A Sensitive Capacitive Biosensor for Protein a Detection Using Human IgG Immobilized on an Electrode Using Layer-by-Layer Applied Gold Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Fabrication of Gold Electrode
2.2.2. Preparation of Layer-by-Layer (LbL) of TU and AuNPs and Human IgG Immobilization
2.2.3. Characterization of LbL Modified Electrodes by Cyclic Voltammetry
2.2.4. Quantification of Immobilized Human IgG
2.2.5. Capacitance Measurement
2.2.6. Regeneration of the Electrode
2.2.7. Reproducibility of Electrode Preparation
3. Results
3.1. AFM Images
3.2. Cyclic Voltammetry
3.3. Amount of Immobilized Human IgG
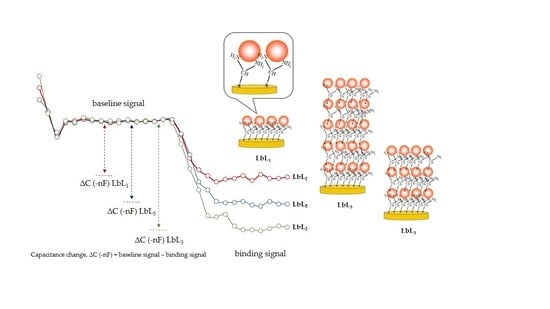
3.4. Capacitive Measurement with Human IgG Modified Electrode
3.5. Sensitivity of the Biosensor Electrodes
3.6. Analytical Characteristics
3.6.1. Linear Range and Limit of Detection
3.6.2. Regeneration of Electrodes
3.6.3. Reproducibility of Electrode Preparation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mattiasson, B.; Teeparuksapun, K.; Hedström, M. Immunochemical binding assays for detection and quantification of trace impurities in biotechnological production. Trends Biotechnol. 2010, 28, 20–27. [Google Scholar] [CrossRef]
- Gilgunn, S.; El-Sabbahy, H.; Albrecht, S.; Gaikwad, M.; Corrigan, K.; Deakin, L.; Jellum, G.; Bones, J. Identification and tracking of problematic host cell proteins removed by a synthetic, highly functionalized nonwoven media in downstream bioprocessing of monoclonal antibodies. J. Chromatogr. A 2019, 1595, 28–38. [Google Scholar] [CrossRef]
- Levy, N.E.; Valente, K.N.; Lee, K.H.; Lenhoff, A.M. Host cell protein impurities in chromatographic polishing steps for monoclonal antibody purification. Biotechnol. Bioeng. 2015, 113, 1260–1272. [Google Scholar] [CrossRef]
- Schenauer, M.; Flynn, G.; Goetze, M. Identification and quantification of host cell protein impurities in biotherapeutics using mass spectrometry. Anal. Biochem. 2012, 428, 150–157. [Google Scholar] [CrossRef]
- Shen, W.; Lai, Y.; Lai, C.; Rakoczy, P. Impurity of recombinant adeno-associated virus type 2 affects the transduction characteristics following subretinal injection in the rat. Vis. Res. 2004, 44, 339–348. [Google Scholar] [CrossRef][Green Version]
- Zhu-Shimonji, J.; Gunawan, F.; Thomas, A.; Vanderlaan, M.; Stults, J. Trace level analysis of leached protein A in bioprocess samples without interference from the large excess of rhMAb IgG. Immunol. Methods 2009, 341, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Rey, G.; Wendeler, M.W. Full automation and validation of a flexible ELISA platform for host cell protein and protein a impurity detection in biopharmaceuticals. J. Pharm. Biomed. 2012, 70, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.I.; Lee, A.; Reddy, B.; Muir, A.; Soong, G.; Pitt, A. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat. Med. 2004, 10, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, W.I.; Buckner, C.D.; Clift, R.A.; Thomas, E.D. Clinical-trials with staphylococcal protein-A. J. Biol. Response Mod. 1984, 3, 347–351. [Google Scholar] [PubMed]
- Bertram, J.H.; Grunberg, S.M.; Shulman, I.; Apuzzo, M.L.J.; Boquiren, D.; Kunkel, L. Staphylococcal protein-A column—Correlation of mitogenicity of perfused plasma with clinical-response. Cancer Res. 1985, 45, 4486–4494. [Google Scholar]
- Luo, Y.; Matejic, T.; Ng, C.K.; Nunnally, B.; Porter, T.; Raso, S.; Rouse, J.; Shang, T.; Steckert, J. Characterization and analysis of biopharmaceutical proteins. Sep. Sci. Technol. 2011, 10, 283–359. [Google Scholar]
