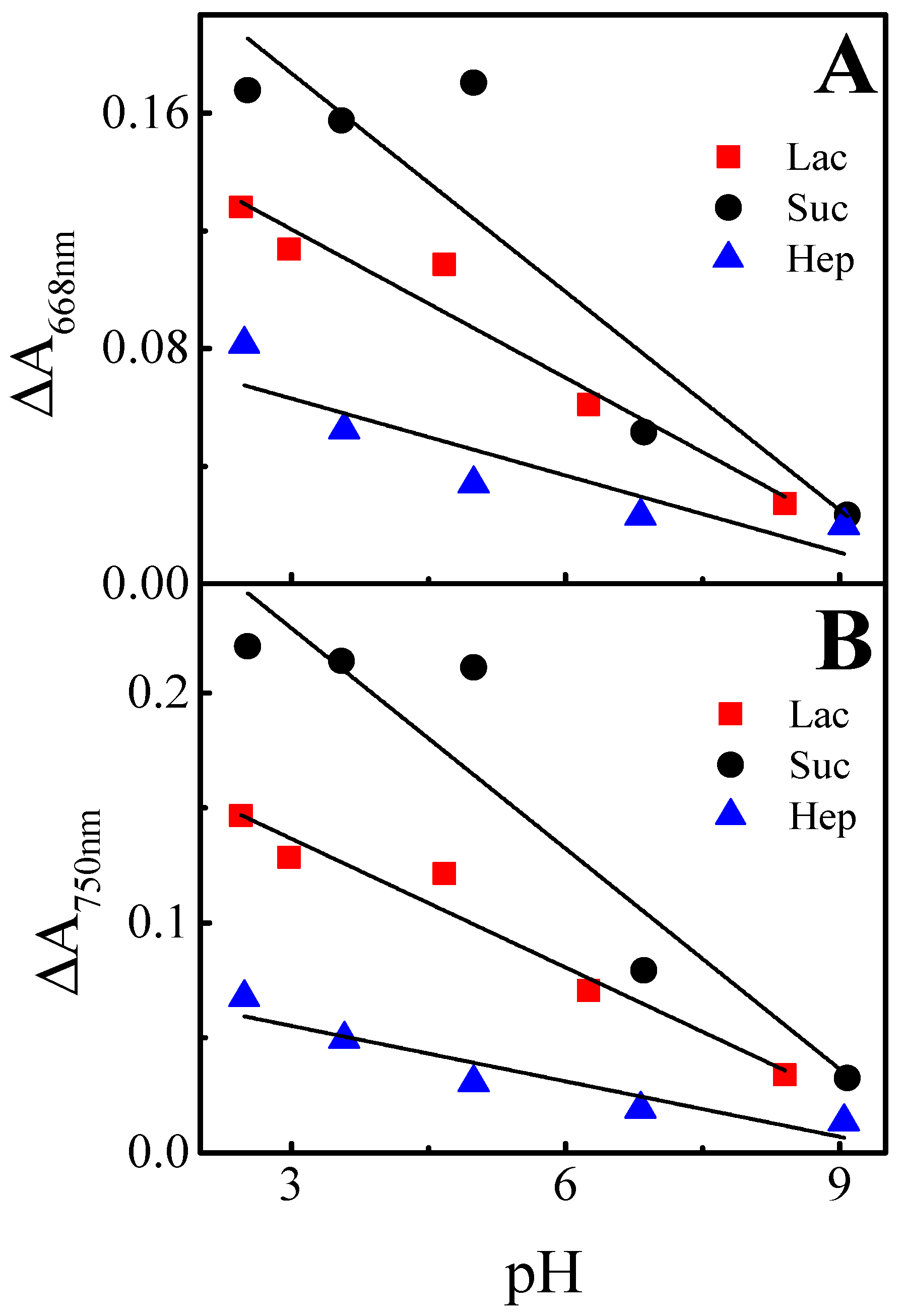

| Wavelength | Layer | Slope | Intercept | R2 |
|---|---|---|---|---|
| 668 nm | (Ppy-PMB)Lac | −0.017 ± 0.002 | 0.171 ± 0.001 | 0.935 |
| (Ppy-PMB)Suc | −0.025 ± 0.006 | 0.248 ± 0.036 | 0.790 | |
| (Ppy-PMB)Hep | −0.009 ± 0.002 | 0.089 ± 0.0145 | 0.742 | |
| 750 nm | (Ppy-PMB)Lac | −0.019 ± 0.002 | 0.193 ± 0.012 | 0.944 |
| (Ppy-PMB)Suc | −0.032 ± 0.006 | 0.324 ± 0.038 | 0.857 | |
| (Ppy-PMB)Hep | −0.008 ± 0.002 | 0.079 ± 0.010 | 0.851 |
| Slope | Intercept | R2 | ||
|---|---|---|---|---|
| ΔI | Theophylline | −0.007 ± 0.0005 | 10.1 ± 0.079 | 0.973 |
| Caffeine | −0.005 ± 0.0004 | 9.35 ± 0.059 | 0.969 | |
| Theobromine | −0.008 ± 0.0021 | 9.48 ± 0.082 | 0.872 | |
| 668 nm | Theophylline | −0.0002 ± 0.00001 | 0.13 ± 0.002 | 0.977 |
| Caffeine | −0.0001 ± 0.00001 | 0.07 ± 0.001 | 0.978 | |
| Theobromine | −0.0005 ± 0.00009 | 0.14 ± 0.003 | 0.939 | |
| 750 nm | Theophylline | −0.0002 ± 0.00001 | 0.17 ± 0.001 | 0.990 |
| Caffeine | −0.0001 ± 0.00001 | 0.08 ± 0.001 | 0.958 | |
| Theobromine | −0.0003 ± 0.00008 | 0.14 ± 0.003 | 0.884 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratautaite, V.; Boguzaite, R.; Mickeviciute, M.B.; Mikoliunaite, L.; Samukaite-Bubniene, U.; Ramanavicius, A.; Ramanaviciene, A. Evaluation of Electrochromic Properties of Polypyrrole/Poly(Methylene Blue) Layer Doped by Polysaccharides. Sensors 2022, 22, 232. https://doi.org/10.3390/s22010232
Ratautaite V, Boguzaite R, Mickeviciute MB, Mikoliunaite L, Samukaite-Bubniene U, Ramanavicius A, Ramanaviciene A. Evaluation of Electrochromic Properties of Polypyrrole/Poly(Methylene Blue) Layer Doped by Polysaccharides. Sensors. 2022; 22(1):232. https://doi.org/10.3390/s22010232
Chicago/Turabian StyleRatautaite, Vilma, Raimonda Boguzaite, Migle Beatrice Mickeviciute, Lina Mikoliunaite, Urte Samukaite-Bubniene, Arunas Ramanavicius, and Almira Ramanaviciene. 2022. "Evaluation of Electrochromic Properties of Polypyrrole/Poly(Methylene Blue) Layer Doped by Polysaccharides" Sensors 22, no. 1: 232. https://doi.org/10.3390/s22010232








