Preparation and Research of a High-Performance ZnO/SnO2 Humidity Sensor
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of ZnO
2.3. Synthesis of ZnO/SnO2 Composites
2.4. Characterizations
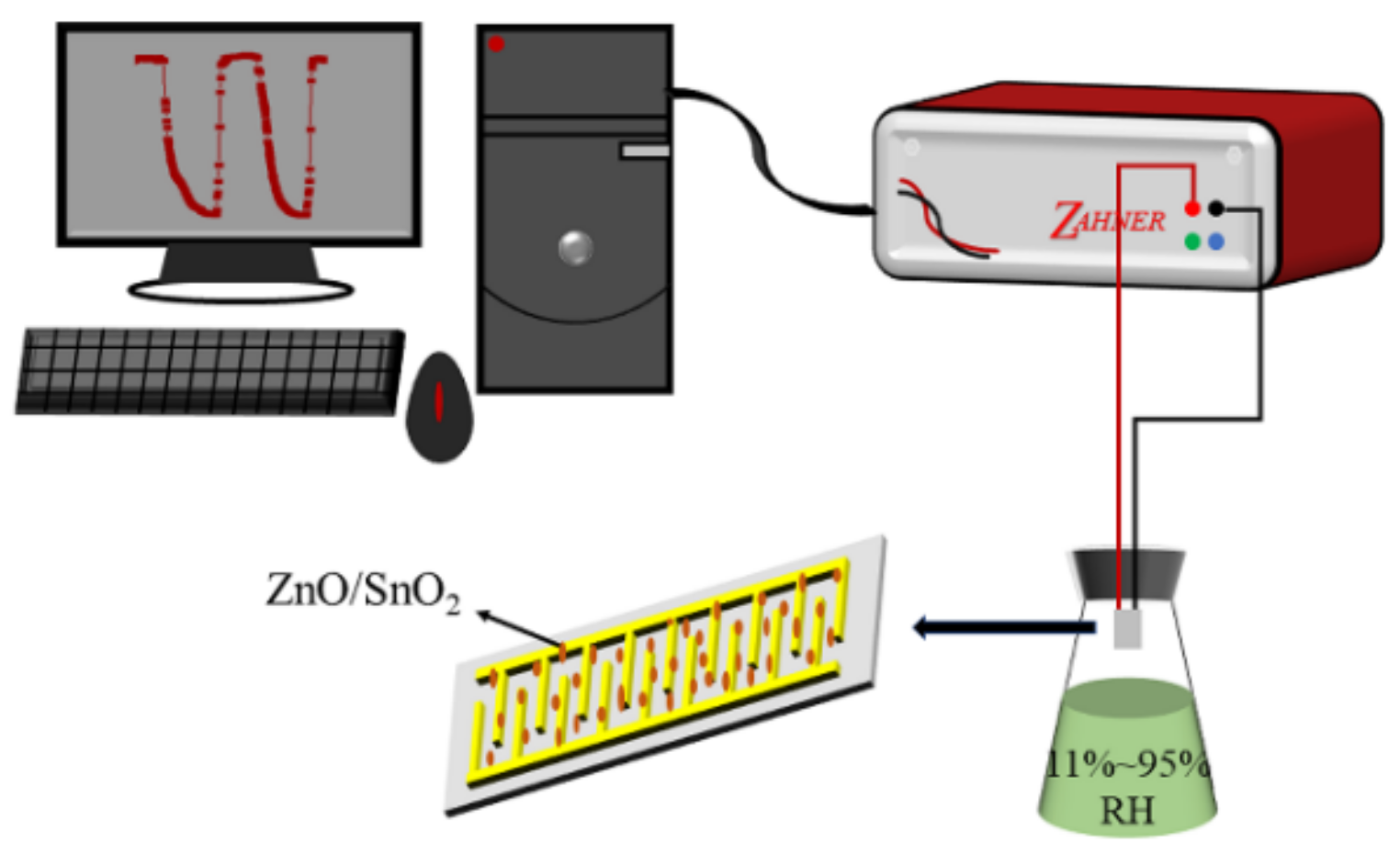
2.5. Design of Humidity Sensor
3. Results and Discussion
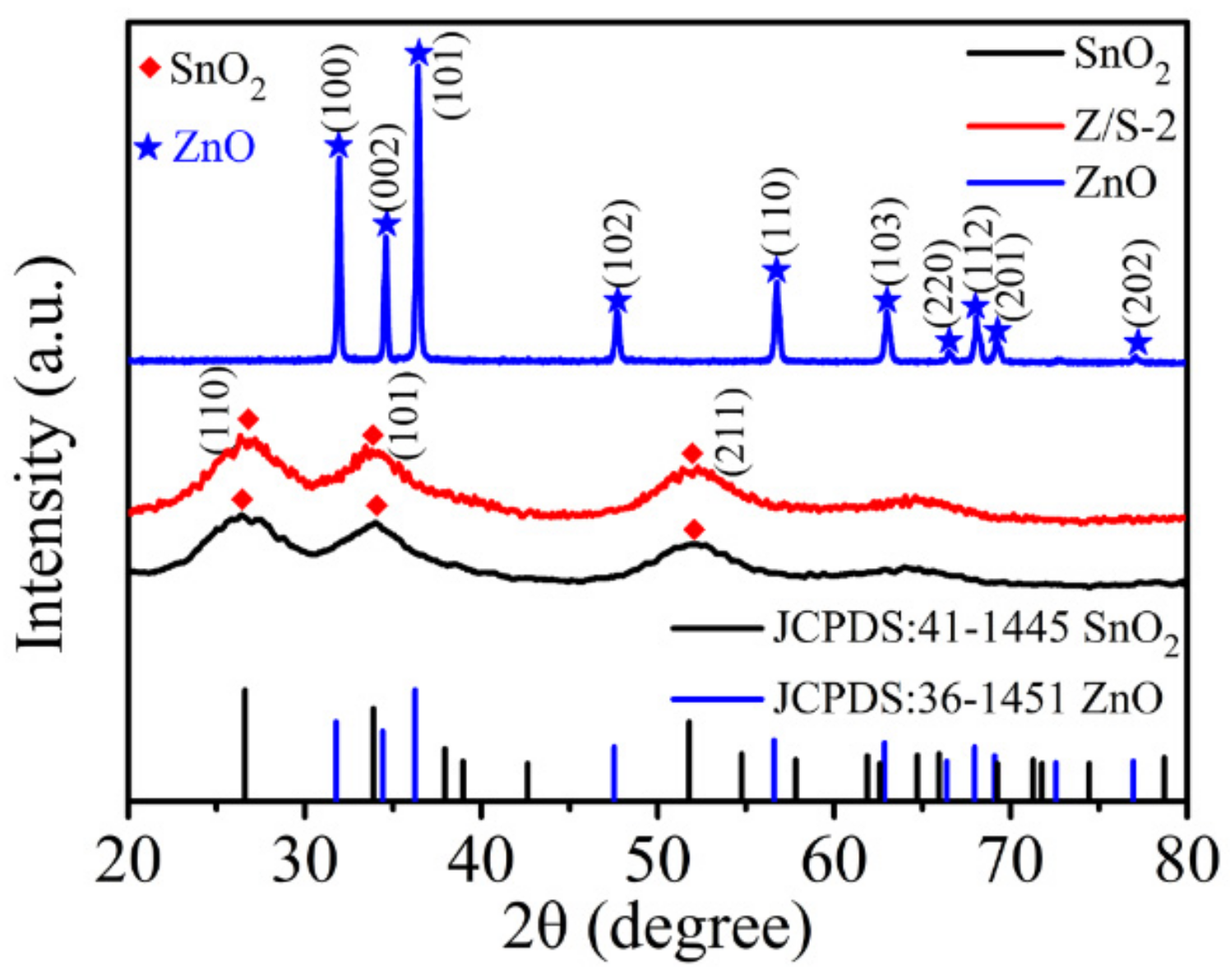
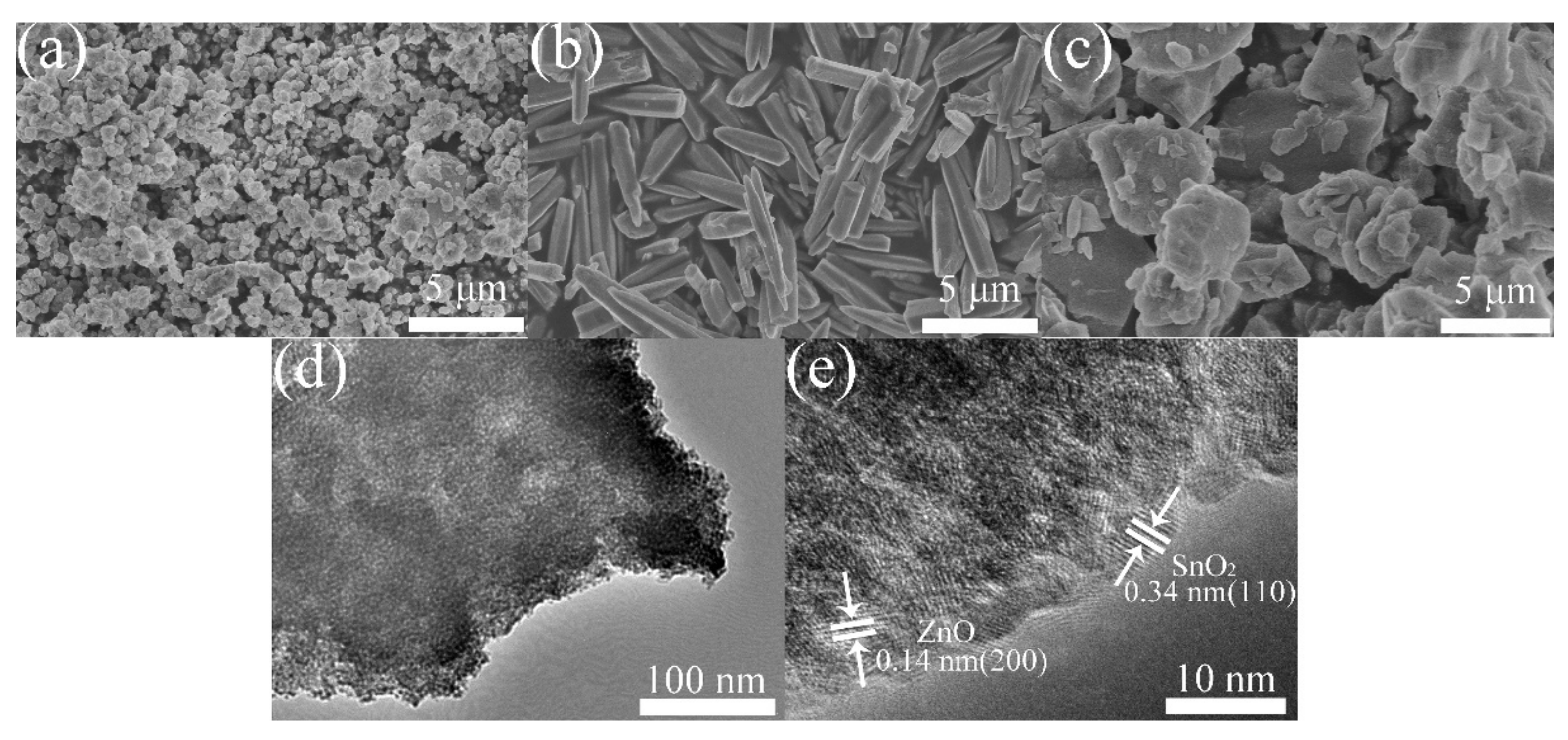
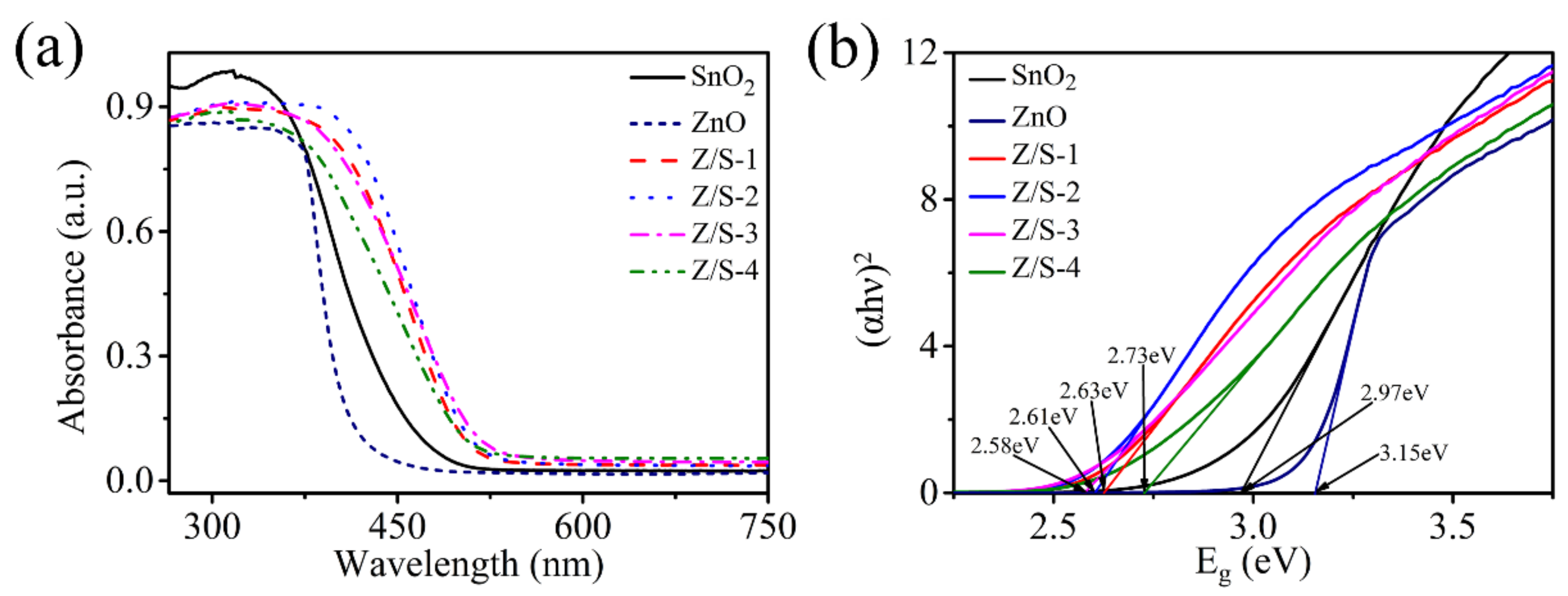
3.1. Material Characterization
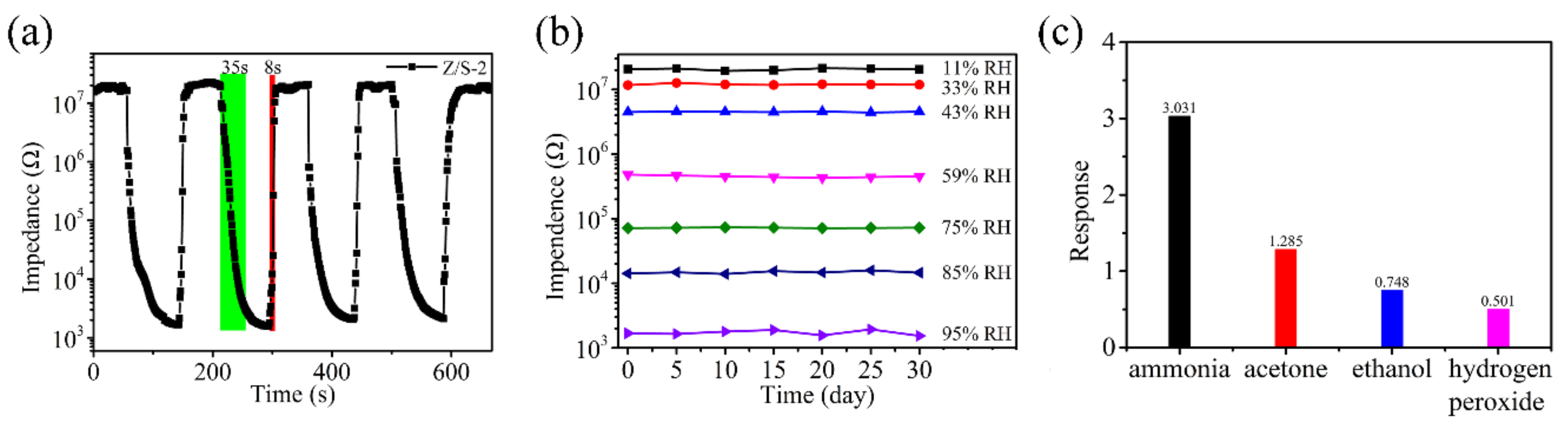
3.2. Sensor Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Sun, Y.E.; Li, P.; Zhang, Y. Facile Fabrication of MoS2-Modified SnO2 Hybrid Nanocomposite for Ultrasensitive Humidity Sensing. ACS Appl. Mater. Interfaces 2016, 8, 14142. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Jiang, Y.; Yan, M.; Wang, S.; Tai, H. Facile, Flexible, Cost-Saving and Environment-Friendly Paper-Based Humidity Sensor for Multifunctional Applications. ACS Appl. Mater. Interfaces 2019, 11, 21840–21849. [Google Scholar] [CrossRef] [PubMed]
- Mogera, U.; Sagade, A.A.; George, S.J.; Kulkarni, G.U. Ultrafast response humidity sensor using supramolecular nanofibre and its application in monitoring breath humidity and flow. Sci. Rep. 2014, 4, 4103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, M.; Wuzella, G.; Lammer, H.; Mahendran, A.R. Smart paper from graphene coated cellulose for high-performance humidity and piezoresistive force sensor. Synth. Metals 2020, 266, 116420. [Google Scholar] [CrossRef]
