Cost Effective Synthesis of Graphene Nanomaterials for Non-Enzymatic Electrochemical Sensors for Glucose: A Comprehensive Review
Abstract
:1. Introduction
1.1. Glucose Electrooxidation Mechanism over Co-Oxides/Graphene (or Its Derivatives)-Based Nanomaterials
1.2. Glucose Electrooxidation over Ni-Oxide Graphene (or Its Derivatives) Electrocatalysts
1.3. Glucose Electrooxidation over Cu-Based Graphene (or Its Derivatives) Electrocatalysts
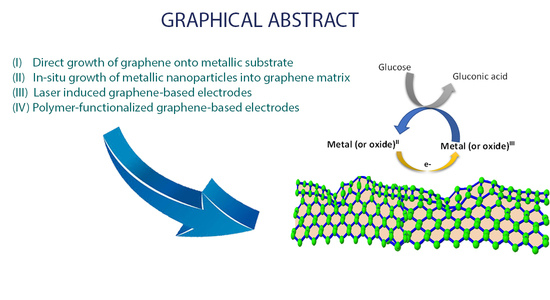
2. Graphene-Based Nanomaterials for Glucose Electrochemical Sensors
2.1. Direct Growth (or Deposition) of Graphene (or Its Oxides) onto Metallic Substrates
2.2. In-Situ Growth of Metallic Nanoparticles into Graphene Nanosheets

2.3. Laser-Induced Graphene-Based Electrode Synthesis and Functionalization
2.4. Polymer Functionalized Graphene-Based Electrodes

3. Challenges and Future Perspectives
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brouzgou, A.; Yan, L.L.; Song, S.Q.; Tsiakaras, P. Glucose electrooxidation over PdxRh/C electrocatalysts in alkaline medium. Appl. Catal. B Environm. 2014, 147, 481–489. [Google Scholar] [CrossRef]
- Yan, L.; Brouzgou, A.; Meng, Y.; Xiao, M.; Tsiakaras, P.; Song, S. Efficient and poison-tolerant PdxAuy/C binary electrocatalysts for glucose electrooxidation in alkaline medium. Appl. Catal. B Environm. 2014, 150–151, 268–274. [Google Scholar] [CrossRef]
- Brouzgou, A.; Song, S.; Tsiakaras, P. Carbon-supported PdSn and Pd3Sn2 anodes for glucose electrooxidation in alkaline media. Appl. Catal. B Environm. 2014, 158–159, 209–216. [Google Scholar] [CrossRef]
- Song, S.; Wang, K.; Yan, L.; Brouzgou, A.; Zhang, Y.; Wang, Y.; Tsiakaras, P. Ceria promoted Pd/C catalysts for glucose electrooxidation in alkaline media. Appl. Catal. B Environm. 2015, 176–177, 233–239. [Google Scholar] [CrossRef]
- Saleh, T.A.; Fadillah, G. Recent trends in the design of chemical sensors based on graphene–metal oxide nanocomposites for the analysis of toxic species and biomolecules. TrAC Trends Anal. Chem. 2019, 120, 115660. [Google Scholar] [CrossRef]
- Mohd Yazid, S.N.A.; Md Isa, I.; Abu Bakar, S.; Hashim, N.; Ab Ghani, S. A review of glucose biosensors based on graphene/metal oxide nanomaterials. Anal. Lett. 2014, 47, 1821–1834. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Yang, Q.; Chen, W. Graphene-based Electrochemical Glucose Sensors: Fabrication and Sensing Properties. Electroanalysis 2018, 30, 2504–2524. [Google Scholar] [CrossRef]
- Brouzgou, A.; Lo Vecchio, C.; Baglio, V.; Aricò, A.S.; Liang, Z.X.; Demin, A.; Tsiakaras, P. Glucose electrooxidation reaction in presence of dopamine and uric acid over ketjenblack carbon supported PdCo electrocatalyst. J. Electroanal. Chem. 2019, 855, 113610. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energ. Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Vassilyev, Y.B.; Khazova, O.A.; Nikolaeva, N.N. Kinetics and mechanism of glucose electrooxidation on different electrode-catalysts: Part II. Effect of the nature of the electrode and the electrooxidation mechanism. J. Electroanal. Chem. Interf. Electrochem. 1985, 196, 127–144. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Reduced Graphene Oxide (rGO)-Loaded Metal-Oxide Nanofiber Gas Sensors: An Overview. Sensors 2021, 21, 1352. [Google Scholar] [CrossRef] [PubMed]
- Brouzgou, A.; Tsiakaras, P. Electrocatalysts for glucose electrooxidation reaction: A review. Top. Catal. 2015, 58, 1311–1327. [Google Scholar] [CrossRef]
