Advances in the Determination of Anabolic-Androgenic Steroids: From Standard Practices to Tailor-Designed Multidisciplinary Approaches
Abstract
:1. Introduction
2. Standard Chromatographic Methods in AAS Determination
3. Antibody-Based Approaches for AAS Determination
3.1. Immunoaffinity Columns
3.2. Enzymatic Immunoassays
3.3. Lateral Flow Immunoassays
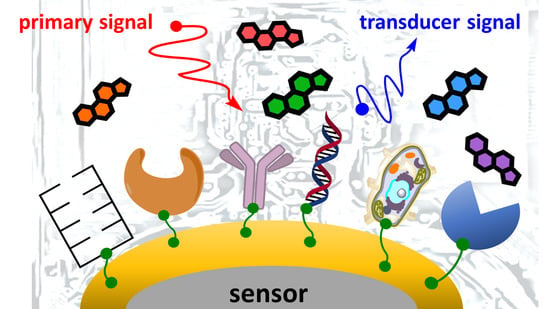
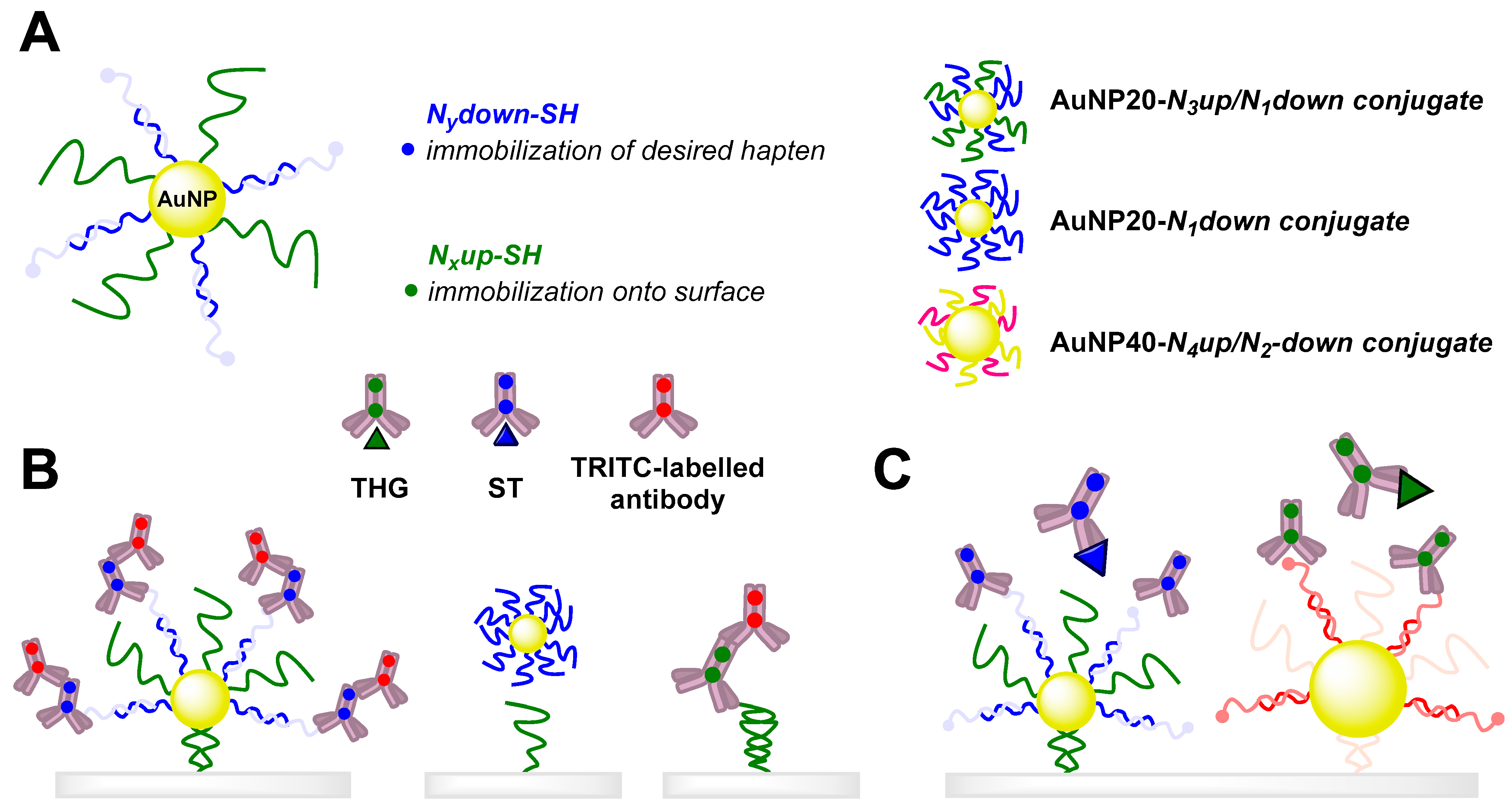
3.4. Immunosensors
3.5. Androgen-Receptor- and Cell-Based Methods for AAS Determination
3.6. Oligonucleotide-Based Approaches for AAS Determination
3.7. Enzyme-Based Sensor for AAS Determination
3.8. Chemically Designed Artificial Sensors for AAS Determination
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AASs | Anabolic-androgenic steroids |
| AcCN | Acetonitrile |
| AIBN | 2,2’-Azobis(2-methylpropionitrile) |
| Ab | Antibody |
| AR | Androgen receptor |
| AREs | Androgen response elements |
| AuNP | Gold nanoparticle |
| BiNb | Biotinylation of a nanobody in vivo |
| BSA | Bovine serum albumin |
| CLEIA | Chemiluminescent enzyme immunoassay |
| CR | Cross-reactivity |
| DHEA | Dehydroepiandrosterone |
| DS | Dietary supplement |
| DSMI | trans-4-[4-(Dimethylamino)styryl]-1-methylpyridinium iodide |
| EC50 | Half-maximal effective concentration |
| EDC | 1-Ethyl-3-(3-dimethyl aminopropyl)carbodiimide |
| EGDMA | Ethylene glycol dimethacrylate |
| EIA | Enzyme immunoassay |
| ELISA | Enzyme-linked immunosorbent assay |
| GCE | Glassy carbon electrode |
| HEMA | 2-Hydroxyethyl methacrylate |
| HPLC | High-performance liquid chromatography |
| HOSu | N-Hydroxysuccinimide |
| HRP | Horseradish peroxidase enzymes |
| HSD | Hydroxysteroid reductase |
| HSPs | Heat shock proteins |
| IAC | Immunoaffinity chromatography |
| IC50 | Half-maximal inhibitory concentration |
| IgG | Immunoglobulin G |
| KLH | Keyhole limpet hemocyanin |
| LFIA | Lateral flow immunoassay |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| LWR | Linear working range |
| MAA | Methacrylic acid |
| mAb | Mouse-derived antibody |
| MIF | Molecularly imprinted polymer film |
| MIP | Molecularly imprinted polymer |
| MT | Methyltestosterone |
| MT-CMO-OVA | A conjugate of methyltestosterone-3-carboxymethyloxime with ovalbumin |
| MWCNTs | Multiwalled carbon nanotubes |
| NAD | Nicotinamide adenine dinucleotide |
| Nb | Nanobody |
| NC | Nitrocellulose |
| OVA | Ovalbumin |
| PSNPs | Polystyrene nanoparticles |
| RSA | Rabbit serum albumin |
| SEAP | Secreted alkaline phosphatase |
| SOI | Silicon-on-insulator wafer |
| SPCEs | Screen-printed carbon electrodes |
| SPEs | Screen-printed electrodes |
| SPR | Surface plasmon resonance |
| ST | Stanozolol |
| THG | Tetrahydrogestrinone |
| TLC | Thin-layer chromatography |
| TPF | Two-photon fluorescence |
| UOC | Under optimal conditions |
| VHH | The antigen-binding fragment of heavy-chain-only antibodies |
| WADA | World Anti-Doping Agency |
References
- de Ronde, W.; Smit, D.L. Anabolic androgenic steroid abuse in young males. Endocr. Connect. 2020, 9, R102–R111. [Google Scholar] [CrossRef] [Green Version]
- Kicman, A.T. Pharmacology of anabolic steroids. Br. J. Pharmacol. 2008, 154, 502–521. [Google Scholar] [CrossRef]
- Venturella, F.; Cancellieri, G.; Giammanco, M.; Di Marco, P.; Catania, F.; Liga, A.V. Amateur doping: A survey on Sicilian population. J. Biol. Res. 2019, 92. [Google Scholar] [CrossRef]
- Börjesson, A.; Lehtihet, M.; Andersson, A.; Dahl, M.; Vicente, V.; Ericsson, M.; Ekström, L. Studies of athlete biological passport biomarkers and clinical parameters in male and female users of anabolic androgenic steroids and other doping agents. Drug Test. Anal. 2020, 12, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Lood, Y.; Eklund, A.; Garle, M.; Ahlner, J. Anabolic androgenic steroids in police cases in Sweden 1999–2009. Forensic Sci. Int. 2012, 219, 199–204. [Google Scholar] [CrossRef]
- Amaral, J.M.; Padilha, M.C.; Chagas, S.V.; Baker, J.S.; Mullen, C.; Neto, L.V.; Neto, F.R.A.; Cruz, M.S. Effective treatment and prevention of attempted suicide, anxiety, and aggressiveness with fluoxetine, despite proven use of androgenic anabolic steroids. Drug Test. Anal. 2020, 13, 197–202. [Google Scholar] [CrossRef]
- Oberlander, J.G.; Henderson, L.P. The Sturm und Drang of anabolic steroid use: Angst, anxiety, and aggression. Trends Neurosci. 2012, 35, 382–392. [Google Scholar] [CrossRef] [Green Version]
- Bond, P.; Llewellyn, W.; Van Mol, P. Anabolic androgenic steroid-induced hepatotoxicity. Med. Hypotheses 2016, 93, 150–153. [Google Scholar] [CrossRef]
- Montisci, M.; El Mazloum, R.; Cecchetto, G.; Terranova, C.; Ferrara, S.D.; Thiene, G.; Basso, C. Anabolic androgenic steroids abuse and cardiac death in athletes: Morphological and toxicological findings in four fatal cases. Forensic Sci. Int. 2012, 217, e13–e18. [Google Scholar] [CrossRef]
- Rahnema, C.D.; Lipshultz, L.I.; Crosnoe, L.E.; Kovac, J.R.; Kim, E.D. Anabolic steroid–induced hypogonadism: Diagnosis and treatment. Fertil. Steril. 2014, 101, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- De Souza, G.L.; Hallak, J. Anabolic steroids and male infertility: A comprehensive review. BJU Int. 2011, 108, 1860–1865. [Google Scholar] [CrossRef]
- Turillazzi, E.; Perilli, G.; Di Paolo, M.; Neri, M.; Riezzo, I.; Fineschi, V. Side effects of AAS abuse: An overview. Mini-Rev. Med. Chem. 2011, 11, 374–389. [Google Scholar] [CrossRef]
- Christoffersen, T.; Andersen, J.T.; Dalhoff, K.P.; Horwitz, H. Anabolic-androgenic steroids and the risk of imprisonment. Drug Alcohol Depend. 2019, 203, 92–97. [Google Scholar] [CrossRef]
- Pope, H.G.; Wood, R.I.; Rogol, A.; Nyberg, F.; Bowers, L.; Bhasin, S. Adverse Health Consequences of Performance-Enhancing Drugs: An Endocrine Society Scientific Statement. Endocr. Rev. 2013, 35, 341–375. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, H.; Andersen, J.T.; Dalhoff, K.P. Health consequences of androgenic anabolic steroid use. J. Intern. Med. 2018, 285, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.J.; Selmer, C.; Østergren, P.B.; Pedersen, K.B.; Schou, M.; Gustafsson, F.; Faber, J.; Juul, A.; Kistorp, C. Former Abusers of Anabolic Androgenic Steroids Exhibit Decreased Testosterone Levels and Hypogonadal Symptoms Years after Cessation: A Case-Control Study. PLoS ONE 2016, 11, e0161208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davani-Davari, D.; Karimzadeh, I.; Khalili, H. The potential effects of anabolic-androgenic steroids and growth hormone as commonly used sport supplements on the kidney: A systematic review. BMC Nephrol. 2019, 20, 198. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Cascio, O.; Bertozzi, G.; Sessa, F.; Messina, A.; Monda, V.; Cipolloni, L.; Biondi, A.; Daniele, A.; Pomara, C. Anabolic androgenic steroids and carcinogenicity focusing on Leydig cell: A literature review. Oncotarget 2018, 9, 19415–19426. [Google Scholar] [CrossRef] [Green Version]