- Dwiputra, M.A.; Fadhila, F.; Imawan, C.; Fauzia, V. The enhanced performance of capacitive-type humidity sensors based on ZnO nanorods/WS2 nanosheets heterostructure. Sens. Actuators 2020, 310, 127810. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, Q.; Wang, S.; Huang, Q.; Yuan, Z.; Zhang, Y.; Jiang, Y.; Tai, H. Halloysite nanotubes: Natural, environmental-friendly and low-cost nanomaterials for high-performance humidity sensor-ScienceDirect. Sens. Actuators B Chem. 2020, 317, 128204. [Google Scholar] [CrossRef]
- Yang, L.; Chao, D.; Yang, M. A novel surface acoustic wave-impedance humidity sensor based on the composite of polyaniline and poly(vinyl alcohol) with a capability of detecting low humidity. Sens. Actuators B Chem. 2012, 165, 7–12. [Google Scholar]
- Borini, S.; White, R.; Wei, D.; Astley, M.; Haque, S.; Spigone, E.; Harris, N.; Kivioja, J.; Ryh Nen, T. Ultrafast Graphene Oxide Humidity Sensors. Acs Nano 2013, 7, 11166–11173. [Google Scholar] [CrossRef]
- Xie, J.; Hu, W.; Lin, Y.; Ying, Z.; Wu, Y. Highly sensitive humidity sensor based on quartz crystal microbalance coated with ZnO colloid spheres. Sens. Actuators B Chem. 2013, 177, 1083–1088. [Google Scholar] [CrossRef]
- Liang, F.; Luo, L.-B.; Tsang, C.-K.; Zheng, L.; Cheng, H.; Li, Y.Y. TiO2 nanotube-based field effect transistors and their application as humidity sensors. Mater. Res. Bull. 2012, 47, 54–58. [Google Scholar] [CrossRef]
- Bda, B.; Dya, B.; Xsa, B.; Yuan, Y.; Dong, M.; Yja, B.; Fla, B. MoS2-based all-fiber humidity sensor for monitoring human breath with fast response and recovery. Sens. Actuators B Chem. 2017, 251, 180–184. [Google Scholar]