- Brouzgou, A.; Gorbova, E.; Wang, Y.; Jing, S.; Seretis, A.; Liang, Z.; Tsiakaras, P. Nitrogen-doped 3D hierarchical ordered mesoporous carbon supported palladium electrocatalyst for the simultaneous detection of ascorbic acid, dopamine, and glucose. Ionics 2019, 25, 6061–6070. [Google Scholar] [CrossRef]
- Lei, H.-Y.; Piao, J.-H.; Brouzgou, A.; Gorbova, E.; Tsiakaras, P.; Liang, Z.-X. Synthesis of nitrogen-doped mesoporous carbon nanosheets for oxygen reduction electrocatalytic activity enhancement in acid and alkaline media. Int. J. Hydrogen Energy 2019, 44, 4423–4431. [Google Scholar] [CrossRef]
- Li, M.; Han, C.; Zhang, Y.; Bo, X.; Guo, L. Facile synthesis of ultrafine Co3O4 nanocrystals embedded carbon matrices with specific skeletal structures as efficient non-enzymatic glucose sensors. Anal. Chim. Acta 2015, 861, 25–35. [Google Scholar] [CrossRef]
- Chen, T.; Li, X.; Qiu, C.; Zhu, W.; Ma, H.; Chen, S.; Meng, O. Electrochemical sensing of glucose by carbon cloth-supported Co3O4/PbO2 core-shell nanorod arrays. Biosens. Bioelectron. 2014, 53, 200–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.-Q.; Bai, Y.; Li, X.; Chu, W. In situ formation of reduced graphene oxide@Co3O4-N-doped carbon and its structure-function relationship for glucose sensing. Appl. Surf. Sci. 2021, 539, 148235. [Google Scholar] [CrossRef]
- Irfan, M.; Liu, X.; Li, S.; Khan, I.U.; Li, Y.; Wang, J.; Wang, X.; Du, X.; Wang, G.; Zhang, P. High-performance glucose fuel cell with bimetallic Ni–Co composite anchored on reduced graphene oxide as anode catalyst. Renew. Energy 2020, 155, 1118–1126. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Mohammadi, A. Reduced graphene oxide nanosheets decorated with cobalt oxide nanoparticles: A nonenzymatic electrochemical approach for glucose detection. Synth. Met. 2020, 269, 116543. [Google Scholar] [CrossRef]
- Hwang, D.-W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Hoa, L.T.; Chung, J.S.; Hur, S.H. A highly sensitive enzyme-free glucose sensor based on Co3O4 nanoflowers and 3D graphene oxide hydrogel fabricated via hydrothermal synthesis. Sens. Actuators B Chem. 2016, 223, 76–82. [Google Scholar] [CrossRef]
- Jiang, D.; Chu, Z.; Peng, J.; Luo, J.; Mao, Y.; Yang, P.; Jin, W. One-step synthesis of three-dimensional Co(OH)2/rGO nano-flowers as enzyme-mimic sensors for glucose detection. Electrochim. Acta 2018, 270, 147–155. [Google Scholar] [CrossRef]
- Du, Y.; He, Y.; Zheng, Z.; Shen, X.; Zhou, Y.; Wang, T.; Zhu, Z.; Wang, C. A renewable platform for high-performance glucose sensor based on Co(OH)2 nanoparticles/three-dimensional graphene frameworks. J. Electrochem. Soc. 2019, 166, B42–B48. [Google Scholar] [CrossRef]
- Dong, M.; Hu, H.; Ding, S.; Wang, C.; Li, L. A facile synthesis of CoMn2O4 nanosheets on reduced graphene oxide for non-enzymatic glucose sensing. Nanotechnology 2020, 32, 055501. [Google Scholar] [CrossRef]
- Tang, B.; Ji, G.; Wang, Z.; Chen, H.; Li, X.; Yu, H.; Li, S.; Liu, H. Three-dimensional graphene networks and reduced graphene oxide nanosheets co-modified dye-sensitized solar cells. RSC Adv. 2017, 7, 45280–45286. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Yu, J.; Lei, Y.; Lu, S.; Wang, C.; Liu, D.; Guo, Q. Synthesis and characterization of nickel oxide hollow spheres–reduced graphene oxide–nafion composite and its biosensing for glucose. Sens. Actuators B Chem. 2015, 208, 90–98. