The Influence of Air Humidity on the Output Signal from an Ionization Smoke Detector in the Presence of Soot Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticle Detector
2.2. Experimental Setup
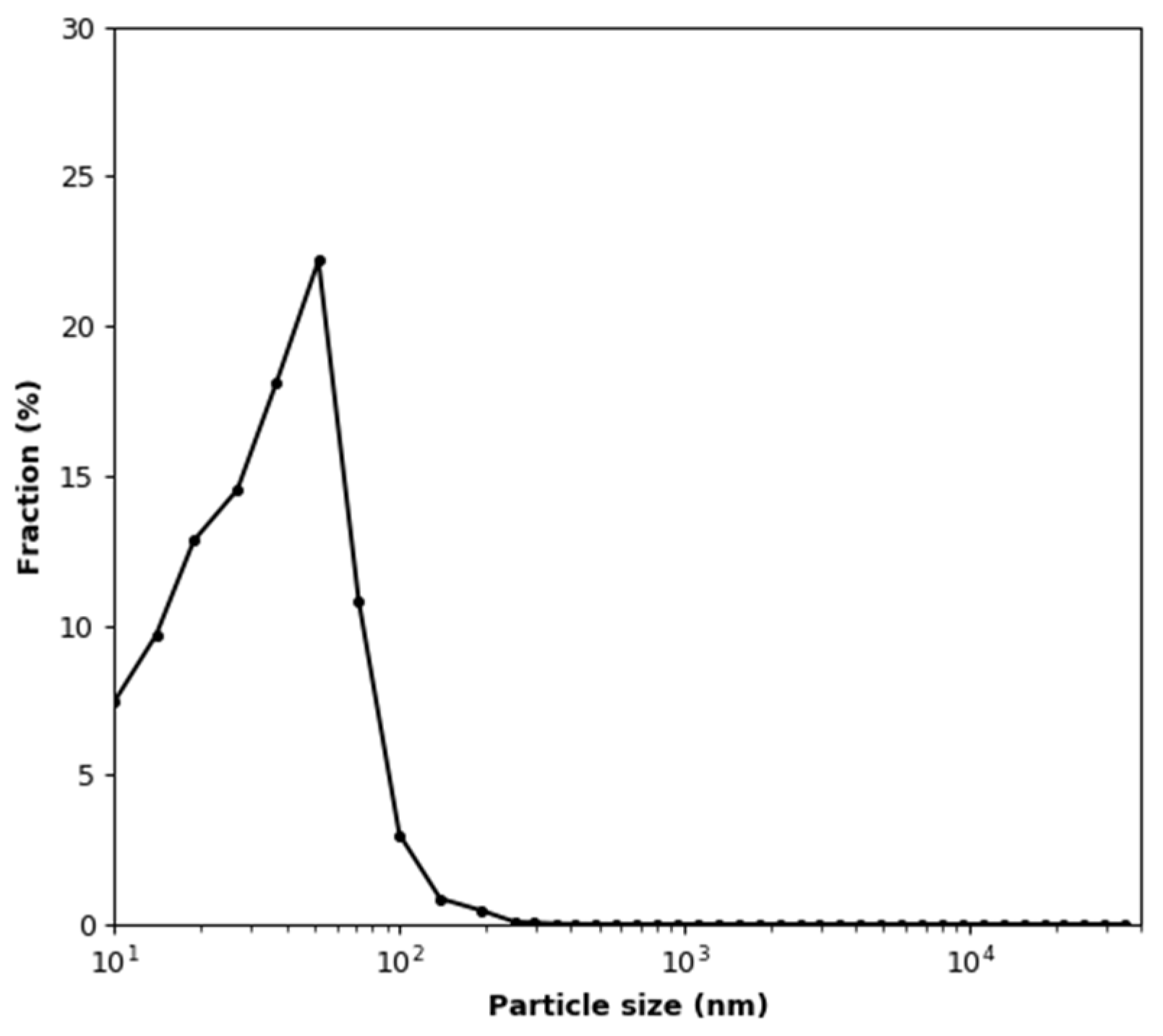
2.3. Methods
3. Results and Discussion
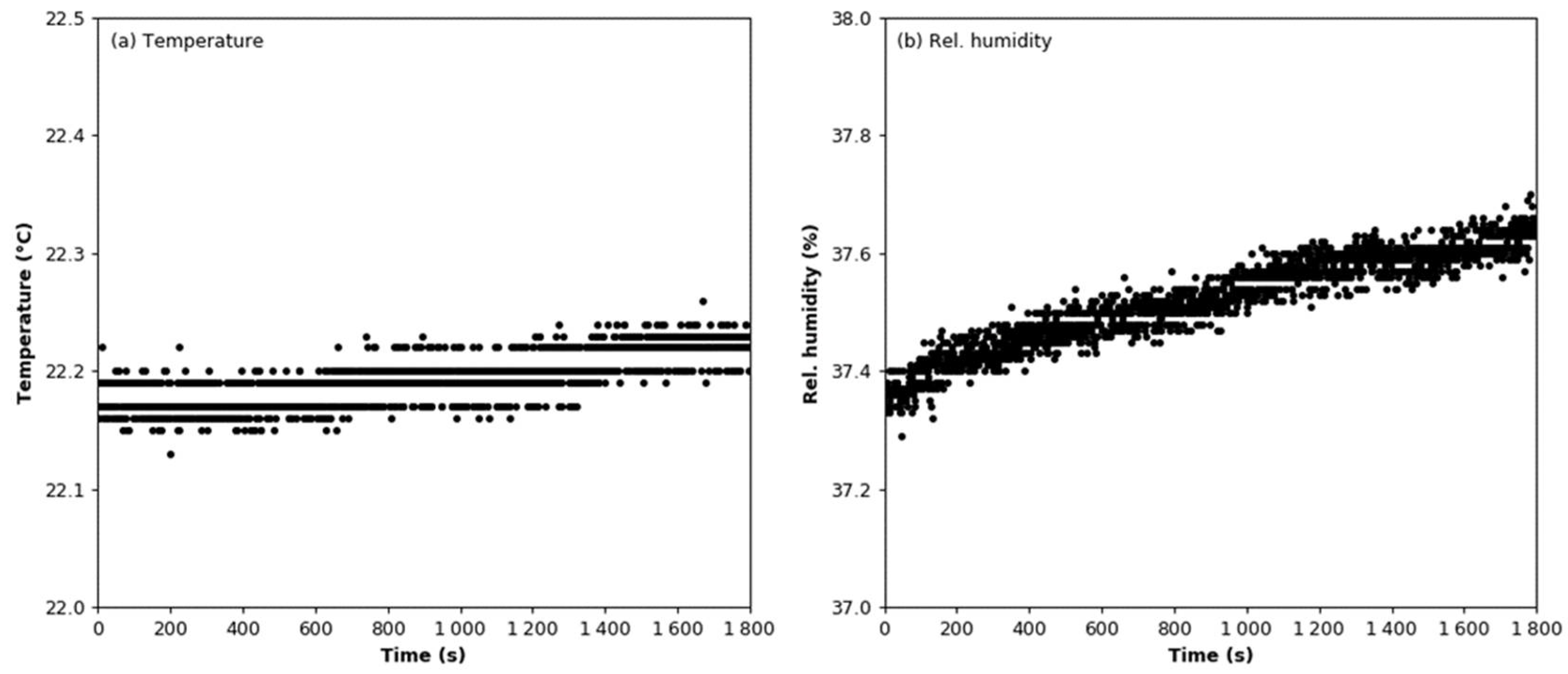
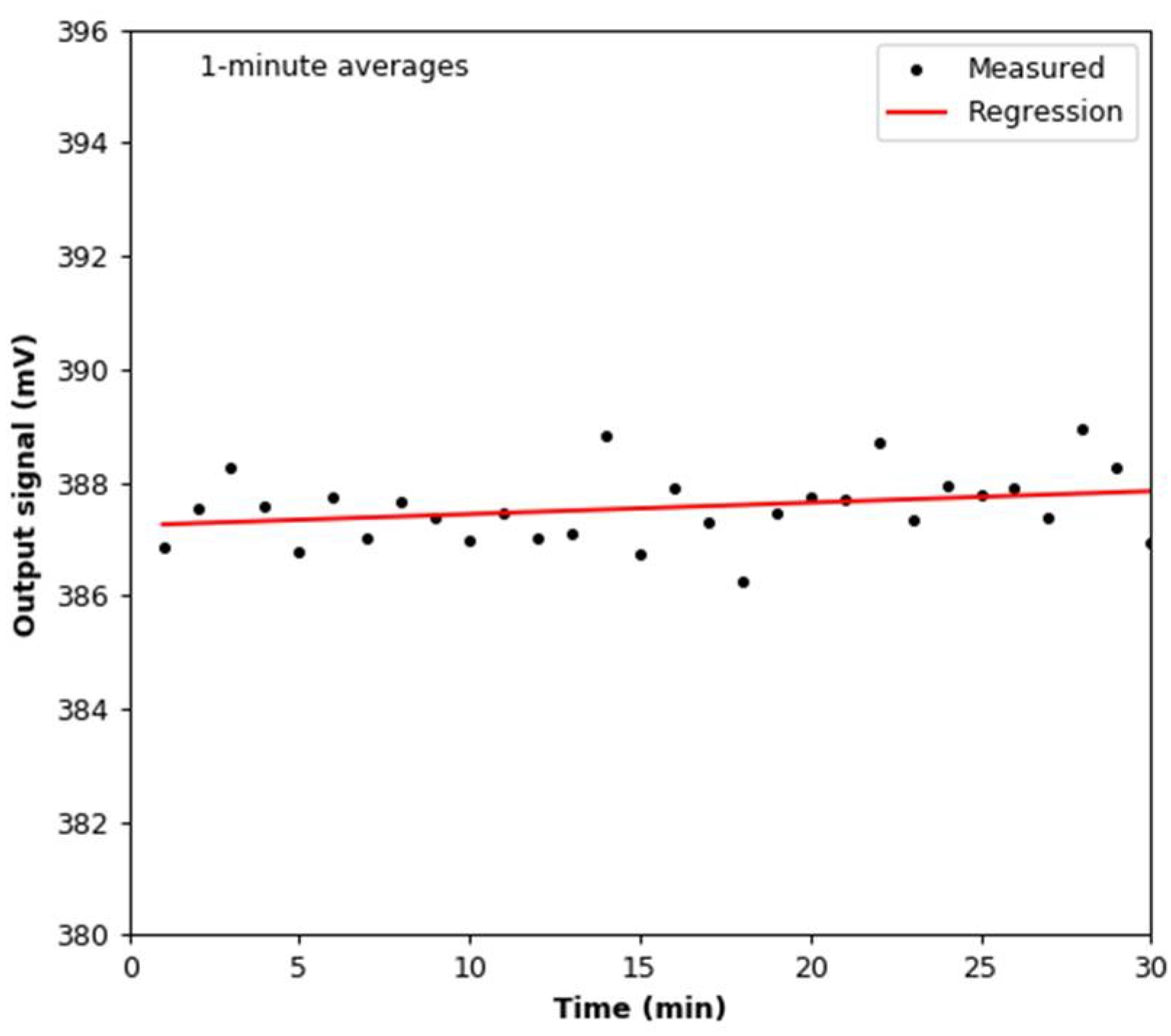
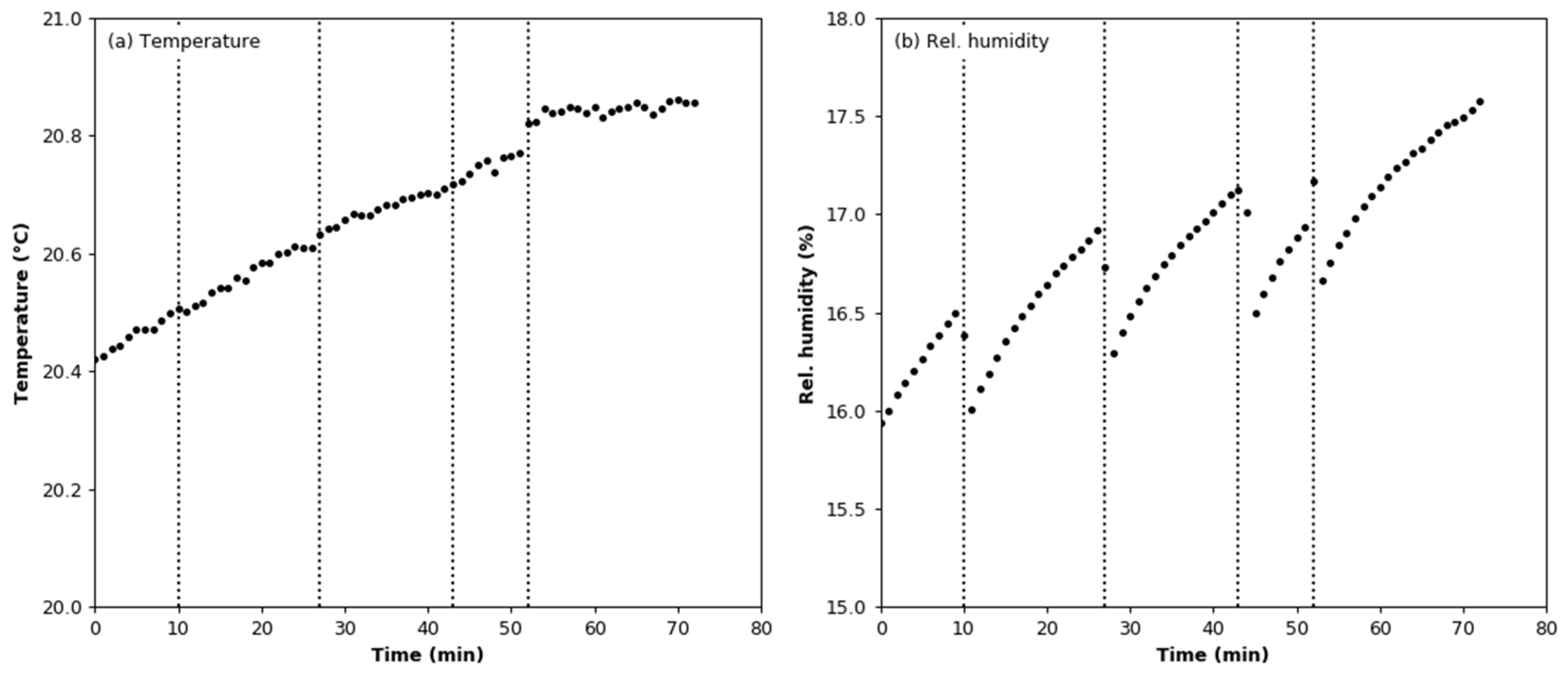
3.1. Signal Stability
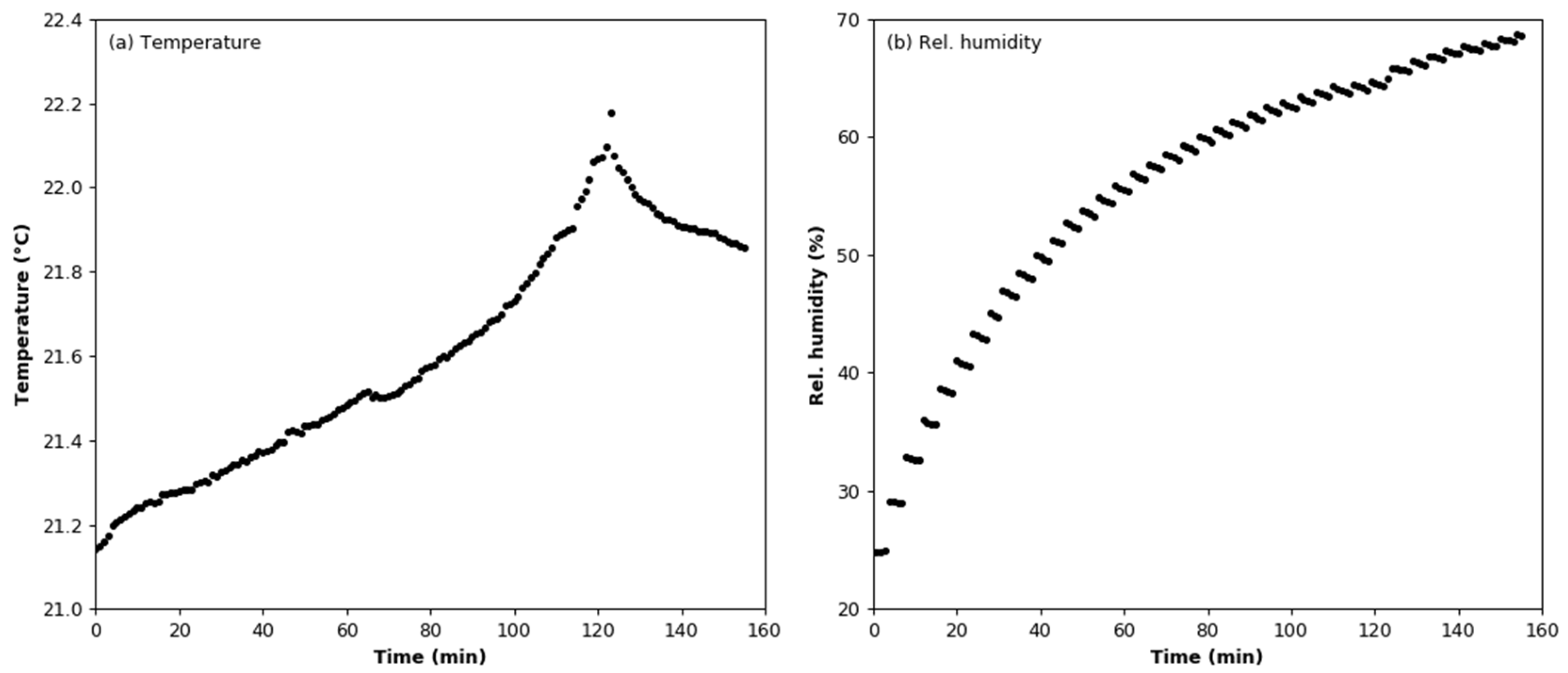
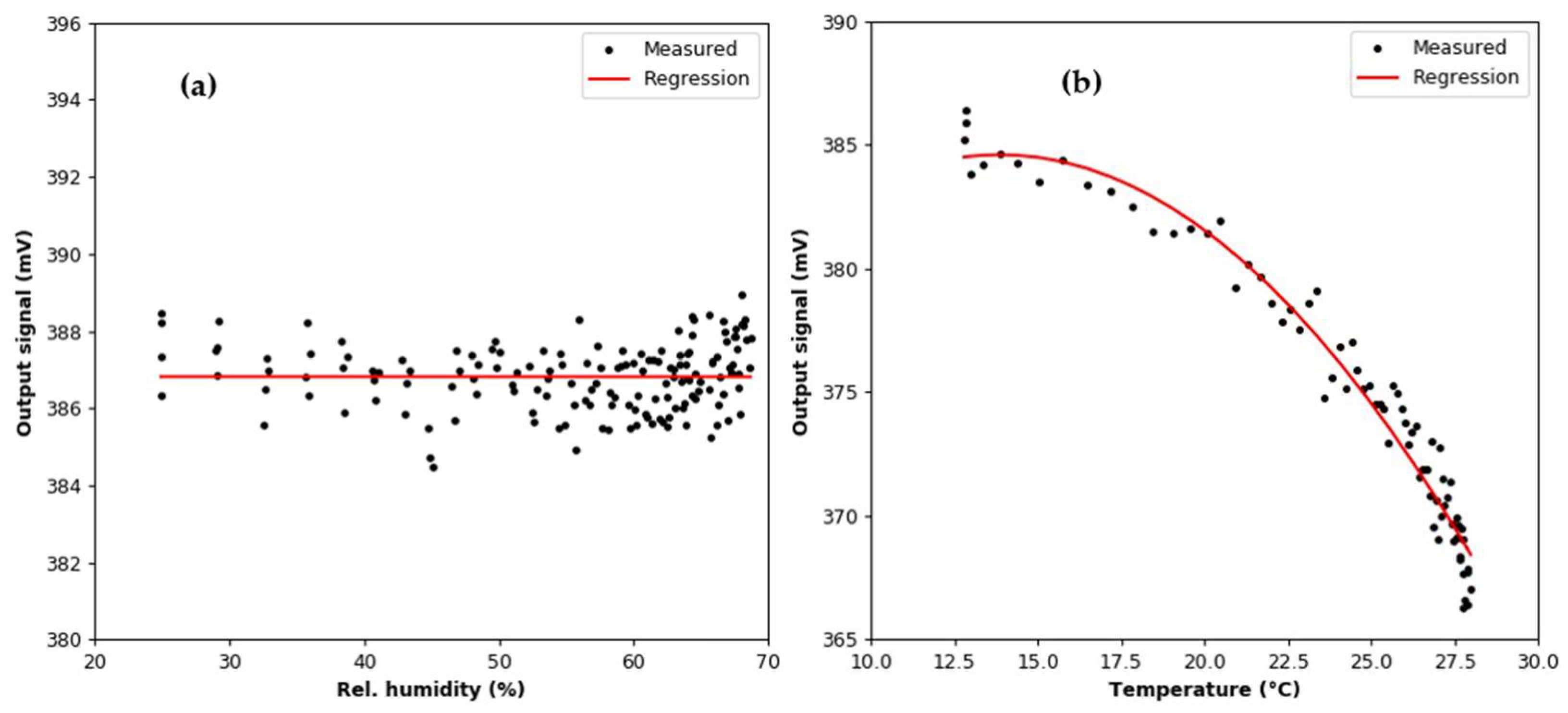
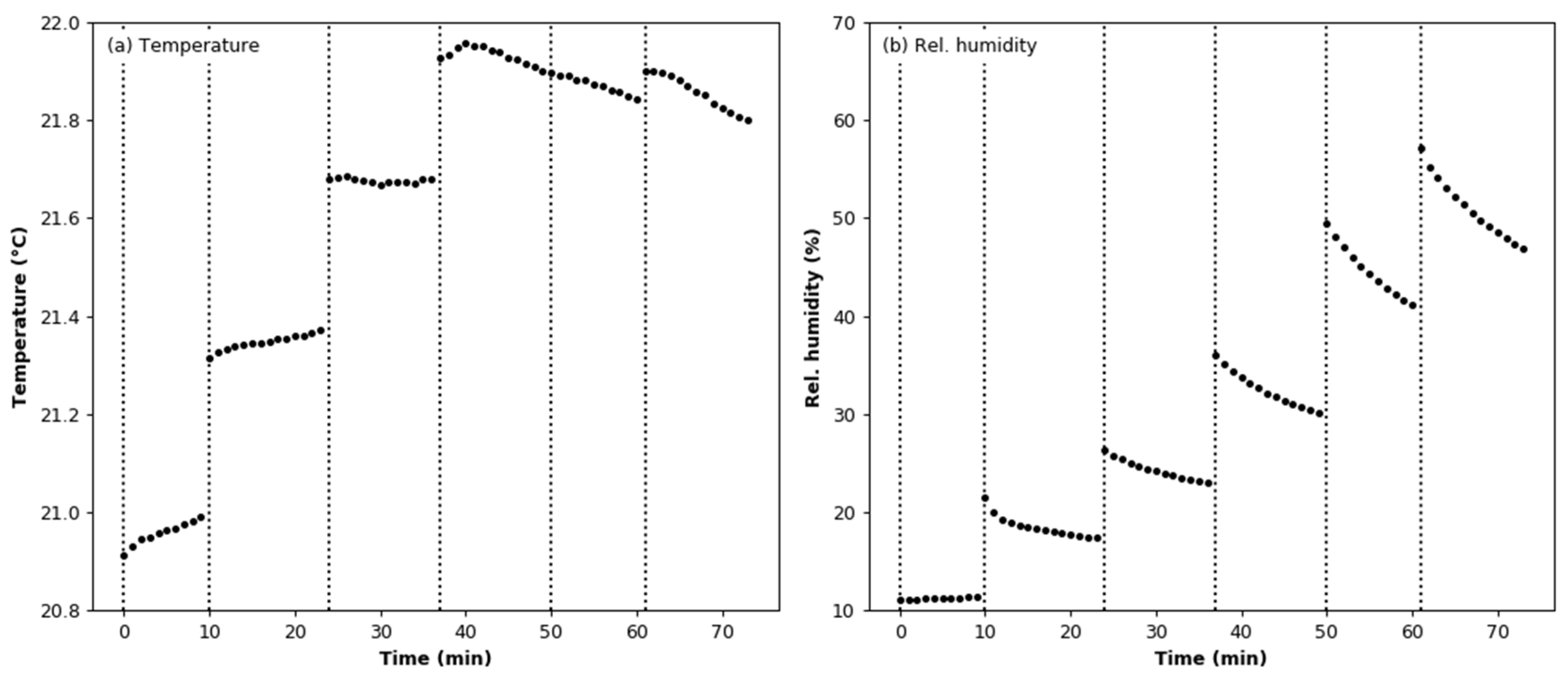
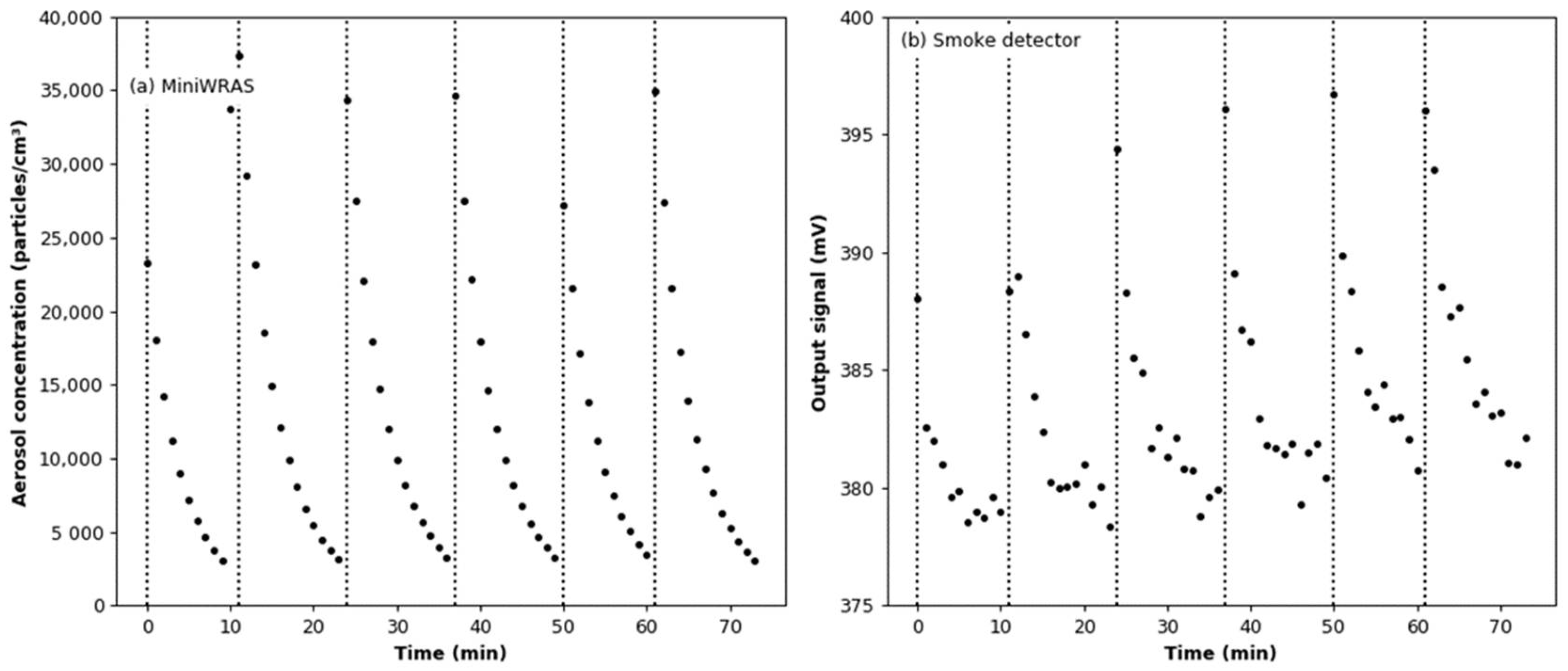
3.2. Humidity Effect Test
3.3. Argon Effect Test
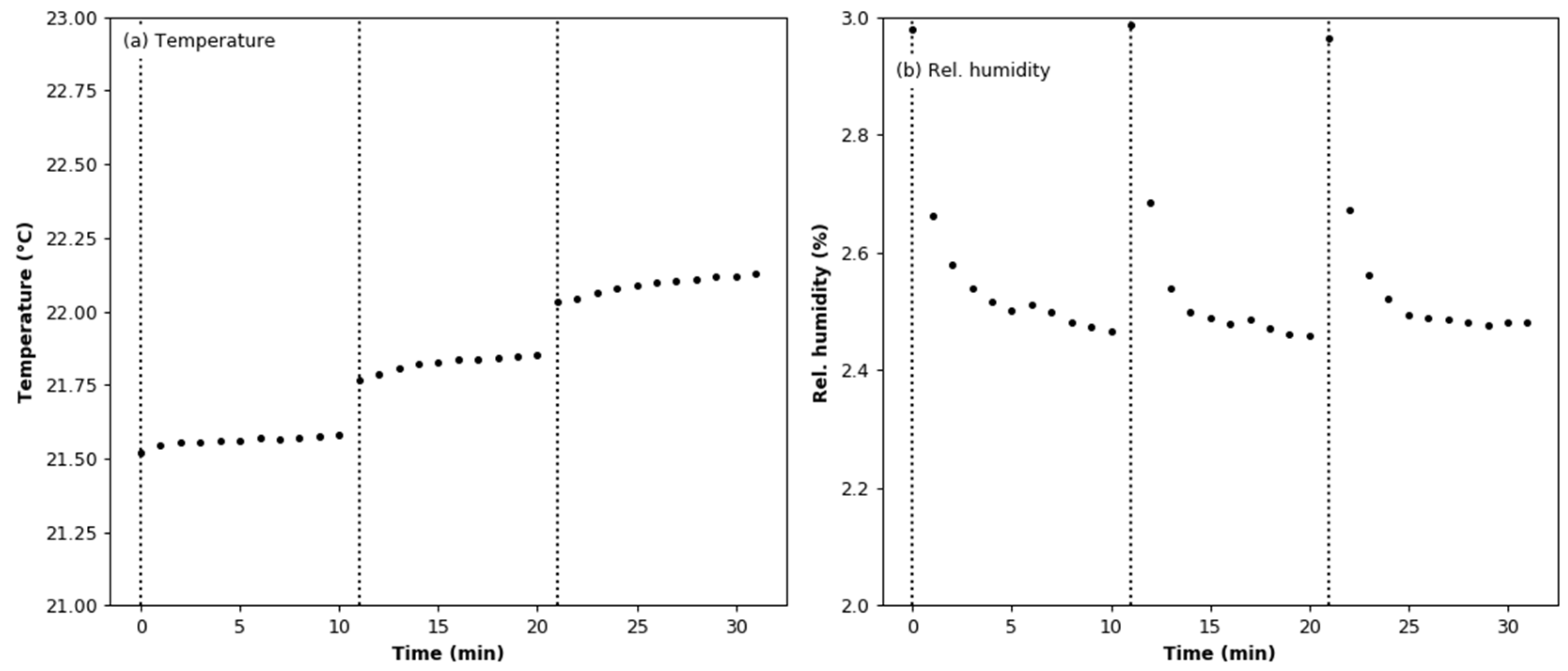
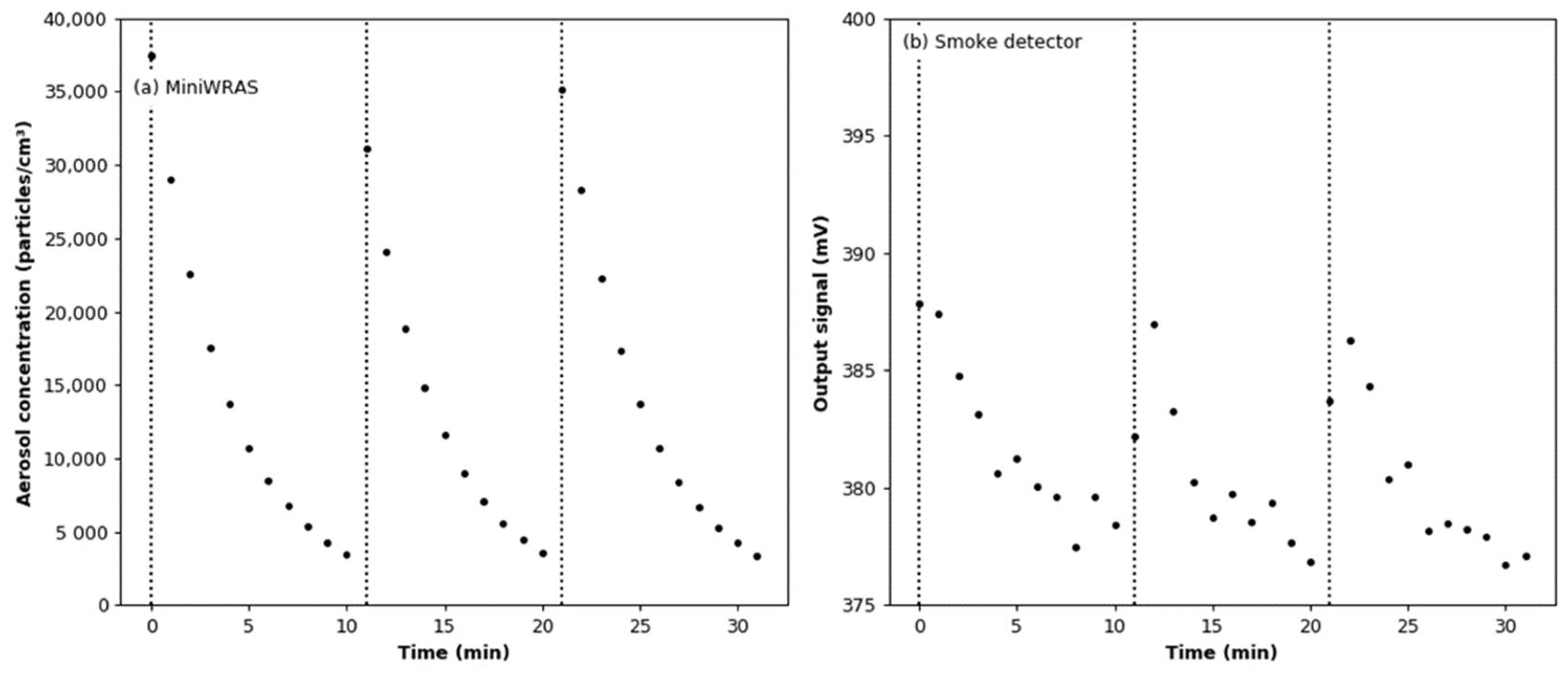