- Zhang, D.; Tong, J.; Xia, B.; Xue, Q. Ultrahigh performance humidity sensor based on layer-by-layer self-assembly of graphene oxide/polyelectrolyte nanocomposite film. Sens. Actuators B Chem. 2014, 203, 263–270. [Google Scholar] [CrossRef]
- Yu, S.; Chen, C.; Zhang, H.; Zhang, J.; Liu, J. Design of High Sensitivity Graphite Carbon Nitride/Zinc Oxide Humidity Sensor for Breath Detection. Sens. Actuators B Chem. 2021, 332, 129536. [Google Scholar] [CrossRef]
- Pawar, M.S.; Late, D.J. Temperature-dependent Raman spectroscopy and sensor applications of PtSe 2 nanosheets synthesized by wet chemistry. Beilstein J. Nanotechnol. 2019, 10, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Xiao, S.; Li, H.; Wang, Y.; Cai, D.; Wang, D.; Wang, L.; Liu, Y.; Li, Q. High performance humidity sensors based on CeO2 nanoparticles. Sens. Actuators B Chem. 2015, 215, 125–132. [Google Scholar]
- Si, R.; Xie, X.; Li, T.; Zheng, J.; Cheng, C.; Huang, S.; Wang, C. TiO2/(K,Na)NbO3 Nanocomposite for Boosting Humidity-Sensing Performances. ACS Sens. 2020, 5, 1345–1353. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H.; Park, S.Y.; Kang, J.H.; Kim, S.J.; Choi, Y.B.; Shin, U.S. Carbon nanotubes immobilized on gold electrode as an electrochemical humidity sensor. Sens. Actuators 2019, 300, 127049. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Sysoev, V.I.; Lobiak, E.V.; Fedoseeva, Y.V.; Makarova, A.A.; Dubois, M.; Flahaut, E.; Okotrub, A.V. Chlorinated holey double-walled carbon nanotubes for relative humidity sensor. Carbon 2019, 148, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Xu, Q.; Xu, H.; Cao, B. Highly sensitive gold-decorated zinc oxide nanorods sensor for triethylamine working at near room temperature. J. Coll. Interface Sci. 2017, 499, 67. [Google Scholar] [CrossRef]
- Gao, N.; Li, H.-Y.; Zhang, W.; Zhang, Y.; Zeng, Y.; Zhixiang, H.; Liu, J.; Jiang, J.; Miao, L.; Yi, F.; et al. QCM-based humidity sensor and sensing properties employing colloidal SnO2 nanowires. Sens. Actuators B Chem. 2019, 293, 129–135. [Google Scholar] [CrossRef]
- Yalei, Z.; Bin, Y.; Jingquan, L. Effect of interdigital electrode gap on the performance of SnO2-modified MoS2 capacitive humidity sensor. Sens. Actuators B Chem. 2018, 271, 256–263. [Google Scholar]
- Tawale, J.S.; Gupta, G.; Mohan, A.; Kumar, A.; Srivastava, A.K. Growth of thermally evaporated SnO2 nanostructures for optical and humidity sensing application. Sens. Actuators B Chem. 2014, 201, 369–377. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S.; Sharma, A.K.; Malik, R.; Nehra, S.P.; Devi, S. One pot synthesis of mesoporous ZnO–SiO2 nanocomposite as high performance humidity sensor. Coll. Surf. A Physicochem. Eng. Asp. 2015, 483, 121–128. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Chen, C.; Lin, C. Investigation of humidity sensor based on Au modified ZnO nanosheets via hydrothermal method and first principle. Sens. Actuators B Chem. 2019, 287, 526–534. [Google Scholar] [CrossRef]