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, L.; Zhang, J.; Dai, W.; Mo, G.; Ye, J. Bifunctional and highly sensitive electrochemical non-enzymatic glucose and hydrogen peroxide biosensor based on NiCo2O4 nanoflowers decorated 3D nitrogen doped holey graphene hydrogel. Mater. Sci. Eng. C 2019, 102, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Asadian, E.; Shahrokhian, S.; Iraji Zad, A. Highly sensitive nonenzymetic glucose sensing platform based on MOF-derived NiCo LDH nanosheets/graphene nanoribbons composite. J. Electroanal. Chem. 2018, 808, 114–123. [Google Scholar] [CrossRef]
- Yang, J.; Ye, H.; Zhang, Z.; Zhao, F.; Zeng, B. Metal–organic framework derived hollow polyhedron CuCo2O4 functionalized porous graphene for sensitive glucose sensing. Sens. Actuators B Chem. 2017, 242, 728–735. [Google Scholar] [CrossRef]
- Justice Babu, K.; Sheet, S.; Lee, Y.S.; Gnana kumar, G. Three-Dimensional Dendrite Cu–Co/Reduced Graphene Oxide Architectures on a Disposable Pencil Graphite Electrode as an Electrochemical Sensor for Nonenzymatic Glucose Detection. ACS Sustain. Chem. Eng. 2018, 6, 1909–1918. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Liao, Q.; Liu, S.; Kang, Z.; Zhang, Y. Nonenzymatic glucose sensor based on in situ reduction of Ni/NiO-graphene nanocomposite. Sensors 2016, 16, 1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurt Urhan, B.; Demir, Ü.; Öznülüer Özer, T.; Öztürk Doğan, H. Electrochemical fabrication of Ni nanoparticles-decorated electrochemically reduced graphene oxide composite electrode for non-enzymatic glucose detection. Thin Solid Films 2020, 693, 137695. [Google Scholar] [CrossRef]
- Darvishi, S.; Souissi, M.; Karimzadeh, F.; Kharaziha, M.; Sahara, R.; Ahadian, S. Ni nanoparticle-decorated reduced graphene oxide for non-enzymatic glucose sensing: An experimental and modeling study. Electrochim. Acta 2017, 240, 388–398. [Google Scholar] [CrossRef]
- Hao, J.; Li, C.; Wu, C.; Wu, K. In-situ synthesis of carbon-encapsulated Ni nanoparticles decorated graphene nanosheets with high reactivity toward glucose oxidation and sensing. Carbon 2019, 148, 44–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.-Q.; Bai, Y.; Chu, W.; Sh, J. Confinement preparation of hierarchical NiO-N-doped carbon@reduced graphene oxide microspheres for high-performance non-enzymatic detection of glucose. Sens. Actuators B Chem. 2020, 309, 127779. [Google Scholar] [CrossRef]
- Subash, V.S.; Alagumalai, K.; Chen, S.-M.; Shanmugam, R.; Shiuan, H.J. Ultrasonication assisted synthesis of NiO nanoparticles anchored on graphene oxide: An enzyme-free glucose sensor with ultrahigh sensitivity. New J. Chem. 2020, 44, 15071–15080. [Google Scholar] [CrossRef]
- Zhuang, X.; Tian, C.; Luan, F.; Wu, X.; Chen, L. One-step electrochemical fabrication of a nickel oxide nanoparticle/polyaniline nanowire/graphene oxide hybrid on a glassy carbon electrode for use as a non-enzymatic glucose biosensor. RSC Adv. 2016, 6, 92541–92546. [Google Scholar] [CrossRef]
- Dong, M.; Hu, H.; Ding, S.; Wang, C.; Li, L. Fabrication of NiMn2O4 nanosheets on reduced graphene oxide for non-enzymatic detection of glucose. Mater. Technol. 2020, 36, 203–211. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Xiang, Y.; Wang, D.; Zhang, P.; Wang, Y.; Lu, S.; Xu, R.; Zhao, J. A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene. Carbon 2020, 156, 506–513. [Google Scholar] [CrossRef]