- Stewart, D.J.; Purvis, D.R.; Pitts, J.M.; Lowe, C.R. Development of an enzyme-linked immunosorbent assay for C.I. Reactive Blue 2 and its application to a comparison of the stability and performance of a perfluorocarbon support with other immobilised C.I. Reactive Blue 2 affinity adsorbents. J. Chromatogr. A 1992, 623, 1–14. [Google Scholar] [CrossRef]
- Loyprasert, S.; Hedström, M.; Thavarungkul, P.; Kanatharana, P.; Mattiasson, B. Sub-attomolar detection of cholera toxin using a label-free capacitive immunosensor. Biosens. Bioelectron. 2010, 25, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Teeparuksapun, K.; Hedström, M.; Wong, E.Y.; Tang, S.; Hewlett, I.K.; Mattiasson, B. Ultrasensitive detection of HIV-1 p24 antigen using nanofunctionalized surfaces in a capacitive immunosensor. Anal. Chem. 2010, 82, 8406–8411. [Google Scholar] [CrossRef] [PubMed]
- Teeparuksapun, K.; Hedström, M.; Kanatharana, P.; Thavarungkul, P.; Mattiasson, B. Capacitive immunosensor for the detection of host cell proteins. J. Biotechnol. 2012, 157, 207–213. [Google Scholar] [CrossRef]
- Berggren, C.; Bjarnason, B.; Johansson, G. Instrumentation for direct capacitive biosensors. Instrum. Sci. Technol. 1999, 27, 131–139. [Google Scholar] [CrossRef]
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Berggren, C.; Johansson, G. Capacitance measurements of antibody−antigen interactions in a flow system. Anal. Chem. 1997, 69, 3651–3657. [Google Scholar] [CrossRef]
- Niyomdecha, S.; Limbut, W.; Numnuam, A.; Kanatharana, P.; Charlermroj, R.; Karoonuthaisiri, N.; Thavarungkul, P. Phage-based capacitive biosensor for Salmonella detection. Talanta 2018, 188, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Valat, C.; Limoges, B.; Huet, D.; Romette, J.L. A disposable protein A-based immunosensor for flow-injection assay with electrochemical detection. Anal. Chim. Acta 2000, 404, 187–194. [Google Scholar] [CrossRef]
- Brogan, K.L.; Wolfe, K.N.; Jones, P.A.; Schoenfisch, M.H. Direct oriented immobilization of F(ab′) antibody fragments on gold. Anal. Chim. Acta 2003, 496, 73–80. [Google Scholar] [CrossRef]
- Kang, J.H.; Choi, H.J.; Hwang, S.Y.; Han, S.H.; Jeon, J.Y.; Lee, E.K. Improving immunobinding using oriented immobilization of an oxidized antibody. J. Chromatogr. A 2007, 1161, 9–14. [Google Scholar] [CrossRef]
- Dawan, S.; Kanatharana, P.; Wongkittisuksa, B.; Limbut, W.; Numnuam, A.; Limsakul, C. Label-free capacitive immunosensors for ultra-trace detection based on the increase of immobilized antibodies on silver nanoparticles. Anal. Chim. Acta 2011, 699, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Hedström, M.; Galaev, I.Y.; Mattiasson, B. Continuous measurements of a binding reaction using a capacitive biosensor. Biosens. Bioelectron. 2005, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Hedström, M.; Amin, M.; Mattiasson, B. A capacitive immunosensor for detection of cholera toxin. Anal. Chim. Acta 2009, 634, 255–261. [Google Scholar] [CrossRef]
- Li, Y.; Schluesenerb, H.J.; Xua, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Narayanan, R. Nanoparticles of different shapes for biosensor applications. ACS Symp. Ser. Am. Chem. Soc. 2012, 1112, 281–292. [Google Scholar]