- Torrisi, M.; Pennisi, G.; Russo, I.; Amico, F.; Esposito, M.; Liberto, A.; Cocimano, G.; Salerno, M.; Rosi, G.L.; Di Nunno, N.; et al. Sudden Cardiac Death in Anabolic-Androgenic Steroid Users: A Literature Review. Medicina 2020, 56, 587. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Guerra, A.I.; Tapia, J.; Menéndez-Quintanal, L.M.; Lucena, J.S. Sudden cardiac death in anabolic androgenic steroids abuse: Case report and literature review. Forensic Sci. Res. 2019, 4, 267–273. [Google Scholar] [CrossRef]
- Lehmann, S.; Thomas, A.; Schiwy-Bochat, K.-H.; Geyer, H.; Thevis, M.; Glenewinkel, F.; Rothschild, M.A.; Andresen-Streichert, H.; Juebner, M. Death after misuse of anabolic substances (clenbuterol, stanozolol and metandienone). Forensic Sci. Int. 2019, 303, 109925. [Google Scholar] [CrossRef]
- Frati, P.; Busardo, F.; Cipolloni, L.; Dominicis, E.; Fineschi, V. Anabolic Androgenic Steroid (AAS) Related Deaths: Autoptic, Histopathological and Toxicological Findings. Curr. Neuropharmacol. 2015, 13, 146–159. [Google Scholar] [CrossRef] [Green Version]
- Tauchen, J.; Jurášek, M.; Huml, L.; Rimpelová, S. Medicinal Use of Testosterone and Related Steroids Revisited. Molecules 2021, 26, 1032. [Google Scholar] [CrossRef]
- World Anti-Doping Agency. 2018 Anti-Doping Testing Figures. Available online: https://www.wada-ama.org/sites/default/files/resources/files/2018_testing_figures_report.pdf (accessed on 15 January 2021).
- Alquraini, H.; Auchus, R.J. Strategies that athletes use to avoid detection of androgenic-anabolic steroid doping and sanctions. Mol. Cell. Endocrinol. 2018, 464, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Pope, H.G.; Kanayama, G.; Athey, A.; Ryan, E.; Hudson, J.I.; Baggish, A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: Current best estimates. Am. J. Addict. 2013, 23, 371–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahin, O.S.C.; De Sousa, E.C.; Santos, A.M. Prevalence of the Use of Anabolic-Androgenic Steroids in Brazil: A Systematic Review. Subst. Use Misuse 2014, 49, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Rachoń, D.; Pokrywka, L.; Suchecka-Rachoń, K. Prevalence and risk factors of anabolic-androgenic steroids (AAS) abuse among adolescents and young adults in Poland. Int. J. Public Health 2006, 51, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Teck, J.T.W.; McCann, M. Tracking internet interest in anabolic-androgenic steroids using Google Trends. Int. J. Drug Policy 2017, 51, 52–55. [Google Scholar] [CrossRef]
- Hullstein, I.R.; Malerod-Fjeld, H.; Dehnes, Y.; Hemmersbach, P. Black market products confiscated in Norway 2011–2014 compared to analytical findings in urine samples. Drug Test. Anal. 2015, 7, 1025–1029. [Google Scholar] [CrossRef]
- Prokudina, E.; Prchalová, J.; Vyšatová, E.; Kuchař, M.; Rajchl, A.; Lapcik, O. Analysis of anabolic androgenic steroids by direct analysis in real time ionization with time-of-flight mass spectrometry. Int. J. Mass Spectrom. 2015, 392, 28–33. [Google Scholar] [CrossRef]
- Weber, C.; Krug, O.; Kamber, M.; Thevis, M. Qualitative and Semiquantitative Analysis of Doping Products Seized at the Swiss Border. Subst. Use Misuse 2017, 52, 742–753. [Google Scholar] [CrossRef]
- Walpurgis, K.; Thomas, A.; Geyer, H.; Mareck, U.; Thevis, M. Dietary Supplement and Food Contaminations and Their Implications for Doping Controls. Foods 2020, 9, 1012. [Google Scholar] [CrossRef]
- Martínez-Sanz, J.M.; Sospedra, I.; Ortiz, C.M.; Baladia, E.; Gil-Izquierdo, A.; Ortiz-Moncada, R. Intended or Unintended Doping? A Review of the Presence of Doping Substances in Dietary Supplements Used in Sports. Nutrients 2017, 9, 1093. [Google Scholar] [CrossRef] [Green Version]
- Odoardi, S.; Castrignanò, E.; Martello, S.; Chiarotti, M.; Strano-Rossi, S. Determination of anabolic agents in dietary supplements by liquid chromatography-high-resolution mass spectrometry. Food Addit. Contam. Part A 2015, 32, 635–647. [Google Scholar] [CrossRef]
- Jurášek, M.; Göselová, S.; Mikšátková, P.; Holubová, B.; Vyšatová, E.; Kuchař, M.; Fukal, L.; Lapcik, O.; Drašar, P. Highly sensitive avidin-biotin ELISA for detection of nandrolone and testosterone in dietary supplements. Drug Test. Anal. 2016, 9, 553–560. [Google Scholar] [CrossRef]
- Fojtíková, L.; Fukal, L.; Blažková, M.; Sýkorová, S.; Kuchař, M.; Mikšátková, P.; Lapčík, O.; Holubová, B. Development of Enzyme-Linked Immunosorbent Assay for Determination of Boldenone in Dietary Supplements. Food Anal. Methods 2016, 9, 3179–3186. [Google Scholar] [CrossRef]
- Sýkorová, S.; Fojtíková, L.; Kuchař, M.; Mikšátková, P.; Karamonová, L.; Fukal, L.; Lapčík, O.; Holubová, B. Sensitive enzyme immunoassay for screening methandienone in dietary supplements. Food Addit. Contam. Part. A 2018, 35, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Holubová, B.; Göselová, S.; Sevcikova, L.; Vlach, M.; Blažková, M.; Lapcik, O.; Fukal, L. Rapid immunoassays for detection of anabolic nortestosterone in dietary supplements. Czech. J. Food Sci. 2013, 31, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Huml, L.; Havlová, D.; Longin, O.; Staňková, E.; Holubová, B.; Kuchař, M.; Prokudina, E.; Rottnerová, Z.; Zimmermann, T.; Drašar, P.; et al. Stanazolol derived ELISA as a sensitive forensic tool for the detection of multiple 17α-methylated anabolics. Steroids 2019, 155, 108550. [Google Scholar] [CrossRef] [PubMed]
- Holubová, B.; Kubešová, P.; Huml, L.; Vlach, M.; Lapčík, O.; Jurášek, M.; Fukal, L. Tailor-Made Immunochromatographic Test for the Detection of Multiple 17α-Methylated Anabolics in Dietary Supplements. Foods 2021, 10, 741. [Google Scholar] [CrossRef]
- Geyer, H.; Parr, M.K.; Koehler, K.; Mareck, U.; Schänzer, W.; Thevis, M. Nutritional supplements cross-contaminated and faked with doping substances. J. Mass Spectrom. 2008, 43, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Czech Agriculture and Food Inspection Authority. V Přípravku Creatine Pyruvate byly Prokázány Nepovolené Anabolické Steroidy. Available online: https://www.szpi.gov.cz/clanek/v-pripravku-creatine-pyruvate-byly-prokazany-nepovolene-anabolicke-steroidy.aspx (accessed on 6 July 2021).