- Khosroshahi, Z.; Karimzadeh, F.; Kharaziha, M.; Allafchian, A. A non-enzymatic sensor based on three-dimensional graphene foam decorated with Cu-xCu2O nanoparticles for electrochemical detection of glucose and its application in human serum. Mater. Sci. Eng. C 2020, 108, 110216. [Google Scholar] [CrossRef]
- Jeong, H.; Kwac, L.K.; Hong, C.G.; Kim, H.G. Direct growth of flower like-structured CuFe oxide on graphene supported nickel foam as an effective sensor for glucose determination. Mater. Sci. Eng. C 2021, 118, 111510. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bao, J.; Qi, Y.; Zhao, J.; Hu, Y.; Wu, W.; Wu, X.; Zhong, D.; Huo, D.; Hou, C. A disposable and sensitive non-enzymatic glucose sensor based on 3D graphene/Cu2O modified carbon paper electrode. Anal. Chim. Acta 2020, 1135, 12–19. [Google Scholar] [CrossRef]
- Zang, G.; Hao, W.; Li, X.; Huang, S.; Gan, J.; Luo, Z.; Zhang, Y. Copper nanowires-MOFs-graphene oxide hybrid nanocomposite targeting glucose electro-oxidation in neutral medium. Electrochim. Acta 2018, 277, 176–184. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, S.; Guo, Q.; Zhang, H.; Li, H.; Wang, Y.; Fu, J.; Wu, X.; Xu, L.; Wang, M. One-step hydrothermal synthesis of Cu2O/CuO hollow microspheres/reduced graphene oxide hybrid with enhanced sensitivity for non-enzymatic glucose sensing. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125076. [Google Scholar] [CrossRef]
- Cui, D.; Su, L.; Li, H.; Li, M.; Li, C.; Xu, S.; Qian, L.; Yang, B. Non-enzymatic glucose sensor based on micro-/nanostructured Cu/Ni deposited on graphene sheets. J. Electroanal. Chem. 2019, 838, 154–162. [Google Scholar] [CrossRef]
- Yan, X.; Yang, J.; Ma, L.; Tong, X.; Wang, Y.; Jin, G.; Guo, X.-Y. Size-controlled synthesis of Cu2O nanoparticles on reduced graphene oxide sheets and their application as non-enzymatic glucose sensor materials. J. Solid State Electrochem. 2015, 19, 3195–3199. [Google Scholar] [CrossRef]
- Zhao, Y.; Bo, X.; Guo, L. Highly exposed copper oxide supported on three-dimensional porous reduced graphene oxide for non-enzymatic detection of glucose. Electrochim. Acta 2015, 176, 1272–1279. [Google Scholar] [CrossRef]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A Critical Review of Glucose Biosensors Based on Carbon Nanomaterials: Carbon Nanotubes and Graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Kumar, S.; Pandey, S.K.; Srivastava, S.; Mishra, M.; Gupta, G.; Malhotra, B.; Tiwari, R.; Srivastava, A. Fabrication of sensitive bioelectrode based on atomically thin CVD grown graphene for cancer biomarker detection. Biosens. Bioelectron. 2018, 105, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, L.; Xu, R.; Ma, Y.; Ma, L. Facile fabrication of biosensors based on Cu nanoparticles modified as-grown CVD graphene for non-enzymatic glucose sensing. J. Electroanal. Chem. 2019, 853, 113527. [Google Scholar] [CrossRef]
- Soganci, T.; Ayranci, R.; Harputlu, E.; Ocakoglu, K.; Acet, M.; Farle, M.; Unlu, C.G.; Ak, M. An effective non-enzymatic biosensor platform based on copper nanoparticles decorated by sputtering on CVD graphene. Sens. Actuators B Chem. 2018, 273, 1501–1507. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, P.; Liu, Y.; Luo, H. A novel non-enzymatic glucose sensor based on a Cu-nanoparticle-modified graphene edge nanoelectrode. Anal. Methods-R. Soc. Chem. 2017, 9, 2205–2210. [Google Scholar] [CrossRef]
- Ren, Z.; Mao, H.; Luo, H.; Liu, Y. Glucose sensor based on porous Ni by using a graphene bottom layer combined with a Ni middle layer. Carbon 2019, 149, 609–617. [Google Scholar] [CrossRef]