- Hamdy, M.E.; Del Carlo, M.; Hussein, H.A.; Salah, T.A.; El-Deeb, A.H.; Emara, M.M.; Pezzoni, G.; Campagnone, D. Development of gold nanoparticles biosensor for ultrasensitive diagnosis of foot and mouth disease virus. J. Nanobiotechnol. 2018, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Yong, K.T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A Review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Feng, J.J.; Zhao, G.; Xu, J.J.; Chen, H.Y. Direct electrochemistry and electrocatalysis of heme proteins immobilized on gold nanoparticles stabilized by chitosan. Anal. Biochem. 2005, 342, 280–286. [Google Scholar] [CrossRef]
- Fu, Y.; Yuan, R.; Tang, D.; Chai, Y.; Xu, L. Study on the immobilization of anti-IgG on Au-colloid modified gold electrode via potentiometric immunosensor, cyclic voltammetry, and electrochemical impedance techniques. Colloids Surf. B Biointerfaces 2005, 40, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Yuan, R.; Chai, Y.; Ou, C.; Cao, S.; Li, X. Amperometric immunosensors based on layer-by-layer assembly of gold nanoparticles and methylene blue on thiourea modified glassy carbon electrode for determination of human chorionic gonadotrophin. Talanta 2008, 74, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yuan, R.; Chai, Y. Quartz crystal microbalance immunoassay for carcinoma antigen 125 based on gold nanowire-functionalized biomimetic interface. Analyst 2008, 133, 933–938. [Google Scholar] [CrossRef]
- Wu, B.Y.; Hou, S.H.; Yin, F.; Li, J.; Zhao, Z.X.; Huang, J.D.; Chen, Q. Amperometric glucose biosensor based on layer-by-layer assembly of multilayer films composed of chitosan, gold nanoparticles and glucose oxidase modified Pt electrode. Biosens. Bioelectron. 2007, 22, 838–844. [Google Scholar] [CrossRef]
- Pichugina, D.A.; Shestakov, A.F.; Kuz’menko, N.E. A theoretical study of the activation of methane by Gold (III) homoleptic complexes. Russ. J. Phys. Chem. 2007, 81, 883–894. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, T.; Zhang, W.; Jiang, C.; Jiao, K. Enhanced sensitivity for deoxyribonucleic acid electrochemical impedance sensor: Gold nanoparticle/polyaniline nanotube membranes. Anal. Chim. Acta 2008, 616, 144–151. [Google Scholar] [CrossRef]
- Masereel, B.; Dinguizli, M.; Bouzin, C.; Moniotte, N.; Feron, O.; Gallez, B.; Borght, T.V.; Michiels, C.; Lucas, S. Antibody immobilization on gold nanoparticles coated layer-by-layer with polyelectrolytes. J. Nanopart. Res. 2010, 13, 1573–1580. [Google Scholar] [CrossRef]
- Chen, S.H.; Chuang, Y.C.; Lu, Y.C.; Lin, H.C.; Yang, Y.L.; Lin, C.S. A method of layer-by-layer gold nanoparticle hybridization in a quartz crystal microbalance DNA sensing system used to detect dengue virus. Nanotechnology 2009, 20, 215501. [Google Scholar] [CrossRef]
- Machado, G.; Feil, A.F.; Migowski, P.; Rossi, L.; Giovanela, M.; Crespo, J.S. Structural control of gold nanoparticles self-assemblies by layer-by-layer process. Nanoscale 2011, 3, 1717–1723. [Google Scholar] [CrossRef]
- Gupta, S.; Jain, U.; Murti, B.T.; Putri, A.D.; Tiwari, A.; Chauhan, N. Nanohybrid-based immunosensor prepared for Helicobacter pylori BabA antigen detection through immobilized antibody assembly with @ Pdnano/rGO/PEDOT sensing platform. Sci. Rep. 2020, 10, 21217. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, T.M.; Gao, J. Layer-by-layer immobilization of horseradish peroxidase on a gold electrode modified with colloidal gold nanoparticles. Microchim. Acta 2008, 161, 241–248. [Google Scholar] [CrossRef]