- Czech Agriculture and Food Inspection Authority. Potravinářská Inspekce Zjistila Doplněk Stravy Škodlivý pro Lidské Zdraví s Množstvím Anabolických Steroidů a Dalších Nepovolených Látek. Available online: https://www.szpi.gov.cz/clanek/potravinarska-inspekce-zjistila-doplnek-stravy-skodlivy-pro-lidske-zdravi-s-mnozstvim-anabolickych-steroidu-a-dalsich-nepovolenych-latek.aspx?q=JmNobnVtPTEmaGw9d2FycmlvciBsYWJz (accessed on 6 July 2021).
- Stárka, L.; Dušková, M.; Kolátorová, L.; Lapčík, O. Anabolic steroid induced hypogonadism in men: Overview and case report. Vnitrni Lek. 2017, 63, 598–603. [Google Scholar] [CrossRef]
- The United States Food and Drug Administration. Available online: https://www.fda.gov/consumers/consumer-updates/caution-bodybuilding-products-can-be-risky (accessed on 15 January 2021).
- Tschmelak, J.; Kumpf, M.; Kappel, N.; Proll, G.; Gauglitz, G. Total internal reflectance fluorescence (TIRF) biosensor for environmental monitoring of testosterone with commercially available immunochemistry: Antibody characterization, assay development and real sample measurements. Talanta 2006, 69, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Jing, L.; Ding, Y.; Wei, T. A novel double-layer molecularly imprinted polymer film based surface plasmon resonance for determination of testosterone in aqueous media. Appl. Surf. Sci. 2015, 342, 84–91. [Google Scholar] [CrossRef]
- Büttler, R.M.; Martens, F.; Kushnir, M.M.; Ackermans, M.T.; Blankenstein, M.; Heijboer, A.C. Simultaneous measurement of testosterone, androstenedione and dehydroepiandrosterone (DHEA) in serum and plasma using Isotope-Dilution 2-Dimension Ultra High Performance Liquid-Chromatography Tandem Mass Spectrometry (ID-LC–MS/MS). Clin. Chim. Acta 2015, 438, 157–159. [Google Scholar] [CrossRef]
- Hirpessa, B.B.; Ulusoy, B.H.; Hecer, C. Hormones and Hormonal Anabolics: Residues in Animal Source Food, Potential Public Health Impacts, and Methods of Analysis. J. Food Qual. 2020, 2020, 5065386. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, M.; Huang, S.; Zhao, J.; Tao, J. Classification and detection of testosterone propionate and nandrolone residues in duck meat using surface-enhanced Raman spectroscopy coupled with multivariate analysis. Poult. Sci. 2020, 100, 296–301. [Google Scholar] [CrossRef]
- Kayani, M.; Parry, J.M. The detection and assessment of the aneugenic potential of selected oestrogens, progestins and androgens using the in vitro cytokinesis blocked micronucleus assay. Mutat. Res. Toxicol. Environ. Mutagen. 2008, 651, 40–45. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, X.; Feng, H.; Shi, H.; Sun, L.; Tao, W.; Xie, Q.; Wang, D. Simultaneous exposure to estrogen and androgen resulted in feminization and endocrine disruption. J. Endocrinol. 2016, 228, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Orlando, E.F.; Kolok, A.S.; Binzcik, G.A.; Gates, J.L.; Horton, M.K.; Lambright, C.S.; Gray, L.E.; Soto, A.M.; Guillette, L.J. Endocrine-disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environ. Health Perspect. 2004, 112, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozo, O.J.; De Brabanter, N.; Fabregat, A.; Segura, J.; Ventura, R.; Van Eenoo, P.; Deventer, K. Current status and bioanalytical challenges in the detection of unknown anabolic androgenic steroids in doping control analysis. Bioanalysis 2013, 5, 2661–2677. [Google Scholar] [CrossRef] [PubMed]
- Anawalt, B.D. Detection of anabolic androgenic steroid use by elite athletes and by members of the general public. Mol. Cell. Endocrinol. 2018, 464, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hampl, R.; Putz, Z.; Bičíková, M.; Stárka, L. Advances in immunoassay of anabolic steroids. In Advances in Steroid Analysis ’84; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Sancho, M.; Arnal, J.M.; Verdú-Martín, G.; Trull-Hernandis, C.; García-Fayos, B. Management of hospital radioactive liquid waste: Treatment proposal for radioimmunoassay wastes. AIMS Environ. Sci. 2021, 8, 449–464. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.-Y.; Lee, S.-K. Development of novel lab-on-a-chip platform for high-throughput radioimmunoassay. Appl. Radiat. Isot. 2020, 168, 109526. [Google Scholar] [CrossRef]
- Clarke, W. Immunoassays for therapeutic drug monitoring and clinical toxicology. In Handbook of Analytical Separations; Elsevier: Amsterdam, The Netherlands, 2020; Volume 7, pp. 97–114. [Google Scholar] [CrossRef]
- O’Kennedy, R.; Murphy, C. Immunoassays: Development, Applications and Future Trends; Pan Stanford Publishing Pte. Ltd.: Singapore, 2017. [Google Scholar]
- Pereira, H.M.G.; Sardela, V.F.; Padilha, M.C.; Mirotti, L.; Casilli, A.; De Oliveira, F.A.; Cavalcanti, G.D.A.; Rodrigues, L.M.L.; De Araujo, A.L.D.; Levy, R.S.; et al. Doping control analysis at the Rio 2016 Olympic and Paralympic Games. Drug Test. Anal. 2017, 9, 1658–1672. [Google Scholar] [CrossRef]
- Bailey, K.; Yazdi, T.; Masharani, U.; Tyrrell, B.; Butch, A.; Schaufele, F. Advantages and Limitations of Androgen Receptor-Based Methods for Detecting Anabolic Androgenic Steroid Abuse as Performance Enhancing Drugs. PLoS ONE 2016, 11, e0151860. [Google Scholar] [CrossRef]
- Thieme, D.; Hemmersbach, P. Doping in Sports; Springer: Heidelberg, Germany, 2009; Volume 195. [Google Scholar]
- Makin, H.L.; Gower, D. Steroid Analysis; Springer Science+Business Media B.V.: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Kirsch, J.; Siltanen, C.; Zhou, Q.; Revzin, A.; Simonian, A.; Katz, E.; Katz, E.; Wang, J.; Bocharova, V.; Wang, J.; et al. Biosensor technology: Recent advances in threat agent detection and medicine. Chem. Soc. Rev. 2013, 42, 8733. [Google Scholar] [CrossRef]
- Nawrot, W.; Drzozga, K.; Baluta, S.; Cabaj, J.; Malecha, K. A Fluorescent Biosensors for Detection Vital Body Fluids’ Agents. Sensors 2018, 18, 2357. [Google Scholar] [CrossRef] [Green Version]
- World Anti-Doping Agency. Technical Document—TD2016EAAS. Available online: https://www.wada-ama.org/sites/default/files/resources/files/wada-td2016eaas-eaas-measurement-and-reporting-en.pdf (accessed on 15 January 2021).