- Usman, M.; Pan, L.; Farid, A.; Riaz, S.; Khan, A.S.; Peng, Z.Y.; Khan, M.A. Ultra-fast and highly sensitive enzyme-free glucose sensor based on 3D vertically aligned silver nanoplates on nickel foam-graphene substrate. J. Electroanal. Chem. 2019, 848, 113342. [Google Scholar] [CrossRef]
- Cusati, T.; Fiori, G.; Gahoi, A.; Passi, V.; Lemme, M.C.; Fortunelli, A.; Iannaccone, G. Electrical properties of graphene-metal contacts. Sci. Rep. 2017, 7, 5109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, M.; Hu, H.; Ding, S.; Wang, C. High-performance non-enzymatic glucose-sensing electrode fabricated by α-nickel hydroxide-reduced graphene oxide nanocomposite on nickel foam substrate. J. Mater. Sci. Mater. Electron. 2021, 32, 19327–19338. [Google Scholar] [CrossRef]
- Wei, S.; Hao, Y.; Ying, Z.; Xu, C.; Wei, Q.; Xue, S.; Cheng, H.-M.; Ren, W.; Ma, L.-P.; Zeng, Y. Transfer-free CVD graphene for highly sensitive glucose sensors. J. Mater. Sci. Technol. 2020, 37, 71–76. [Google Scholar] [CrossRef]
- Fatema, K.N.; Oh, W.-C. A comparative electrochemical study of non-enzymatic glucose, ascorbic acid, and albumin detection by using a ternary mesoporous metal oxide (ZrO2, SiO2 and In2O3) modified graphene composite based biosensor. RSC Adv. 2021, 11, 4256–4269. [Google Scholar] [CrossRef]
- Chen, H.-C.; Su, W.-R.; Yeh, Y.-C. Functional channel of SWCNTs/Cu2O/ZnO NRs/graphene hybrid electrodes for highly sensitive nonenzymatic glucose sensors. ACS Appl. Mater. Interfaces 2020, 12, 32905–32914. [Google Scholar] [CrossRef]
- Brouzgou, A.; Podias, A.; Tsiakaras, P. PEMFCs and AEMFCs directly fed with ethanol: A current status comparative review. J. Appl. Electrochem. 2013, 43, 119–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Xia, J.; Zhang, F.; Wang, Z. MOF-Derived Porous Ni2P/Graphene Composites with Enhanced Electrochemical Properties for Sensitive Nonenzymatic Glucose Sensing. ACS Appl. Mater. Interfaces 2018, 10, 39151–39160. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Liu, H.; Zhai, Y.; Li, L.; Wen, H. 2D MOF with electrochemical exfoliated graphene for nonenzymatic glucose sensing: Central metal sites and oxidation potentials. Anal. Chim. Acta 2020, 1122, 9–19. [Google Scholar] [CrossRef]
- Chen, X.; Liu, D.; Cao, G.; Tang, Y.; Wu, C. In situ synthesis of a sandwich-like graphene@ ZIF-67 heterostructure for highly sensitive nonenzymatic glucose sensing in human serums. ACS Appl. Mater. Interfaces 2019, 11, 9374–9384. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.M.; Goudini, L.; Piri, F.; Zamani, A.; Saadati, F. Molecular imprinting method for fabricating novel glucose sensor: Polyvinyl acetate electrode reinforced by MnO2/CuO loaded on graphene oxide nanoparticles. Food Chem. 2016, 194, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; He, H.; Ye, Z.; Huang, J. Three-dimensional graphene foam integrated with Ni(OH)2 nanosheets as a hierarchical structure for non-enzymatic glucose sensing. J. Electroanal. Chem. 2019, 832, 275–283. [Google Scholar] [CrossRef]
- Cui, N.; Guo, P.; Yuan, Q.; Ye, C.; Yang, M.; Yang, M.; Chee, K.W.A.; Wang, F.; Fu, L.; Wei, Q.; et al. Single-Step Formation of Ni Nanoparticle-Modified Graphene–Diamond Hybrid Electrodes for Electrochemical Glucose Detection. Sensors 2019, 19, 2979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, M.; Mehrabadi, Z.; Farsadrooh, M.; Bafkary, R.; Derikvandi, H.; Hayati, P.; Mohammadi, K. Chapter 4—Metal–organic framework. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: London, UK, 2021; Volume 33, pp. 279–387. [Google Scholar]