- Mahmood, H.; Khan, M.A.; Mohuddin, B.; Iqbal, T. Solution-phase growth of tin oxide (SnO2) nanostructures: Structural, optical and photocatalytic properties. Mater. Sci. Eng. B 2020, 258, 114568. [Google Scholar] [CrossRef]
- Khudiar, A.I.; Khalaf, M.K.; Ofui, A.M. Improvement of the sensing characterizations of ZnO nanostructure by using thermal annealing prepared through R. F. magnetron sputtering technique. Opt. Mater. 2021, 114, 110885. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Guo, Y.; Lv, X.; Dou, X. 2D TiO2 nanosheets for ultrasensitive humidity sensing application benefited by abundant surface oxygen vacancy defects. Sens. Actuators B Chem. 2018, 262, 350–358. [Google Scholar] [CrossRef]
- Ma, X.; Song, H.; Guan, C. Enhanced ethanol sensing properties of ZnO-doped porous SnO2 hollow nanospheres. Sens. Actuators B Chem. 2013, 188, 193–199. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hsueh, Y.C.; Lee, P.S.; Wang, C.; Wu, J.M.; Perng, T.P.; Shih, H.C. Fabrication of tin dioxide nanowires with ultrahigh gas sensitivity by atomic layer deposition of platinum. J. Mater. Chem. 2011, 21, 10552–10558. [Google Scholar] [CrossRef]
- Babar, A.R.; Shinde, S.S.; Moholkar, A.V.; Bhosale, C.H.; Kim, J.H.; Rajpure, K.Y. Structural and optoelectronic properties of antimony incorporated tin oxide thin films. J. Alloys Compd. 2010, 505, 416–422. [Google Scholar] [CrossRef]