- Thevis, M.; Kuuranne, T.; Geyer, H. Annual banned-substance review—Analytical approaches in human sports drug testing. Drug Test. Anal. 2019, 12, 7–26. [Google Scholar] [CrossRef] [Green Version]
- Thevis, M.; Walpurgis, K.; Thomas, A. Analytical Approaches in Human Sports Drug Testing: Recent Advances, Challenges, and Solutions. Anal. Chem. 2019, 92, 506–523. [Google Scholar] [CrossRef]
- Balcells, G.; Pozo, O.J.; Ventura, R. High-resolution mass spectrometry in doping control. In Applications of Time-of-Flight and Orbitrap Mass Spectrometry in Environmental, Food, Doping, and Forensic Analysis; Perez, S., Eichhorn, P., Barcelo, D., Eds.; Elsevier Science BV: Amsterdam, The Netherlands, 2016; Volume 71, pp. 91–117. [Google Scholar]
- Marcos, J.; Pozo, O.J. Current LC–MS methods and procedures applied to the identification of new steroid metabolites. J. Steroid Biochem. Mol. Biol. 2016, 162, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Balcells, G.; Pozo, O.J.; Esquivel, A.; Kotronoulas, A.; Joglar, J.; Segura, J.; Ventura, R. Screening for anabolic steroids in sports: Analytical strategy based on the detection of phase I and phase II intact urinary metabolites by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2015, 1389, 65–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhawani, S.; Sulaiman, O.; Hashim, R.; Ibrahim, M.N.M. Thin-Layer Chromatographic Analysis of Steroids: A Review. Trop. J. Pharm. Res. 2010, 9, 301–313. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Arfeen, Q.U.; Mazhar, W.; Kanwal, N. A validated stability-indicating TLC-densitometric method for the determination of stanozolol in pharmaceutical formulations. Chem. Cent. J. 2013, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoli-Diva, M.; Pourghazi, K. Gold nanoparticles grafted modified silica gel as a new stationary phase for separation and determination of steroid hormones by thin layer chromatography. J. Food Drug Anal. 2015, 23, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, A.C.; Hage, D.S. Immunoaffinity chromatography: An introduction to applications and recent developments. Bioanalysis 2010, 2, 769–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichon, V. 6—Aptamer-based and immunosorbents. In Solid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 151–183. [Google Scholar] [CrossRef]
- Rodriguez, E.L.; Poddar, S.; Iftekhar, S.; Suh, K.; Woolfork, A.G.; Ovbude, S.; Pekarek, A.; Walters, M.; Lott, S.; Hage, D.S. Affinity chromatography: A review of trends and developments over the past 50 years. J. Chromatogr. B 2020, 1157, 122332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, E.-L.; Xu, Y.; Wu, J.; Dong, Y. Improved preparation of a chitosan-based immunoaffinity column using antibody against methandrostenolone as ligand. Food Agric. Immunol. 2013, 25, 149–159. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Zhang, X.; Wang, E.; Dong, Y. Development and characterization of a chitosan-supported immunoaffinity chromatography column for the selective extraction of methandrostenolone from food and feed samples. Int. J. Biol. Macromol. 2011, 49, 428–432. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Li, X.; Wang, X.; Zhengguo, L.; Wu, J.; Xi, C.; Li, Z. Preparation and Characterization of an Immunoaffinity Column for the Selective Extraction of Salbutamol from Pork Sample. J. Chromatogr. Sci. 2011, 49, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Xu, L.; Cui, Y.-R.; Deng, Q.-P.; Wang, W.; Chen, H.-X.; Zhang, X.-X. Pseudo-homogeneous immunoextraction of epitestosterone from human urine samples based on gold-coated magnetic nanoparticles. Talanta 2010, 81, 819–823. [Google Scholar] [CrossRef]
- Salvador, J.-P.; Sanchez-Baeza, F.; Marco, M.-P. A high-throughput screening (HTS) immunochemical method for the analysis of stanozolol metabolites in cattle urine samples. J. Chromatogr. B 2010, 878, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-P.; Wang, Y.-C.; Liu, C.-H.; Li, Y.-K. Development of chemiluminescence detection of gold nanoparticles in biological conjugates for immunoassay. Anal. Chim. Acta 2005, 551, 85–91. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, X.; Li, Z.-J.; Ren, S.-Q.; Chen, G.-N.; Ying, X.-T.; Lin, J.-M. Development of a sensitive, rapid, biotin–streptavidin based chemiluminescent enzyme immunoassay for human thyroid stimulating hormone. Talanta 2008, 75, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cheng, G.; Wang, H.; Chen, T.; Xu, C.; Lv, H.; Zhang, H.; Hou, R.; Wang, Y.; Peng, D.; et al. Development of a broad-spectrum monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for screening of androgens in animal edible tissues. Microchem. J. 2020, 160, 105683. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Wu, X.; Wang, J.; Liu, Z.; Sun, Y.; Shen, X.; Lei, H. Rapid detection of adulteration of dehydroepiandrosterone in slimming products by competitive indirect enzyme-linked immunosorbent assay and lateral flow immunochromatography. Food Agric. Immunol. 2019, 30, 123–139. [Google Scholar] [CrossRef] [Green Version]
- Holubová, B.; Mikšátková, P.; Kuchař, M.; Karamonová, L.; Lapčík, O.; Fukal, L. Immunochemical techniques for anabolic androgenic steroid: Matrix effects study for food supplements. Eur. Food Res. Technol. 2018, 245, 1011–1019. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, L.; Dong, Y.; Song, Z.; Wang, Y.; Meng, M.; Ren, L.; Eremin, S.A.; Deng, C.; Yin, Y.; et al. Establishment of Enhanced Chemiluminescent Immunoassay Formats for Stanozolol Detection in animal-derived foodstuffs and Other Matrices. Food Anal. Methods 2015, 9, 1284–1292. [Google Scholar] [CrossRef]
- Kong, N.; Song, S.; Peng, J.; Liu, L.; Kuang, H.; Xu, C. Sensitive, Fast, and Specific Immunoassays for Methyltestosterone Detection. Sensors 2015, 15, 10059–10073. [Google Scholar] [CrossRef] [Green Version]
- Tort, N.; Salvador, J.-P.; Marco, M.-P. Multiplexed immunoassay to detect anabolic androgenic steroids in human serum. Anal. Bioanal. Chem. 2012, 403, 1361–1371. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Z.; Zhang, H.; Zhang, X.; Liu, X.; Wang, S. Monoclonal Antibody-Based ELISA and Colloidal Gold Immunoassay for Detecting 19-Nortestosterone Residue in Animal Tissues. J. Agric. Food Chem. 2011, 59, 9763–9769. [Google Scholar] [CrossRef]
- Calvo, D.; Tort, N.; Salvador, J.P.; Marco, M.-P.; Centi, F.; Marco, S. Preliminary study for simultaneous detection and quantification of androgenic anabolic steroids using ELISA and pattern recognition techniques. Analyst 2011, 136, 4045–4052. [Google Scholar] [CrossRef]