3.4. Effect of Aerosol under Constant Humidity Conditions
3.5. Effect of Aerosol under Variable Humidity Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEN | European Committee for Standardization |
| NOAA | Nano-objects and their aggregates and agglomerates |
| NPs | Nanoparticles |
| ISO | International Organization for Standardization |
| UFPs | Ultrafine particles |
| PM 0.1 | Fraction of ambient particulate matter 0.1 µm |
| CPC | Condensation particle counter |
| DSC | Diffusion size classifier |
| DMA | Differential mobility analyzer |
| SMPS | Scanning mobility particle sizer |
| ELPI | Electrical low pressure impactor |
| KCl | Potassium chloride |
| HEPA filter | High-efficiency particulate air filter |
| ADC | An analog to digital converter |
| PC | Personal computer |
| WHO | World Health Organisation |
| SER | Sociaal-Economische Raad |
References
- Bhardwaj, V.; Kaushik, A. Biomedical Applications of Nanotechnology and Nanomaterials. Micromachines 2017, 8, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maynard, A.D.; Kuempel, E.D. Airborne Nanostructured Particles and Occupational Health. J. Nanopart. Res. 2005, 7, 587–614. [Google Scholar] [CrossRef]
- Bakand, S.; Hayes, A.; Dechsakulthorn, F. Nanoparticles: A review of particle toxicology following inhalation exposure. Inhal. Toxicol. 2012, 24, 125–135. [Google Scholar] [CrossRef]