- Lvov, Y.; Decher, G.; Sukhorukov, G. Assembly of thin-films by means of successive deposition of alternate layers of DNA and poly (Allylamine). Macromolecules 1993, 26, 5396–5399. [Google Scholar] [CrossRef]
- Lvov, Y.; Ariga, K.; Ichinose, I.; Kunitake, T. Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J. Am. Chem. Soc. 1995, 117, 6117–6123. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Wang, X.; Zhao, B.; Zhang, J.; Li, C. Preparation of hollow capsule-stabilized gold nanoparticles through the encapsulation of the dendrimer. J. Colloid Interface Sci. 2006, 302, 142–148. [Google Scholar] [CrossRef]
- Ma, H.Y.; Zhang, L.P.; Pan, Y.; Zhang, K.Y.; Zhang, Y.Z. A novel electrochemical DNA biosensor fabricated with layer-by-layer covalent attachment of multiwalled carbon nanotubes and gold nanoparticles. Electroanalysis 2008, 20, 1220–1226. [Google Scholar] [CrossRef]
- Wang, Y.; Byrne, J.D.; Napier, M.E.; DeSimone, J.M. Engineering nanomedicines using stimuli-responsive biomaterials. Adv. Drug Deliv. Rev. 2012, 64, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Limbut, W.; Kanatharana, P.; Mattiasson, B.; Asawatreratanakul, P.; Thavarungkul, P. A reusable capacitive immunosensor for carcinoembryonic antigen (CEA) detection using thiourea modified gold electrode. Anal. Chim. Acta 2006, 561, 55–61. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recommendations for nomenclature of ion-selective electrodes (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Mattiasson, B.; Teeparuksapun, K.; Lebogang, L.; Hedström, M. Nanoenvironmental effects dramatically influence the sensitivity of immunoassays. Trends Biotechnol. 2017, 35, 1021–1024. [Google Scholar] [CrossRef]





| Electrode Modifications | Sensitivity (−nF/cm2/log M) |
|---|---|
| SAM 2 h | 2.31 ± 0.23 |
| SAM 24 h | 5.11 ± 0.23 |
| LbL1 | 5.48 ± 0.31 |
| LbL2 | 7.71 ± 0.30 |
| LbL3 | 7.66 ± 0.43 |
| LbL4 | 7.69 ± 0.37 |
| LbL5 | 6.64 ± 0.42 |
| Analyte | Detection Method | Detection Range | LOD | References |
|---|---|---|---|---|
| carcinoma antigen 125 | quartz crystal microbalance | 1.5–180 U ml−1 | 0.5 mL−1 | [33] |
| glucose | amperometric | 0.5–16 mM | 7.0 µM | [34] |
| DNA | impedance | 1.0 × 10−12 to 1.0 × 10−6 M | 3.1 × 10−13 M | [36] |
| virus | quartz crystal microbalance | 2 × 100 to 2 × 106 PFU mL−1 | 2 PFU mL−1 | [38] |
| protein A | capacitive | 1 × 10−16 to 1 × 10−13 M | 9 × 10−17 M | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teeparuksapun, K.; Hedström, M.; Mattiasson, B. A Sensitive Capacitive Biosensor for Protein a Detection Using Human IgG Immobilized on an Electrode Using Layer-by-Layer Applied Gold Nanoparticles. Sensors 2022, 22, 99. https://doi.org/10.3390/s22010099
Teeparuksapun K, Hedström M, Mattiasson B. A Sensitive Capacitive Biosensor for Protein a Detection Using Human IgG Immobilized on an Electrode Using Layer-by-Layer Applied Gold Nanoparticles. Sensors. 2022; 22(1):99. https://doi.org/10.3390/s22010099
Chicago/Turabian StyleTeeparuksapun, Kosin, Martin Hedström, and Bo Mattiasson. 2022. "A Sensitive Capacitive Biosensor for Protein a Detection Using Human IgG Immobilized on an Electrode Using Layer-by-Layer Applied Gold Nanoparticles" Sensors 22, no. 1: 99. https://doi.org/10.3390/s22010099
APA StyleTeeparuksapun, K., Hedström, M., & Mattiasson, B. (2022). A Sensitive Capacitive Biosensor for Protein a Detection Using Human IgG Immobilized on an Electrode Using Layer-by-Layer Applied Gold Nanoparticles. Sensors, 22(1), 99. https://doi.org/10.3390/s22010099