- Bulut, U.; Şanli, S.; Cevher, S.C.; Cirpan, A.; Donmez, S.; Timur, S. A biosensor platform based on amine functionalized conjugated benzenediamine-benzodithiophene polymer for testosterone analysis. J. Appl. Polym. Sci. 2020, 137, 49332. [Google Scholar] [CrossRef]
- Lim, S.A.; Ahmed, M.U. Chapter 1. Introduction to immunosensors. In Immunosensors; RSC Publishing: London, UK, 2019; pp. 1–20. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural Single-Domain Antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Xu, Q.; Wu, Y.; Jiang, H.; Wei, W.; Zulipikaer, A.; Guo, Y.; Jirimutu; Chen, J. Nanobodies derived from Camelids represent versatile biomolecules for biomedical applications. Biomater. Sci. 2020, 8, 3559–3573. [Google Scholar] [CrossRef]
- Lee, K.M.; Kim, K.H.; Yoon, H.; Kim, H. Chemical Design of Functional Polymer Structures for Biosensors: From Nanoscale to Macroscale. Polymers 2018, 10, 551. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z. Electrochemical Investigation of Testosterone Using a AuNPs Modified Electrode. Int. J. Electrochem. Sci. 2017, 11224–11234. [Google Scholar] [CrossRef]
- Li, G.; Zhu, M.; Ma, L.; Yan, J.; Lu, X.; Shen, Y.; Wan, Y. Generation of Small Single Domain Nanobody Binders for Sensitive Detection of Testosterone by Electrochemical Impedance Spectroscopy. ACS Appl. Mater. Interfaces 2016, 8, 13830–13839. [Google Scholar] [CrossRef]
- Eguílaz, M.; Moreno-Guzmán, M.; Campuzano, S.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M.; Ruiz, S.C. An electrochemical immunosensor for testosterone using functionalized magnetic beads and screen-printed carbon electrodes. Biosens. Bioelectron. 2010, 26, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Muriano, A.; Salvador, J.-P.; Galve, R.; Marco, M.-P.; Thayil, A.K.N.; Loza-Alvarez, P.; Soria, S. High-sensitive nonlinear detection of steroids by resonant double grating waveguide structures-based immunosensors. In In Proceedings of the SPIE OPTO, San Francisco, CA, USA, 22–27 January 2011; Volume 7941, p. 794114. [Google Scholar] [CrossRef]
- Serafín, V.; Eguílaz, M.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. An Electrochemical Immunosensor for Testosterone Using Gold Nanoparticles—Carbon Nanotubes Composite Electrodes. Electroanalysis 2010, 23, 169–176. [Google Scholar] [CrossRef]
- Laczka, O.; del Campo, F.J.; Muñoz-Pascual, F.X.; Baldrich, E. Electrochemical Detection of Testosterone by Use of Three-Dimensional Disc–Ring Microelectrode Sensing Platforms: Application to Doping Monitoring. Anal. Chem. 2011, 83, 4037–4044. [Google Scholar] [CrossRef]
- Martínez, M.T.; Tseng, Y.-C.; Salvador, J.P.; Marco, M.P.; Ormategui, N.; Loinaz, I.; Bokor, J. Electronic Anabolic Steroid Recognition with Carbon Nanotube Field-Effect Transistors. ACS Nano 2010, 4, 1473–1480. [Google Scholar] [CrossRef]
- Mitchell, J.S.; Lowe, T.E. Ultrasensitive detection of testosterone using conjugate linker technology in a nanoparticle-enhanced surface plasmon resonance biosensor. Biosens. Bioelectron. 2009, 24, 2177–2183. [Google Scholar] [CrossRef]
- Liang, K.-Z.; Qi, J.-S.; Mu, W.-J.; Chen, Z.-G. Biomolecules/gold nanowires-doped sol–gel film for label-free electrochemical immunoassay of testosterone. J. Biochem. Biophys. Methods 2008, 70, 1156–1162. [Google Scholar] [CrossRef]
- Conneely, G.; O’Mahony, D.; Lu, H.; Guilbault, G.G.; Pravda, M.; Aherne, M. An Immunosensor for the Detection of Stanozolol in Bovine Urine. Anal. Lett. 2007, 40, 1280–1293. [Google Scholar] [CrossRef]
- Conneely, G.; Aherne, M.; Lu, H.; Guilbault, G. Electrochemical immunosensors for the detection of 19-nortestosterone and methyltestosterone in bovine urine. Sens. Actuators B Chem. 2007, 121, 103–112. [Google Scholar] [CrossRef]
- Lu, H.; Kreuzer, M.P.; Takkinen, K.; Guilbault, G.G. A recombinant Fab fragment-based electrochemical immunosensor for the determination of testosterone in bovine urine. Biosens. Bioelectron. 2007, 22, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC Trends Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Mistry, K.K.; Layek, K.; Mahapatra, A.; RoyChaudhuri, C.; Saha, H. A review on amperometric-type immunosensors based on screen-printed electrodes. Analyst 2014, 139, 2289–2311. [Google Scholar] [CrossRef] [PubMed]
- Cadwallader, A.B.; Lim, C.S.; Rollins, D.E.; Botrè, F. The Androgen Receptor and Its Use in Biological Assays: Looking Toward Effect-Based Testing and Its Applications. J. Anal. Toxicol. 2011, 35, 594–607. [Google Scholar] [CrossRef] [Green Version]
- Cooper, E.R.; McGrath, K.C.Y.; Heather, A.K. In Vitro Androgen Bioassays as a Detection Method for Designer Androgens. Sensors 2013, 13, 2148–2163. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2016, 16, 181–202. [Google Scholar] [CrossRef] [Green Version]
- Skouridou, V.; Rubio, M.J.; Ballester, P.; Bashammakh, A.S.; El-Shahawi, M.S.; Alyoubi, A.O.; O’Sullivan, C.K. Selection and characterization of DNA aptamers against the steroid testosterone. Microchim. Acta 2017, 184, 1631–1639. [Google Scholar] [CrossRef]
- Darmostuk, M.; Rimpelova, S.; Gbelcova, H.; Ruml, T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol. Adv. 2015, 33, 1141–1161. [Google Scholar] [CrossRef]
- Bai, W.; Zhu, C.; Liu, J.; Yan, M.; Yang, S.; Chen, A. Split aptamer-based sandwich fluorescence resonance energy transfer assay for 19-nortestosterone. Microchim. Acta 2016, 183, 2533–2538. [Google Scholar] [CrossRef]
- Tort, N.; Salvador, J.-P.; Marco, M.-P.; Eritja, R.; Poch, M.; Martínez, E.; Samitier, J. Fluorescence site-encoded DNA addressable hapten microarray for anabolic androgenic steroids. TrAC Trends Anal. Chem. 2009, 28, 718–728. [Google Scholar] [CrossRef] [Green Version]
- Tort, N.; Salvador, J.-P.; Aviñó, A.; Eritja, R.; Comelles, J.; Martínez, E.; Samitier, J.; Marco, M.-P. Synthesis of Steroid–Oligonucleotide Conjugates for a DNA Site-Encoded SPR Immunosensor. Bioconjug. Chem. 2012, 23, 2183–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tort, N.; Salvador, J.-P.; Marco, M.-P. Multimodal plasmonic biosensing nanostructures prepared by DNA-directed immobilization of multifunctional DNA-gold nanoparticles. Biosens. Bioelectron. 2017, 90, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundaca, R.; Moreno-Guzmán, M.; Eguílaz, M.; Yáñez-Sedeño, P.; Pingarrón, J. Enzyme biosensor for androsterone based on 3α-hydroxysteroid dehydrogenase immobilized onto a carbon nanotubes/ionic liquid/NAD+ composite electrode. Talanta 2012, 99, 697–702. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2018, 119, 94–119. [Google Scholar] [CrossRef]