- Zapór, L.; Oberbek, P. Nanomaterials in the Work Environment. In Emerging Chemical Risks in the Work Environment, 1st ed.; Pośniak, M., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 5–73. [Google Scholar] [CrossRef]
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdorster, G.; Philbert, M.A.; Ryan, J.; Seaton, A.; Stone, V.; et al. Safe handling of nanotechnology. Nature 2006, 444, 267–269. [Google Scholar] [CrossRef]
- SCENIHR. Risk Assessment of Products of Nanotechnologies; Scientific Committee on Emerging and Newly Identified Health Risks; European Commission: Brussels, Belgium, 2009. [Google Scholar]
- SCENIHR. The Appropriateness of the Risk Assessment Methodology in Accordance with the Technical Guidance Documents for New and Existing Substances for Assessing the Risks of Nanomaterials; Scientific Committee on Emerging and Newly Identified Health Risks; European Commission: Brussels, Belgium, 2007. [Google Scholar]
- Semmler, M.; Seitz, J.; Erbe, F.; Mayer, P.; Heyder, J.; Oberdorster, G.; Kreyling, W.G. Long-term clearance kinetics of inhaled ultrafine insoluble iridium particles from the rat lung, including transient translocation into secondary organs. Inhal. Toxicol. 2004, 16, 453–459. [Google Scholar] [CrossRef]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Al-Sid-Cheikh, M.; Rowland, S.J.; Stevenson, K.; Rouleau, C.; Henry, T.B.; Thompson, R.C. Uptake, Whole-Body Distribution, and Depuration of Nanoplastics by the Scallop Pecten maximus at Environmentally Realistic Concentrations. Environ. Sci. Technol. 2018, 52, 14480–14486. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.-C.; Jiang, X.; Wang, J.; Chen, C.; Liu, R.-S. Nano-bio effects: Interaction of nanomaterials with cells. Nanoscale 2013, 5, 3547–3569. [Google Scholar] [CrossRef]
- Mc Carthy, D.J.; Malhotra, M.; O’Mahony, A.M.; Cryan, J.F.; O’Driscoll, C.M. Nanoparticles and the Blood-Brain Barrier: Advancing from In-Vitro Models Towards Therapeutic Significance. Pharm. Res. 2015, 32, 1161–1185. [Google Scholar] [CrossRef]
- ISO/TS 80004-2:2015; Nanotechnologies—Vocabulary—Part 2: Nano-Objects. International Organization for Standardization: Geneva, Switzerland, 2015.