- Yan, X.; Gu, Y.; Li, C.; Zheng, B.; Li, Y.; Zhang, T.; Zhang, Z.; Yang, M. A non-enzymatic glucose sensor based on the CuS nanoflakes–reduced graphene oxide nanocomposite. Anal. Methods-R. Soc. Chem. 2018, 10, 381–388. [Google Scholar] [CrossRef]
- Chen, X.; Gao, J.; Zhao, G.; Wu, C. In situ growth of FeOOH nanoparticles on physically-exfoliated graphene nanosheets as high performance H2O2 electrochemical sensor. Sens. Actuators B Chem. 2020, 313, 128038. [Google Scholar] [CrossRef]
- Sun, S.; Tang, Y.; Wu, C.; Wan, C. Phytic acid functionalized ZIF-67 decorated graphene nanosheets with remarkably boosted electrochemical sensing performance. Anal. Chim. Acta 2020, 1107, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Jothi, L.; Jayakumar, N.; Jaganathan, S.K.; Nageswaran, G. Ultrasensitive and selective non-enzymatic electrochemical glucose sensor based on hybrid material of graphene nanosheets/graphene nanoribbons/nickel nanoparticle. Mater. Res. Bull. 2018, 98, 300–307. [Google Scholar] [CrossRef]
- Huang, L.; Su, J.; Song, Y.; Ye, R. Laser-Induced Graphene: En Route to Smart Sensing. Nano-Micro Lett. 2020, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.D.; Khalelov, A.; Postnikov, P.S.; Lipovka, A.; Dorozhko, E.; Amin, I.; Murastov, G.V.; Chen, J.-J.; Sheng, W.; Trusova, M.E. Beyond graphene oxide: Laser engineering functionalized graphene for flexible electronics. Mater. Horiz. 2020, 7, 1030–1041. [Google Scholar] [CrossRef]
- Reghunath, R.; devi, K.; Singh, K.K. Recent advances in graphene based electrochemical glucose sensor. Nano-Struct. Nano-Objects 2021, 26, 100750. [Google Scholar] [CrossRef]
- Yang, G.-h.; Bao, D.-d.; Liu, H.; Zhang, D.-q.; Wang, N.; Li, H.-t. Functionalization of Graphene and Applications of the Derivatives. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1129–1141. [Google Scholar] [CrossRef]
- Gao, J.; He, S.; Nag, A. Electrochemical Detection of Glucose Molecules Using Laser-Induced Graphene Sensors: A Review. Sensors 2021, 21, 2818. [Google Scholar] [CrossRef] [PubMed]
- Hyeong, S.-K.; Choi, K.-H.; Park, S.-W.; Bae, S.; Park, M.; Ryu, S.; Lee, J.-H.; Lee, S.-K. Review of the Direct Laser Synthesis of Functionalized Graphene and its Application in Sensor Technology. Appl. Sci. Converg. Technol. 2019, 28, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Settu, K.; Chiu, P.-T.; Huang, Y.-M. Laser-Induced Graphene-Based Enzymatic Biosensor for Glucose Detection. Polymers 2021, 13, 2795. [Google Scholar] [CrossRef]
- Vivaldi, F.M.; Dallinger, A.; Bonini, A.; Poma, N.; Sembranti, L.; Biagini, D.; Salvo, P.; Greco, F.; Di Francesco, F. Three-Dimensional (3D) Laser-Induced Graphene: Structure, Properties, and Application to Chemical Sensing. ACS Appl. Mater. Interfaces 2021, 13, 30245–30260. [Google Scholar] [CrossRef]
- Zhu, B.; Yu, L.; Beikzadeh, S.; Zhang, S.; Zhang, P.; Wang, L.; Travas-Sejdic, J. Disposable and portable gold nanoparticles modified-laser-scribed graphene sensing strips for electrochemical, non-enzymatic detection of glucose. Electrochim. Acta 2021, 378, 138132. [Google Scholar] [CrossRef]
- Qu, L.; Dai, L. Substrate-Enhanced Electroless Deposition of Metal Nanoparticles on Carbon Nanotubes. J. Am. Chem. Soc. 2005, 127, 10806–10807. [Google Scholar] [CrossRef]