- Liu, K.-H.; O’Hare, D.; Thomas, J.L.; Guo, H.-Z.; Yang, C.-H.; Lee, M.-H. Self-assembly Synthesis of Molecularly Imprinted Polymers for the Ultrasensitive Electrochemical Determination of Testosterone. Biosensors 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Haynes, A.Z.; Levine, M. Detection of anabolic steroids via cyclodextrin-promoted fluorescence modulation. RSC Adv. 2020, 10, 25108–25115. [Google Scholar] [CrossRef]
- Gill, A.D.; Perez, L.; Salinas, I.N.Q.; Byers, S.; Liu, Y.; Hickey, B.L.; Zhong, W.; Hooley, R.J. Selective Array-Based Sensing of Anabolic Steroids in Aqueous Solution by Host–Guest Reporter Complexes. Chem. Eur. J. 2018, 25, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, M.; Tsutsui, T.; Sei, Y.; Akita, M.; Yoshizawa, M. A polyaromatic receptor with high androgen affinity. Sci. Adv. 2019, 5, eaav3179. [Google Scholar] [CrossRef] [Green Version]
- Kellens, E.; Bové, H.; Vandenryt, T.; Lambrichts, J.; Dekens, J.; Drijkoningen, S.; D’Haen, J.; De Ceuninck, W.; Thoelen, R.; Junkers, T.; et al. Micro-patterned molecularly imprinted polymer structures on functionalized diamond-coated substrates for testosterone detection. Biosens. Bioelectron. 2018, 118, 58–65. [Google Scholar] [CrossRef]
- Luo, M.; Hua, Y.; Liang, Y.; Han, J.; Liu, D.; Zhao, W.; Wang, P. Synthesis of novel β-cyclodextrin functionalized S, N codoped carbon dots for selective detection of testosterone. Biosens. Bioelectron. 2017, 98, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, Y.; Sun, G.; Wang, S.; Deng, J.; Wei, H. Molecularly imprinted polymers on graphene oxide surface for EIS sensing of testosterone. Biosens. Bioelectron. 2017, 92, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Heidarimoghadam, R.; Akhavan, O.; Ghaderi, E.; Hashemi, E.; Mortazavi, S.S.; Farmany, A. Graphene oxide for rapid determination of testosterone in the presence of cetyltrimethylammonium bromide in urine and blood plasma of athletes. Mater. Sci. Eng. C 2016, 61, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Lazar, A.I.; Biedermann, F.; Mustafina, K.R.; Assaf, K.I.; Hennig, A.; Nau, W.M. Nanomolar Binding of Steroids to Cucurbit[n]urils: Selectivity and Applications. J. Am. Chem. Soc. 2016, 138, 13022–13029. [Google Scholar] [CrossRef]
- Levent, A.; Altun, A.; Taş, S.; Yardım, Y.; Şentürk, Z. Voltammetric Behavior of Testosterone on Bismuth Film Electrode: Highly Sensitive Determination in Pharmaceuticals and Human Urine by Square-Wave Adsorptive Stripping Voltammetry. Electroanalysis 2015, 27, 1219–1228. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Shen, X.; Chang, Z.; Tang, L.; Dong, W.-F.; Li, M.; He, J.-J. Ultrasensitive Detection of Testosterone Using Microring Resonator with Molecularly Imprinted Polymers. Sensors 2015, 15, 31558–31565. [Google Scholar] [CrossRef] [Green Version]
- Levent, A.; Altun, A.; Yardım, Y.; Şentürk, Z. Sensitive voltammetric determination of testosterone in pharmaceuticals and human urine using a glassy carbon electrode in the presence of cationic surfactant. Electrochim. Acta 2014, 128, 54–60. [Google Scholar] [CrossRef]
- Moura, S.L.; de Moraes, R.R.; dos Santos, M.A.P.; Pividori, M.I.; Lopes, J.A.D.; Moreira, D.D.L.; Zucolotto, V.; Júnior, J.R.D.S. Electrochemical detection in vitro and electron transfer mechanism of testosterone using a modified electrode with a cobalt oxide film. Sens. Actuators B Chem. 2014, 202, 469–474. [Google Scholar] [CrossRef]
- Zhang, Q.; Jing, L.; Zhang, J.; Ren, Y.; Wang, Y.; Wang, Y.; Wei, T.; Liedberg, B. Surface plasmon resonance sensor for femtomolar detection of testosterone with water-compatible macroporous molecularly imprinted film. Anal. Biochem. 2014, 463, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Betatache, A.; Lagarde, F.; Sanglar, C.; Bonhomme, A.; Leonard, D.; Jaffrezic-Renault, N. Gold electrodes modified with molecular imprinted acrylate polymer for impedimetric determination of testosterone. Sens. Transducers 2014, 27, 92. [Google Scholar]
- Goyal, R.N.; Gupta, V.K.; Chatterjee, S. Electrochemical investigations of corticosteroid isomers—testosterone and epitestosterone and their simultaneous determination in human urine. Anal. Chim. Acta 2010, 657, 147–153. [Google Scholar] [CrossRef]
- Goyal, R.N.; Chatterjee, S.; Bishnoi, S. Effect of substrate and embedded metallic impurities of fullerene in the determination of nandrolone. Anal. Chim. Acta 2009, 643, 95–99. [Google Scholar] [CrossRef]
- Chang, K.S.; Chen, C.C.; Sheu, J.T.; Li, Y.-K. Detection of an uncharged steroid with a silicon nanowire field-effect transistor. Sens. Actuators B Chem. 2009, 138, 148–153. [Google Scholar] [CrossRef]
- Kreuzer, M.P.; Quidant, R.; Salvador, J.-P.; Marco, M.-P.; Badenes, G. Colloidal-based localized surface plasmon resonance (LSPR) biosensor for the quantitative determination of stanozolol. Anal. Bioanal. Chem. 2008, 391, 1813–1820. [Google Scholar] [CrossRef]









| Compound of Interest | Approach and Ab Used | Analytical Characteristics | Matrix | Ref. |
|---|---|---|---|---|
| Methandienone | Monoclonal Ab covalently bound to chitosan by a glutaraldehyde linker | MBC of an adsorbent was 3900 ng·mL−1 | Spiked animal tissue and feed samples | [80] |
| Methandienone | Monoclonal Ab against methandienone-KLH coupled to CNBr-activated Sepharose 4B (commercially available) | MBC of an adsorbent was 4760 ng·mL−1 | Spiked animal tissue and feed samples | [81] |
| Methandienone | Polyclonal | MBC of an adsorbent was 334 ng·mL−1 | Spiked animal tissue and feed samples | [82] |
| Epitestosterone | Half-IgG of anti-epitestosterone monoclonal antibodies were covalently immobilized onto Fe3O4 magnetic nanoparticles coated with gold | Pretreatment of urine samples by this novel immunoaffinity column led to an increase in the sensitivity of HPLC analysis by two orders of magnitude (LOD = 60 pg·mL−1) | Human urine | [83] |
| A Compound of Interest/EIA Format | Immunogen/Coating Antigen | Antibodies | Analytical Characteristics of the Most Sensitive System | Specificity of the Most Sensitive System/Determined Cross-Reactants > 1% | Matrix | Ref. |
|---|---|---|---|---|---|---|
| Stanozolol/ ELISA | Three different BSA-derived immunogens/ biotinylated, BSA- and RSA-derived antigens | Eight batches of rabbit polyclonal Ab | IC50RSA = 0.32 ng·mL−1 LODRSA = 20 pg·mL−1 LWRRSA = 0.03–3.53 ng·mL−1 IC50Biotin = 3.9 ng·mL−1 LODBiotin = 570 pg·mL−1 LWRBiotin = 1.1–24.5 ng·mL−1 | Group-specific to 17α-methylated AAS | Dietary supplements | [40] |
| Methyltestosterone/ ELISA | BSA-derived immunogen/ OVA-derived antigen | Eight murine polyclonal/ one monoclonal Ab | IC50 = 0.3–4.4 µg·L−1 LOD = 37.2–697.8 ng·L−1 LOQ = 70.0–1524.0 ng·L−1 | Nortestosterone, testosterone, and trenbolone | 11 types of animal tissues | [87] |