- Boverhof, D.R.; Bramante, C.M.; Butala, J.H.; Clancy, S.F.; Lafranconi, M.; West, J.; Gordon, S.C. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 2015, 73, 137–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaldson, K.; Stone, V.; Clouter, A.; Renwick, L.; MacNee, W. Ultrafine particles. Occup. Environ. Med. 2001, 58, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.-S.; Ryu, M.H.; Carlsten, C. Ultrafine particles: Unique physicochemical properties relevant to health and disease. Exp. Mol. Med. 2020, 52, 318–328. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler, M.; Erbe, F.; Mayer, P.; Takenaka, S.; Schulz, H.; Oberdörster, G.; Ziesenis, A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health Part A 2002, 65, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nalwa, H.S. Nanotechnology and health safety—Toxicity and risk assessments of nanostructured materials on human health. J. Nanosci. Nanotechnol. 2007, 7, 3048–3070. [Google Scholar] [CrossRef] [PubMed]
- Marra, J.; van den Brink, W.; Goossens, H.; Kessels, S. Nanoparticle monitoring for exposure assessment. IEEE Nanotechnol. Mag. 2009, 3, 6–37. [Google Scholar] [CrossRef]
- ISO 7708:1995; Air Quality—Particle Size Fraction Definitions for Health-Related Sampling. Volume Reviewed and confirmed in 2017; International Organization for Standardization: Geneva, Switzerland, 1995.
- ISO (2012) 13138:2012; Air Quality—Sampling Conventions for Airborne Particle Deposition in the Human Respiratory System. International Standard Organization: Geneva, Switzerland, 2012.
- HSE. General Methods for Sampling and Gravimetric Analysis of Respirable, Thoracic and Inhalable Aerosols; Health and Safety Laboratory: Derbyshire, UK, 2014.
- Lavoie, J.H. A review of nanomaterial safety concerns. Process Saf. Progress 2010, 29, 182–185. [Google Scholar] [CrossRef]
- Maynard, A.D.; Aitken, R.J. Assessing exposure to airborne nanomaterials: Current abilities and future requirements. Nanotoxicology 2007, 1, 26–41. [Google Scholar] [CrossRef]
- Mark, D. Chapter 4: Occupational Exposure to Nanoparticles and Nanotubes. In Nanotechnology: Consequences for Human Health and the Environment; The Royal Society of Chemistry: London, UK, 2007; Volume 24, pp. 50–80. [Google Scholar]
- Hodson, L.; Methner, M.; Zumwalde, R.D. Approaches to Safe Nanotechnology: Managing the Health and Safety Concerns Associated with Engineered Nanomaterials; DHHS (NIOSH) Publication Number 2009–125; National Institute for Occupational Safety and Health: Washington, DC, USA, 2009.
- Wang, J.; Asbach, C.; Fissan, H.; Hülser, T.; Kuhlbusch, T.A.J.; Thompson, D.; Pui, D.Y.H. How can nanobiotechnology oversight advance science and industry: Examples from environmental, health, and safety studies of nanoparticles (nano-EHS). J. Nanopart. Res. 2011, 13, 1373–1387. [Google Scholar] [CrossRef]
- Leskinen, J.; Joutsensaari, J.; Lyyränen, J.; Koivisto, J.; Ruusunen, J.; Järvelä, M.; Tuomi, T.; Hämeri, K.; Auvinen, A.; Jokiniemi, J. Comparison of nanoparticle measurement instruments for occupational health applications. J. Nanopart. Res. 2012, 14, 718. [Google Scholar] [CrossRef]
- Zimmerman, N.; Pollitt, K.J.G.; Jeong, C.-H.; Wang, J.M.; Jung, T.; Cooper, J.M.; Wallace, J.S.; Evans, G.J. Comparison of three nanoparticle sizing instruments: The influence of particle morphology. Atmos. Environ. 2014, 86, 140–147. [Google Scholar] [CrossRef]
- Bau, S.; Zimmermann, B.; Payet, R.; Witschger, O. A laboratory study of the performance of the handheld diffusion size classifier (DiSCmini) for various aerosols in the 15–400 nm range. Environ. Sci. Process. Impacts 2015, 17, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kaliszewski, M.; Włodarski, M.; Młyńczak, J.; Kopczyński, K. Comparison of Low-Cost Particulate Matter Sensors for Indoor Air Monitoring during COVID-19 Lockdown. Sensors 2020, 20, 7290. [Google Scholar] [CrossRef] [PubMed]
- Markowicz, K.M.; Chiliński, M.T. Evaluation of Two Low-Cost Optical Particle Counters for the Measurement of Ambient Aerosol Scattering Coefficient and Ångström Exponent. Sensors 2020, 20, 2617. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, D.; Zhu, R.; Yang, W.; Jiang, P. A Miniature Aerosol Sensor for Detecting Polydisperse Airborne Ultrafine Particles. Sensors 2017, 17, 929. [Google Scholar] [CrossRef] [Green Version]
- Litton, C.D.; Smith, K.R.; Edwards, R.; Allen, T. Combined Optical and Ionization Measurement Techniques for Inexpensive Characterization of Micrometer and Submicrometer Aerosols. Aerosol Sci. Technol. 2004, 38, 1054–1062. [Google Scholar] [CrossRef] [Green Version]
- Edwards, R.; Smith, K.R.; Kirby, B.; Allen, T.; Litton, C.D.; Hering, S. An Inexpensive Dual-Chamber Particle Monitor: Laboratory Characterization. J. Air Waste Manag. Assoc. 2006, 56, 789–799. [Google Scholar] [CrossRef]
- Litton, C.D. The use of light scattering and ion chamber responses for the detection of fires in diesel contaminated atmospheres. Fire Saf. J. 2002, 37, 409–425. [Google Scholar] [CrossRef]
- Dahl, A.; Gudmundsson, A.; Bohgard, M. A low cost nanoparticle monitor for screening measurements in indoor environments. In Proceedings of the 11th International Conference on Indoor Air Quality and Climate, Copenhagen, Denmark, 17–22 August 2008. [Google Scholar]
- Data Sheet for the DIO-40 Ionization Smoke Detector. Available online: https://polon-alfa.pl/sites/default/files/aat/download/DIO-40.pdf (accessed on 14 April 2022).