- Li, H.; Chu, S.; Ma, Q.; Li, H.; Yang, P. Multi-Level Effective Heterojunctions Based on SnO2/ZnO 1D Fibrous Hierarchical Structure with Unique Interface Electronic Effects. ACS Appl. Mater. Interfaces 2019, 11, 31551–31561. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Tang, P.; Wang, Y.; Feng, Y.; Chen, A.; Luo, R.; Li, D. Facile synthesis and gas sensing properties of tubular hierarchical ZnO self-assembled by porous nanosheets. Sens. Actuators B Chem. 2015, 215, 231–240. [Google Scholar] [CrossRef]
- Gharagozlou, M.; Naghibi, S. Sensitization of ZnO nanoparticle by vitamin B12: Investigation of microstructure, FTIR and optical properties. Mater. Res. Bull. 2016, 84, 71–78. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, R.; Chauhan, M.S.; Al-Hadeethi, Y. ZnO–SnO2 nanocubes for fluorescence sensing and dye degradation applications. Ceram. Int. 2020, 47, 3201–3210. [Google Scholar] [CrossRef]
- Cebi, N.; Sagdic, O.; Arici, M. The Famous Turkish Rose Essential Oil: Characterization and Authenticity Monitoring by FTIR, Raman and GC–MS Techniques Combined with Chemometrics; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Lan, G.; Li, J.; Zhang, G.; Ruan, J.; Lu, Z.; Jin, S.; Cao, D.; Wang, J. Thermal decomposition mechanism study of 3-nitro-1,2,4-triazol-5-one (NTO): Combined TG-FTIR-MS techniques and ReaxFF reactive molecular dynamics simulations. Fuel 2021, 295, 102655. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Z.; Qi, D.; Zhao, C. High sensitive and fast response humidity sensor based on polymer composite nanofibers for breath monitoring and non-contact sensing. Sens. Actuators B Chem. 2020, 330, 129239. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Hariharan, V.; Sekar, C. High-sensitivity humidity sensor based on SnO2 nanoparticles synthesized by microwave irradiation method. Mater. Sci. Eng. C 2011, 31, 840–844. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, J.; Li, H.; Zhu, Y.; Xu, J. Synthesis of mesoporous SnO2–SiO2 composites and their application as quartz crystal microbalance humidity sensor. Sens. Actuators B Chem. 2014, 193, 320–325. [Google Scholar] [CrossRef]
- Toloman, D.; Popa, A.; Stan, M.; Socaci, C.; Biris, A.R.; Katona, G.; Tudorache, F.; Petrila, I.; Iacomi, F. Reduced graphene oxide decorated with Fe doped SnO2 nanoparticles for humidity sensor. Appl. Surf. Sci. 2017, 402, 410–417. [Google Scholar] [CrossRef]
- Pascariu, P.; Airinei, A.; Olaru, N.; Petrila, I.; Nica, V.; Sacarescu, L.; Tudorache, F. Microstructure, electrical and humidity sensor properties of electrospun NiO–SnO2 nanofibers. Sens. Actuators B Chem. 2016, 222, 1024–1031. [Google Scholar] [CrossRef]
- Zhang, J.; Zhen, Y.; Xue, H.; Gao, X.; Wang, W.; Li, Y.; Alharbi, N.S. An urchin-like SnO2/NaNbO3 nanocomposite with stable humidity-sensing properties at room temperature. Sens. Actuators B Chem. 2019, 283, 643–650. [Google Scholar] [CrossRef]
- Jiang, K.; Zhao, H.; Dai, J.; Kuang, D.; Fei, T.; Zhang, T. Excellent Humidity Sensor Based on LiCl Loaded Hierarchically Porous Polymeric Microspheres. ACS Appl Mater. Interfaces 2016, 8, 25529–25534. [Google Scholar] [CrossRef]