- Tehrani, F.; Bavarian, B. Facile and scalable disposable sensor based on laser engraved graphene for electrochemical detection of glucose. Sci. Rep. 2016, 6, 27975. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zheng, C.; Gao, J.; Gui, J.; Deng, L.; Wang, Y.; Xu, R. Co3O4 nanoparticles embedded in laser-induced graphene for a flexible and highly sensitive enzyme-free glucose biosensor. Sens. Actuators B Chem. 2021, 347, 130653. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Hu, Z.; Zhang, X.; Yi, N.; Tang, K.; Dexheimer, M.G.; Lian, X.; Wang, Q.; Yang, J.; et al. Laser-induced graphene non-enzymatic glucose sensors for on-body measurements. Biosens. Bioelectron. 2021, 193, 113606. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y.J.A.M. Recent progress in graphene/polymer nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef] [PubMed]
- Apátiga, J.L.; Del Castillo, R.M.; Del Castillo, L.F.; Calles, A.G.; Espejel-Morales, R.; Favela, J.F.; Compañ, V. Non-Covalent Interactions on Polymer-Graphene Nanocomposites and Their Effects on the Electrical Conductivity. Polymers 2021, 13, 1714. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Q.; Yin, W.; Jiang, T.; Zhao, D.; Jiang, L. A novel nonenzymatic glucose sensor based on functionalized PDDA-graphene/CuO nanocomposites. Sens. Actuators B Chem. 2017, 253, 1087–1095. [Google Scholar] [CrossRef]
- Thakur, S.; Verma, A.; Alsanie, W.F.; Christie, G.; Thakur, V.K. On the graphene and its derivative based polymer nanocomposites for glucose sensing. Mater. Lett. 2022, 307, 130971. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Kang, B.-C.; Ha, T.-J. Non-enzymatic electrochemical glucose sensors based on polyaniline/reduced-graphene-oxide nanocomposites functionalized with silver nanoparticles. J. Mater. Chem. C 2020, 8, 5112–5123. [Google Scholar] [CrossRef]
- Bairagi, P.K.; Verma, N. Electro-polymerized polyacrylamide nano film grown on a Ni-reduced graphene oxide-polymer composite: A highly selective non-enzymatic electrochemical recognition element for glucose. Sens. Actuators B Chem. 2019, 289, 216–225. [Google Scholar] [CrossRef]
- Batool, R.; Akhtar, M.A.; Hayat, A.; Han, D.; Niu, L.; Ahmad, M.A.; Nawaz, M.H. A nanocomposite prepared from magnetite nanoparticles, polyaniline and carboxy-modified graphene oxide for non-enzymatic sensing of glucose. Microchim. Acta 2019, 186, 267. [Google Scholar] [CrossRef]
- Anand, V.K.; Bhatt, K.; Kumar, S.; Archana, B.; Sharma, S.; Singh, K.; Gupta, M.; Goyal, R.; Virdi, G.S. Sensitive and Enzyme-Free Glucose Sensor Based on Copper Nanowires/Polyaniline/Reduced Graphene Oxide Nanocomposite Ink. Int. J. Nanosci. 2021, 20, 2150020. [Google Scholar] [CrossRef]
- Luan, F.; Zhang, S.; Chen, D.; Wei, F.; Zhuang, X. Ni3S2/ionic liquid-functionalized graphene as an enhanced material for the nonenzymatic detection of glucose. Microchem. J. 2018, 143, 450–456. [Google Scholar] [CrossRef]
- Samoson, K.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. A Nonenzymatic Glucose Sensor Based on the Excellent Dispersion of a Graphene Oxide-Poly(acrylic acid)-Palladium Nanoparticle-Modified Screen-Printed Carbon Electrode. J. Electrochem. Soc. 2019, 166, B1079–B1087. [Google Scholar] [CrossRef]