| DHEA/ ELISA | Rabbit polyclonal Ab | IC50 = 4.89 ng·mL−1 LOD = 0.1 ng·mL−1 LWR = 0.41–58.77 ng·mL−1 | Androstenedione | Slimming products (teas, capsules, tablets) | [88] | |
| Mesterolone/ ELISA | BSA-derived immunogen and antigen | IC50 = 4.2 ng·mL−1 LOD = 10 pg·mL−1 LWR = 1–34 ng·mL−1 | Dihydrotestosterone, testosterone, progesterone, boldenone sulfate, 4-androstene-3,17-dione, nandrolone, methandienone, boldenone undecanoate, epitestosterone, oxandrolone, trenbolone, dehydroepiandrosterone | Dietary supplements | [89] | |
| Methandienone/ELISA | BSA-derived immunogen/ OVA-derived antigen | IC50 = 1.54 ng·mL−1 LOD = 40 pg·mL−1 LWR = 0.2–12 ng·mL−1 | Boldenone and its derivatives, testosterone and its derivatives, 4-androstene-19-ol-3,17-dione, cortisone, 4-androsten-3,17-dione, 11-deoxycorticosterone | [38] | ||
| Nandrolone and testosterone/ ELISA | Four BSA-derived immunogens/ linker-optimized biotinylated nandrolone and testosterone as antigens | Four batches of rabbit polyclonal Ab | The most sensitive nandrolone-based system: IC50 = 180 pg·mL−1 LOD = 4 pg·mL−1 LWR = 0.02–1.38 ng·mL−1 | CR in respect to nandrolone: testosterone, dihydrotestosterone, drostanolone, trenbolone, boldenone | [39] | |
| Boldenone/ ELISA | BSA-derived immunogen/ OVA-derived antigen | Rabbit polyclonal Ab | IC50 = 293 pg·mL−1 LOD = 14 pg·mL−1 LWR = 0.065–1.52 ng·mL−1 | Boldenone and its derivatives, dihydrotestosterone, methandienone, testosterone | [37] | |
| Stanozolol/ CLEIA using luminol | Two batches of rabbit polyclonal Ab | IC50 = 340 pg·mL−1 LOD = 70 pg·mL−1 | Oxymetholone, testosterone | Various plant and animal tissues | [90] | |
| Methyltestosterone/ELISA | Murine monoclonal Ab | IC50 = 260 pg·mL−1 LOD = 45 pg·mL−1 LWR = 0.02–1.38 ng·mL−1 | Testosterone, nortestosterone | Animal feed | [91] | |
| Methandienone/ELISA | BSA-derived immunogen/KLH-derived immunogen | Murine monoclonal Ab | IC50 = 7.89 ng·mL−1 LOD = 0.17 ng·mL−1 | n.a. | n.a. | [81] |
| Stanozolol, boldenone and tetrahydrogestrinone/ELISA | Multianalyte ELISA/four BSA-derived immunogens/three BSA-derived antigens | Cocktail of three rabbit polyclonal Abs | IC50 = 0.16–9.75 ng·mL−1 LOD = 20–340 ng·mL−1 | Detection of up to 11 AASs | Human serum | [92] |
| Nandrolone/ ELISA | BSA-derived immunogen/OVA-derived antigen | Murine monoclonal Ab | IC50 = 0.52 ng·mL−1 LOD = 0.01 ng·mL−1 LWR = 0.03–38 ng·mL−1 | 17α-Nortestosterone, trenbolone, β-boldenone | Beef and pork tissues | [93] |
| Stanozolol, boldenone, methylboldeno-ne, tetrahydrogestrinone/ELISA | Multiple ELISA (combination of 8 assays)/ 8 BSA-derived antigens/multiple component analyses calculation | Six rabbit polyclonal Abs | IC50 = 0.38–2.60 nM LOD = 0.1–316 nM | Detection of up to 23 AASs | Human serum and urine | [94] |
| Stanozolol, 6β-hydroxy-stanozolol/ ELISA | Immunosorbent solid phase as a pre-step/BSA- derived immunogen/ coated with antiserum | Two rabbit polyclonal Abs | Values for stanozolol: IC50 = 550 ng·mL−1 LOD = 36 ng·mL−1 LWR = 104–2720 ng·mL−1 | CR in respect to stanozolol: 16β-hydroxystanozolol, norstanozolol, 3′-hydroxystanazolol, boldenone, methylboldenone | Cow urine | [84] |
| Compound of Interest | Approach and Used Ab | Analytical Characteristics | Matrix | Ref. |
|---|---|---|---|---|
| 17α-Methylated AASs | Gold-labeled rabbit polyclonal | LOD = 0.7 ng·mL−1 | Dietary supplements | [41] |
| Dehydroepiandrosterone | LOD = 500 µg·kg−1 | Slimming products (herbal teas, capsules, pills) | [88] | |
| Mesterolone | LOD = 50 ng·mL−1 | Dietary supplements | [89] | |
| Methyltestosterone | Gold-labeled murine monoclonal | LOD = 1 ng·mL−1 | Animal feed | [91] |
| Nandrolone | Gold-labeled rabbit polyclonal | LOD = 1 ng·mL−1 | Dietary supplements | [39] |
| Nandrolone | Gold-labeled murine monoclonal | LOD = 1 ng·mL−1 | Beef and pork tissues | [93] |
| Compound of Interest | Type of Transduction and Its Principle | Description of Methods and Materials Used | Analytical Characteristics | Matrix | Ref. |
|---|---|---|---|---|---|
| Testosterone, DHEA | Electrochemical/ amperometric | Anti-testosterone Abs/glutaraldehyde/the polymer drop-coated screen-printed carbon electrode surface | LOD = 16.7 ng·mL−1 LWR = 10–500 ng·mL−1 | Synthetic urine and synthetic serum | [95] |
| Testosterone | Electrochemical/ impedance spectroscopy | Anti-testosterone Abs/Au(3-mercaptopropionic acid)/ (3-aminopropyl) triethoxysilane/indium tin oxide glass electrode | LOD = 3.9 ng·mL−1 LWR = 10–500 ng·mL−1 | Saliva | [100] |
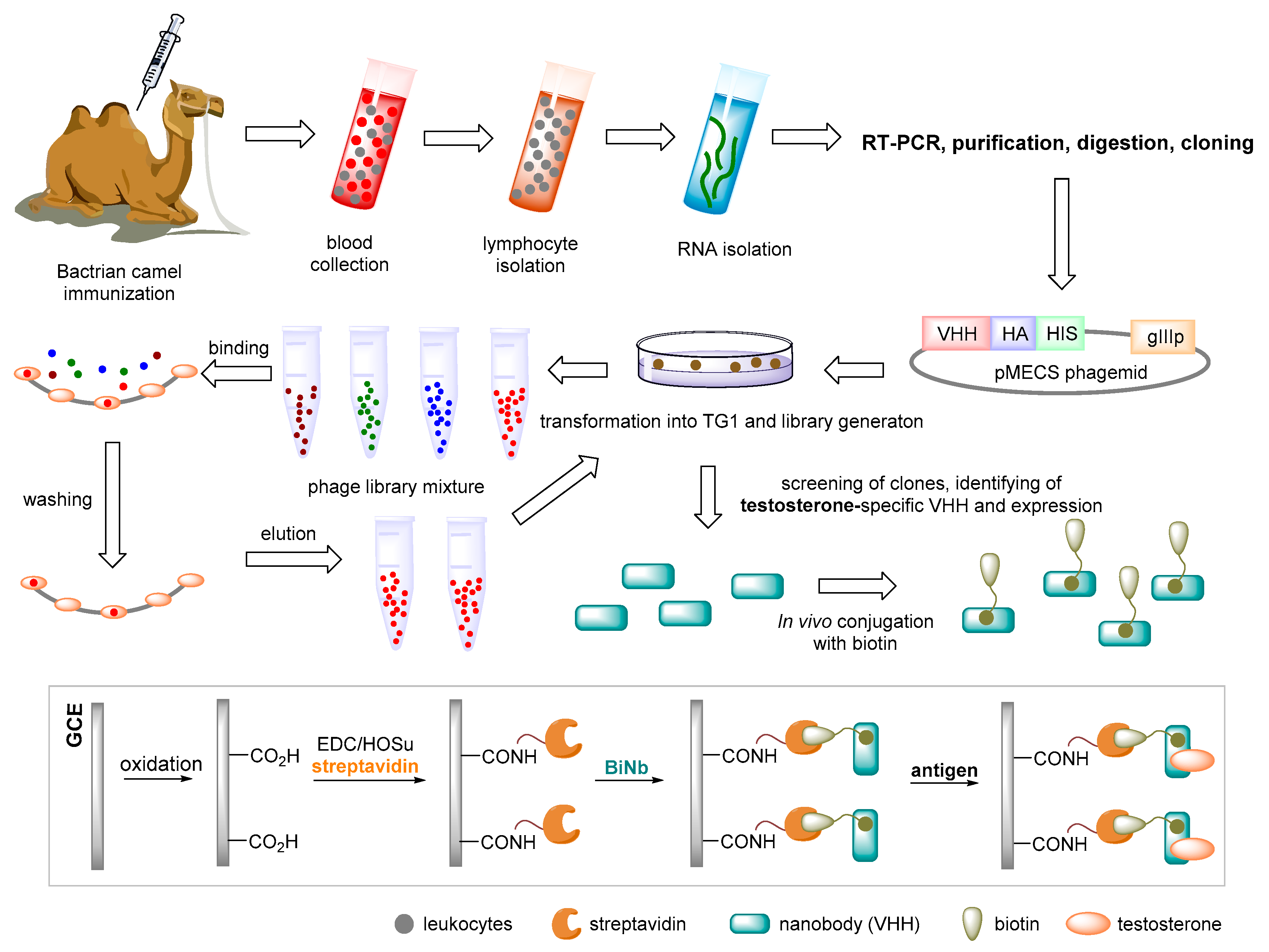
| Testosterone | Electrochemical/ impedance spectroscopy | Isolation of Bactrian nanobody from an immune phage display library/ biotinylation/glassy carbon electrode | LOD = 0.045 ng·mL−1 LWR = 0.05–5 ng·mL−1 | Serum | [101] |
| Testosterone | Electrochemical/ amperometric | Screen-printed carbon electrodes and protein-A-functionalized magnetic beads/testosterone labeled with HRP/ hydroquinone as the redox mediator | LOD = 1.7 pg·mL−1 LWR = 0.005–50 ng·mL−1 EC50 = 250 pg·mL−1 | Human serum | [102] |
| Methylboldenone | Optical/ two-photon fluorescence emission | Immunoreagents/immobilized onto a resonant Ta2O5 double -grating waveguide structure | LOD = 0.1 ng·mL−1 IC50 = 4.6 ng·mL−1 | Buffer | [103] |
| Testosterone | Electrochemical/ amperometric | Testosterone and HRP-testosterone/Abs on AuNPs/MWCNTs/Teflon electrodes/H2O2 with catechol as redox mediator | LOD = 85 pg·mL−1 LWR = 0.1–10 ng·mL−1 | Human serum | [104] |
| Testosterone | Electrochemical/ chronoamperometric | 3D competitive sensing platforms/gold disc-ring microelectrode array for immunofunctionalization/near second microelectrode array for electrochemical monitoring | LOD = 12.5 pg·mL−1 LWR = 0.01–10 ng·mL−1 | Human saliva | [105] |
| Stanozolol and methylboldenone | Electrochemical/ amperometric, voltammetric | Two specific Abs/arrays of carbon nanotube field-effect transistors | Only recognition | Optimal conditions | [106] |
| Testosterone | Optical/ surface plasmon resonance | Testosterone/oligoethylene glycol/ surface plasmon resonance biosensor/secondary Abs and AuNP signal enhancement | LOD = 15.4 pg·mL−1 LWR = 29–290 pg·mL−1 | Human saliva | [107] |
| Testosterone | Electrochemical/ potentiometric | Anti-testosterone Abs/polyvinyl butyral sol–gel film doped with gold nanowires | LOD = 0.1 ng·mL−1 LWR = 1.2–83.5 ng·mL−1 | Human serum | [108] |
| Stanozolol | Electrochemical/ chronoamperometric | Immobilized antigen–protein conjugate on screen-printed electrodes | LOD = 41.6 pg·mL−1 LWR = 0.2–500 ng·mL−1 EC50 = 2.15 ng·mL−1 | Bovine urine | [109] |