- Zhang, D.; Zong, X.; Wu, Z.; Yong, Z. Hierarchical Self-Assembled SnS2 Nanoflower/Zn2SnO4 Hollow Sphere Nanohybrid for Humidity Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 32631–32639. [Google Scholar] [CrossRef]
- Song, X.; Qi, Q.; Tong, Z.; Wang, C. A humidity sensor based on KCl-doped SnO2 nanofibers. Sens. Actuators B Chem. 2009, 138, 368–373. [Google Scholar] [CrossRef]

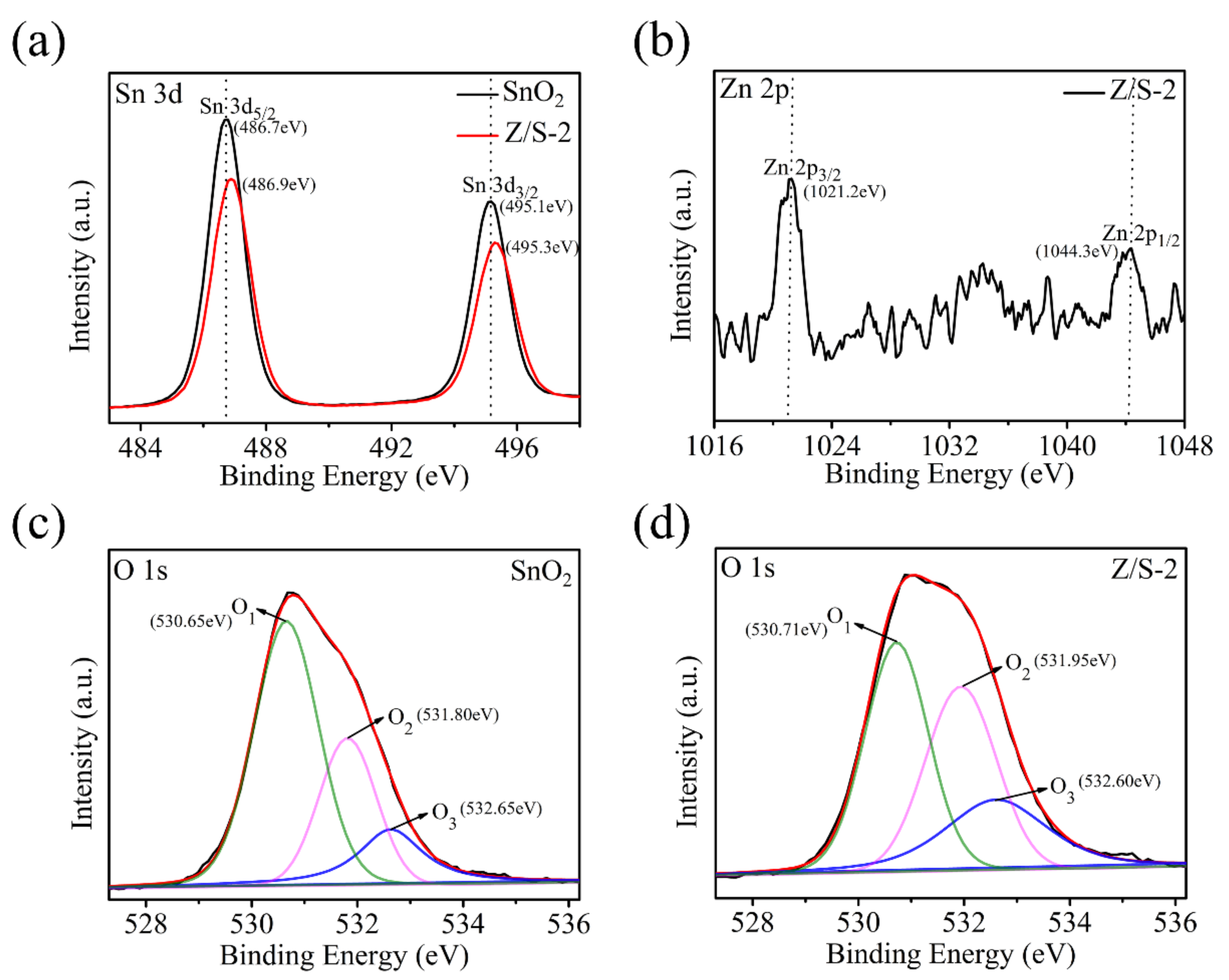
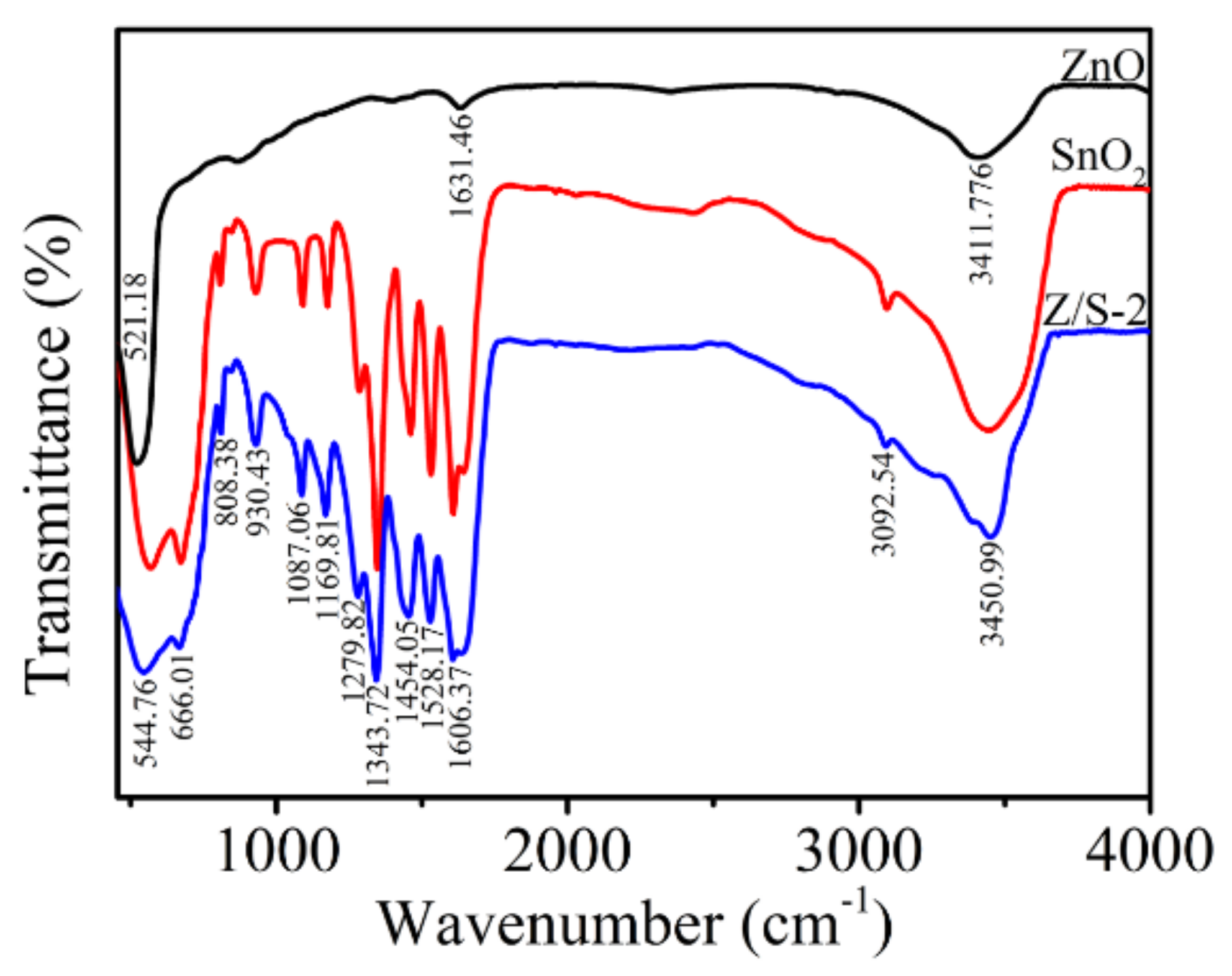
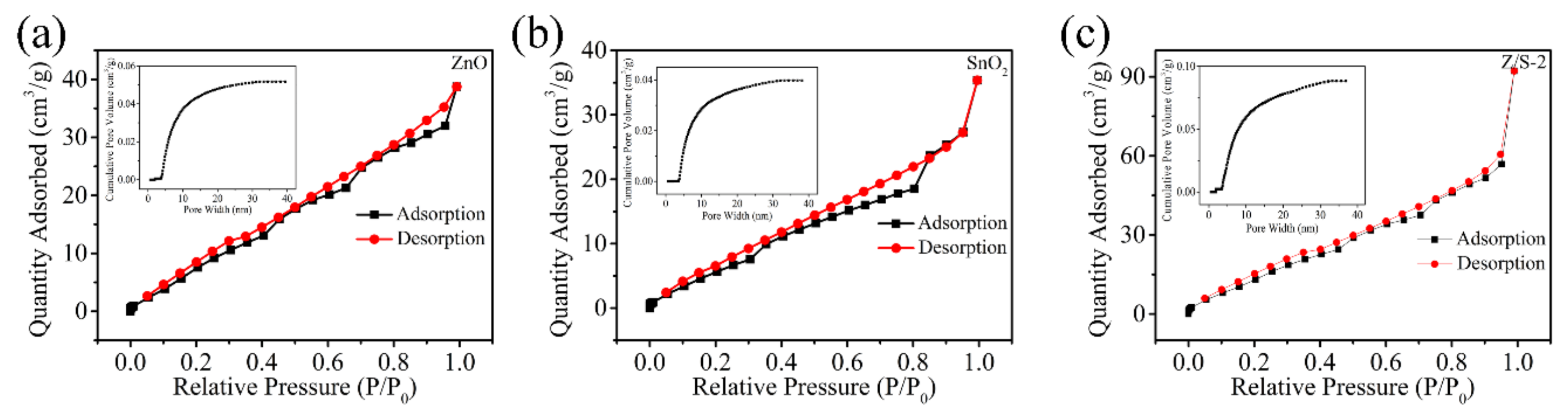
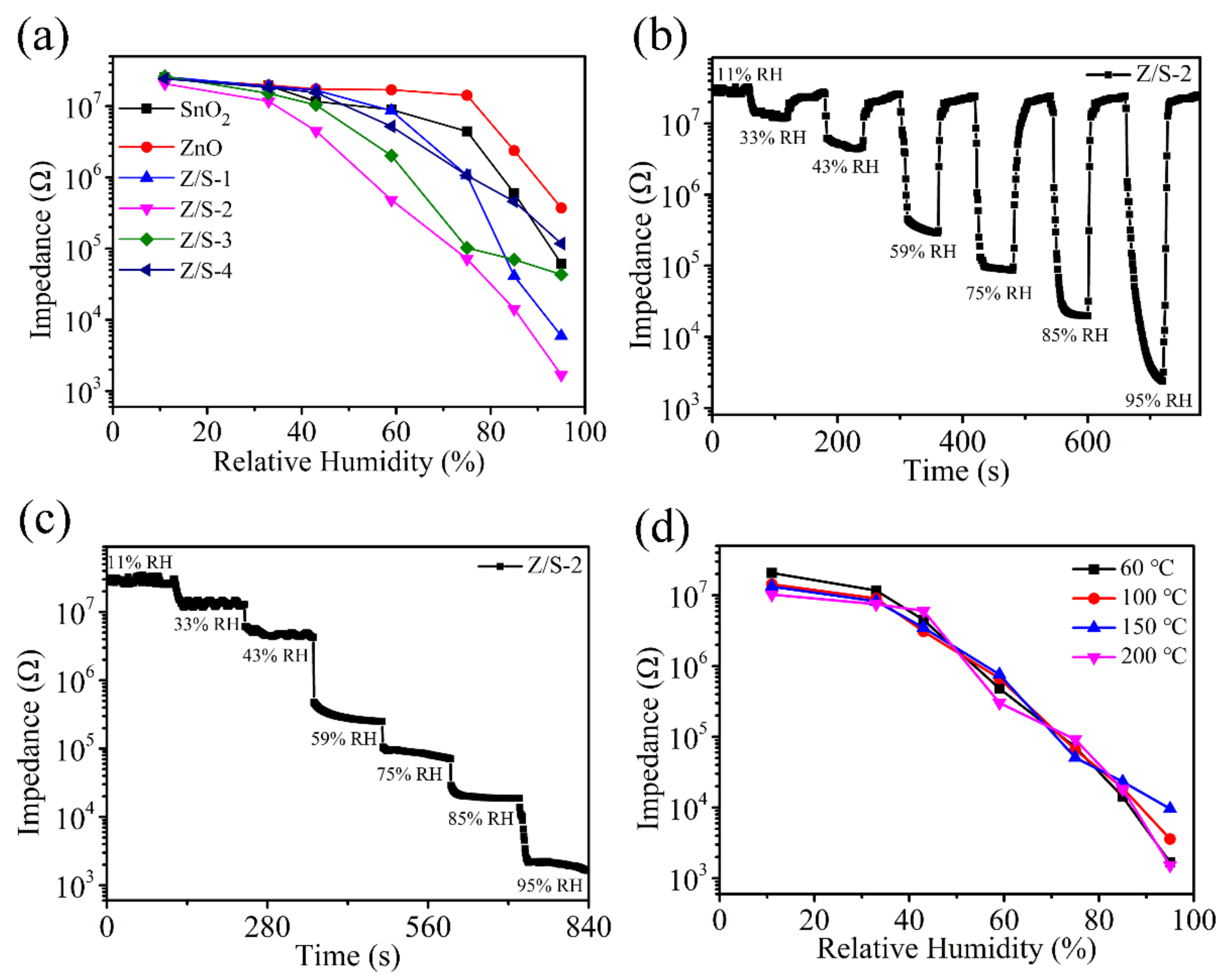


| Sample | Oxygen Species | Binding Energy (eV) | Relative Percentage (%) |
|---|---|---|---|
| SnO2 | O1 | 530.65 | 56.27 |
| O2 | 531.80 | 26.83 | |
| O3 | 532.65 | 16.90 | |
| Z/S-2 | O1 | 530.71 | 40.57 |
| O2 | 531.95 | 35.22 | |
| O3 | 532.60 | 24.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Li, P.; Zhang, H. Preparation and Research of a High-Performance ZnO/SnO2 Humidity Sensor. Sensors 2022, 22, 293. https://doi.org/10.3390/s22010293
Li F, Li P, Zhang H. Preparation and Research of a High-Performance ZnO/SnO2 Humidity Sensor. Sensors. 2022; 22(1):293. https://doi.org/10.3390/s22010293
Chicago/Turabian StyleLi, Fan, Peng Li, and Hongyan Zhang. 2022. "Preparation and Research of a High-Performance ZnO/SnO2 Humidity Sensor" Sensors 22, no. 1: 293. https://doi.org/10.3390/s22010293
APA StyleLi, F., Li, P., & Zhang, H. (2022). Preparation and Research of a High-Performance ZnO/SnO2 Humidity Sensor. Sensors, 22(1), 293. https://doi.org/10.3390/s22010293