- Anand, V.K.; Bukke, A.; Bhatt, K.; Kumar, S.; Sharma, S.; Goyal, R.; Virdi, G. Highly sensitive and reusable Cu+2/polyaniline/reduced graphene oxide nanocomposite ink-based non-enzymatic glucose sensor. Appl. Phys. A 2020, 126, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhu, H.; Li, S.; Jiang, C.; Blue, R.J.; Su, Y. Functionalization of the support material based on N-doped carbon-reduced graphene oxide and its influence on the non-enzymatic detection of glucose. J. Alloys Compd. 2019, 780, 98–106. [Google Scholar] [CrossRef]
- Lin, S.; Feng, W.; Miao, X.; Zhang, X.; Chen, S.; Chen, Y.; Wang, W.; Zhang, Y. A flexible and highly sensitive nonenzymatic glucose sensor based on DVD-laser scribed graphene substrate. Biosens. Bioelectron. 2018, 110, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yang, G.; Xu, M.; Bo, X. Universal laser-assisted growth of transition metal nanoparticles on a flexible graphene electrode for a nonenzymatic glucose sensor. New J. Chem. 2020, 44, 17954–17960. [Google Scholar] [CrossRef]
- Prabhakaran, A.; Nayak, P. Surface engineering of laser-scribed graphene sensor enables non-enzymatic glucose detection in human body fluids. ACS Appl. Nano Mater. 2019, 3, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.-C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef] [PubMed]


| Electrocatalyst | Sensitivity (μAmM−1cm−2) | LOD (μM) | Potential (V vs. Ag/AgCl) | Synthesis Method | Ref. |
|---|---|---|---|---|---|
| (Ag⊥@NFG)S | 2 × 1011 | 0.002 | 0.050 | 2.1 | [54] |
| CuFe-O/Gr/NF hybrid | 368,000 | 0.008 | 0.650 | 2.1 | [41] |
| NiNPs/ERGO | 185,200 | 0.040 | 0.550 | 2.1 | [32] |
| Ni3S2/IL-graphene | 25,343 | 0.161 | 0.370 | 2.4 | [93] |
| Ni2P/G | 7234 | 0.440 | 0.300 | 2.2 | [61] |
| Cu/Ni-EG/pNi | 6161 | 0.460 | 0.550 | 2.1 | [53] |
| PDDA-graphene/CuO | 4982.2 | 0.200 | 0.580 | 2.4 | [87] |
| DLEG-CuNCs | 4532.2 | 0.250 | 0.550 | 2.3 | [82] |
| NiO-NC-rGO | 4254 | 0.071 | 0.500 | 2.4 | [35] |
| Cu+2/PANI/rGO/FR4 | 4168.37 | 4.930 | 0.660 | 2.4 | [95] |
| Co(OH)2/rGO film | 3354 | 1.000 | 0.500 | 2.2 | [22] |
| CoO-Co-NC-rGO | 3172 | 0.340 | 0.600 | 2.2 | [96] |
| Ag–PANI/rGO | 2766.4 | 0.790 | 0.500 | 2.3 | [89] |
| ZIF67@GIS | 2718.3 | 0.086 | 0.600 | 2.2 | [70] |
| rGO@Co3O4-NC | 2563 | 0.0504 | 0.650 | 2.2 | [17] |
| CuCo2O4/PrGO-10 | 2426 | 0.150 | 0.592 | 2.2 | [29] |
| Ni(OH)2/3DGF | 2366 | 0.320 | 0.454 | 2.2 | [65] |
| GS/GNR/Ni | 2300 | 0.0025 | 0.500 | 2.2 | [71] |
| Ni/NiO-rGO | 1997 | 1.800 | 0.550 | 2.2 | [31] |
| GS@ZIF-67 | 1521.1 | 0.360 | 0.592 | 2.2 | [63] |
| LSG/Cu-NPs | 1518 | 0.350 | 0.642 | 2.3 | [97] |
| Co/3D Gr | 1411.2 | 2.700 | 0.600 | 2.3 | [98] |
| Ni/C/rGO | 1211.41 | 0.340 | 0.592 | 2.2 | [34] |
| CuO0.1/LSG | 764.331 | 0.100 | 0.400 | 2.3 | [99] |
| PEDOT-ERGO | 696.90 | 0.120 | −0.200 | 2.4 | [100] |
| Cu NPs-LIG | 495.0 | 0.390 | 0.500 | 2.3 | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balkourani, G.; Damartzis, T.; Brouzgou, A.; Tsiakaras, P. Cost Effective Synthesis of Graphene Nanomaterials for Non-Enzymatic Electrochemical Sensors for Glucose: A Comprehensive Review. Sensors 2022, 22, 355. https://doi.org/10.3390/s22010355
Balkourani G, Damartzis T, Brouzgou A, Tsiakaras P. Cost Effective Synthesis of Graphene Nanomaterials for Non-Enzymatic Electrochemical Sensors for Glucose: A Comprehensive Review. Sensors. 2022; 22(1):355. https://doi.org/10.3390/s22010355
Chicago/Turabian StyleBalkourani, Georgia, Theodoros Damartzis, Angeliki Brouzgou, and Panagiotis Tsiakaras. 2022. "Cost Effective Synthesis of Graphene Nanomaterials for Non-Enzymatic Electrochemical Sensors for Glucose: A Comprehensive Review" Sensors 22, no. 1: 355. https://doi.org/10.3390/s22010355