| Nandrolone and methyltestosterone | 19-Nortestosterone: LOD = 10.5 pg·mL−1 EC50 = 936 pg·mL−1 methyltestosterone: LOD = 14.8 pg·mL−1 EC50 = 274 pg·mL−1 | [110] | |||
| Testosterone | Immobilized testosterone conjugate on screen-printed electrodes/ anti-testosterone Abs fragments | LOD = 90 pg·mL−1 LWR = 0.3–40 ng·mL−1 | [111] |
| Compound of Interest | Principle of Transduction or Detection | Description of Method and Used Materials | Analytical Characteristics | Matrix | Ref. |
|---|---|---|---|---|---|
| Testosterone | Cyclic voltammetry | Synthetic self-assembly of poly(aniline-co-metanilic acid) and testosterone forming imprinted electronically conductive polymers on sensing electrodes | LOD = units of pM LWR = 0.1–100 pg·mL−1 | Urine | [125] |
| Mesterolone, oxandrolone, oxymetholone, stanozolol, trenbolone | Fluorescence modulation | β-Cyclodextrin-promoted interactions between the analyte of interest and fluorescent rhodamine 6G, leading to analyte-specific changes in the fluorophore emission signal | LOD = 0.775–17 µM specificity = 100% differentiation between structurally similar analytes | Citrate buffer | [126] |
| Stanozolol, 17α-methyltestosterone, methandienone | Arrayed complexes of host-guest cavitands using two fluorescent indicators and a low amount of small metal ions | LOD = 10 µM; highly selective, able to discriminate between structures varying only by a single π bond | Human urine | [127] | |
| Testosterone | Fluorescent detection | Fluorescent detection of testosterone by a receptor-dye complex. The emission of a fluorescent coumarin derivative as a dye guest is displaced by a more hydrophobic hormone guest | Discrimination between testosterone and female hormones in the order of molecule units | Water | [128] |
| Electrochemical impedance spectroscopy | Microstructures of molecularly imprinted polymers on functionalized nanocrystalline diamond/ testosterone target molecule/ N,O-bismethacryloyl ethanolamine as a bifunctional monomer | LOD = 0.5 nM LWR = 0.5–20 nM | Human urine and saliva | [129] | |
| A photoinduced electron transfer fluorescent probe system | Covalently linking β-cyclodextrin to the surface of N, S co-doped carbon dots/carbon dot and (ferrocenylmethyl)trimethylammonium iodide (Fc+) | LOD = 0.51 μM LWR = 0–280 μM | Water and cytoplasm | [130] | |
| Testosterone | Electrochemical impedance spectroscopy | Nanosized molecularly imprinted polymer film that was electrochemically grafted on a graphene oxide sheet/modified glassy carbon electrode | LOD = 0.4 fM LWR = 1 fM–1 µm | Human serum | [131] |
| Differential pulse voltammetry | Electrochemical reduction of testosterone in the presence of a cationic surfactant using graphene oxide/glassy carbon electrode | LOD = 0.1 nM LWR = 2–210 nM | Human plasma and urine | [132] | |
| Testosterone, nandrolone, nandrolone-17- propionate | Fluorescence emission-based binding assays | Cucurbit[n]urils as a high-binding -capacity host provide water-soluble formulations for an analyte of interest. Displacement of a fluorescent dye by various steroidal analytes provides a distinct and measurable fluorescent response | LOD = units of µM | Water, buffer, gastric acid, blood serum | [133] |
| Testosterone | Square-wave adsorptive stripping voltammetry | Bismuth film/ glassy carbon electrode | LWR = 1–45 nmol·L−1 LOD = 0.3 nmol·L−1 and 0.09 ng·mL−1 | Oil-based pharmaceuticals and human urine | [134] |
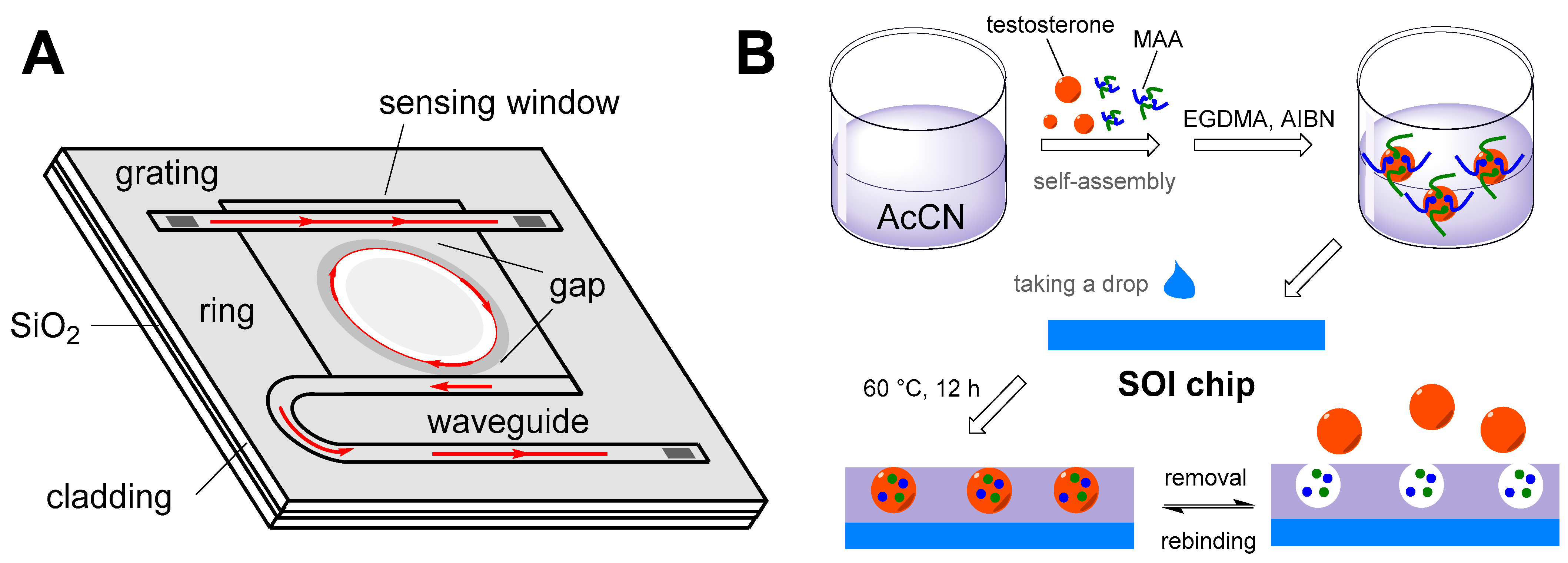
| Testosterone | Resonant wavelength shift | Micro-ring resonator sensor with MIP | LWR = 0.05–10 ng·mL−1 LOD = 48.7 pg·mL−1 | Deionized water | [135] |
| Testosterone | Surface plasmon resonance | Double photografting polymerization of 1-dodecanethiol leading to a double layer of MIF on the gold surface of SPR sensor chips | LWR = 1 × 10−12–1 × 10−8 mol·L−1 LOD = 10−12 mol·L−1 | Seawater | [48] |
| Square-wave adsorptive stripping voltammetry | Glassy carbon electrode in the presence of cationic surfactant | LWR = 10–70 nM LOD = 1.2 nM | Oil-based pharmaceuticals and human urine | [136] | |
| Cyclic voltammetry | Oxidation of testosterone at the plane glassy carbon electrode modified with cobalt oxide | LWR = 0.33 to 2.00 µM LOD = 0.16 µM | Supporting electrolyte (0.10 M NaOH) | [137] | |
| Testosterone | Surface plasmon resonance | Gold-chip-based macroporous molecularly imprinted film in combination with polystyrene nanoparticles | LOD = units of fg·mL−1 | Artificial urine and human urine | [138] |
| Testosterone | Electrochemical impedance spectroscopy | MIP was synthetized at the surface of gold electrodes via a photoradical initiator covalently coupled with a self-assembled monolayer of amine-terminated alkanethiol | Linearity up to 50 µg·L−1 LOD = 103 ng·L −1 | PBS buffer | [139] |
| Testosterone, epitestosterone | Square-wave voltammetry | Bare and single-wall carbon nanotubes modified an edge plane of a pyrolytic graphite electrode | LODT = 2.8 × 10−9 M LODET = 4.1 × 10−9 M LWRT&ET = 5–1000 nM | Human urine | [140] |
| Nandrolone | Fullerene modified an edge plane of a pyrolytic graphite electrode | LWR = 0.01–50 nM LOD = 1.5 × 10−11 M | Medicinal samples | [141] | |
| 19-Norandrostendione | Conductance | Chemically modified Δ5-3-ketosteroid isomerase immobilized on the surface of a silicon nanowire | LOD = units of fM | n.a. | [142] |
| Stanozolol | Localized SPR | Functionalized glass substrates by noble metal gold colloid | LOD = 0.7 μg·L−1 Dt = 2 min | Buffer solution | [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huml, L.; Tauchen, J.; Rimpelová, S.; Holubová, B.; Lapčík, O.; Jurášek, M. Advances in the Determination of Anabolic-Androgenic Steroids: From Standard Practices to Tailor-Designed Multidisciplinary Approaches. Sensors 2022, 22, 4. https://doi.org/10.3390/s22010004
Huml L, Tauchen J, Rimpelová S, Holubová B, Lapčík O, Jurášek M. Advances in the Determination of Anabolic-Androgenic Steroids: From Standard Practices to Tailor-Designed Multidisciplinary Approaches. Sensors. 2022; 22(1):4. https://doi.org/10.3390/s22010004
Chicago/Turabian StyleHuml, Lukáš, Jan Tauchen, Silvie Rimpelová, Barbora Holubová, Oldřich Lapčík, and Michal Jurášek. 2022. "Advances in the Determination of Anabolic-Androgenic Steroids: From Standard Practices to Tailor-Designed Multidisciplinary Approaches" Sensors 22, no. 1: 4. https://doi.org/10.3390/s22010004
APA StyleHuml, L., Tauchen, J., Rimpelová, S., Holubová, B., Lapčík, O., & Jurášek, M. (2022). Advances in the Determination of Anabolic-Androgenic Steroids: From Standard Practices to Tailor-Designed Multidisciplinary Approaches. Sensors, 22(1), 4. https://doi.org/10.3390/s22010004