- Datasheet Mini Wide Range Aerosol Spectrometer. Available online: https://www.grimm-aerosol.com/fileadmin/files/grimm-aerosol/3%20Products/Indoor%20Air%20Quality/The%20Wide%20Range%20Hybrid/1371/Product%20PDFs/Datasheet_1371miniwras_ENG.pdf (accessed on 14 April 2022).
- Datasheet GFG 1000/3000 Aerosol Generator. Available online: http://www.anpico.com/en/ProductDetails_432.html# (accessed on 14 April 2022).


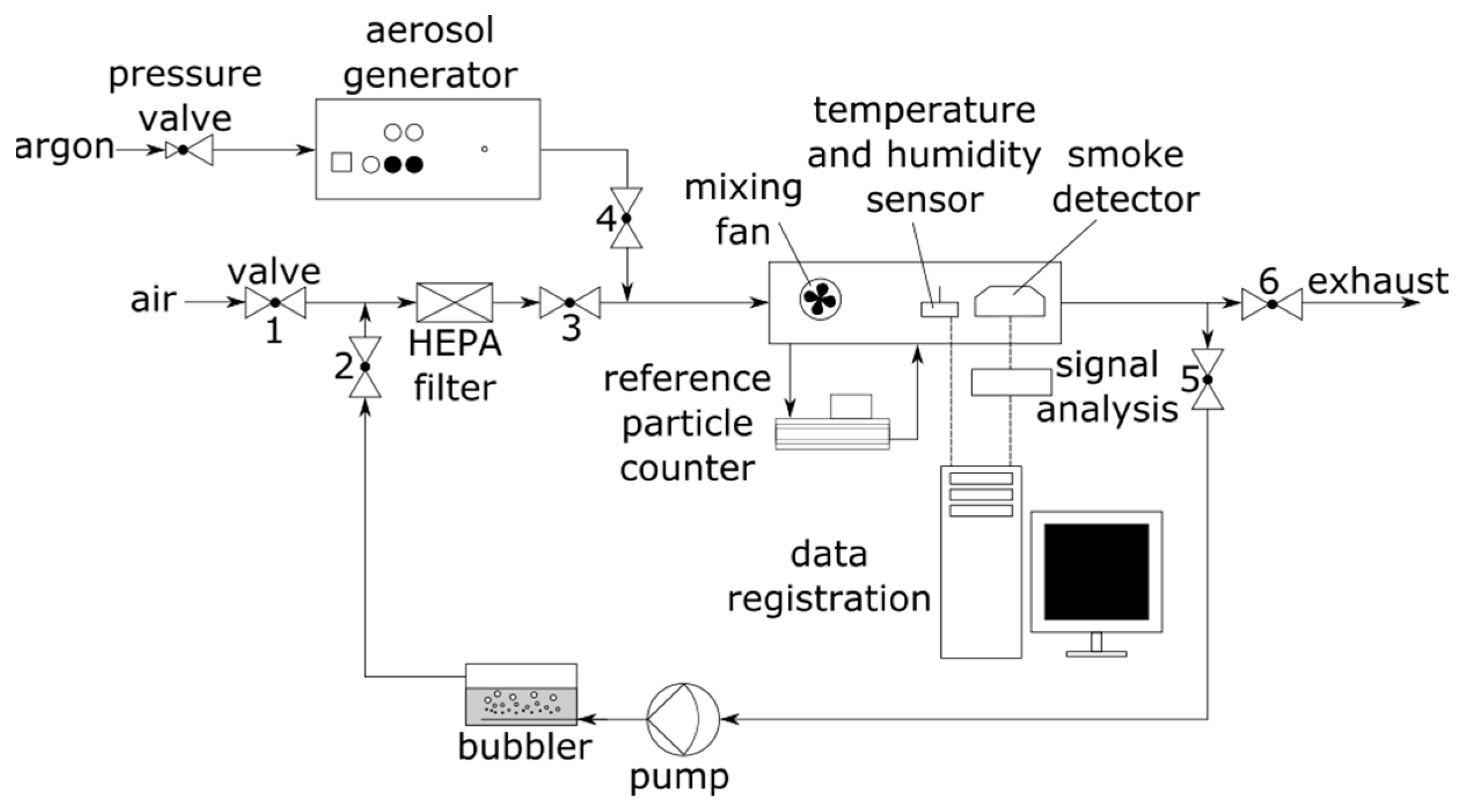




| Valve | Air Preparation Stage (Air Flushing of the Chamber) | Aerosol Generating Stage (Adding Test Aerosol, Change in Aerosol Concentration) | Air Humidification Stage (Change in Air Humidity) |
|---|---|---|---|
| 1 | opened | closed | closed |
| 2 | closed | closed | opened |
| 3 | closed | closed | closed |
| 4 | closed | opened | closed |
| 5 | closed | closed | opened |
| 6 | opened | opened | closed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankowski, T.; Sobiech, P.; Jakubiak, S. The Influence of Air Humidity on the Output Signal from an Ionization Smoke Detector in the Presence of Soot Nanoparticles. Sensors 2022, 22, 3639. https://doi.org/10.3390/s22103639
Jankowski T, Sobiech P, Jakubiak S. The Influence of Air Humidity on the Output Signal from an Ionization Smoke Detector in the Presence of Soot Nanoparticles. Sensors. 2022; 22(10):3639. https://doi.org/10.3390/s22103639
Chicago/Turabian StyleJankowski, Tomasz, Piotr Sobiech, and Szymon Jakubiak. 2022. "The Influence of Air Humidity on the Output Signal from an Ionization Smoke Detector in the Presence of Soot Nanoparticles" Sensors 22, no. 10: 3639. https://doi.org/10.3390/s22103639
APA StyleJankowski, T., Sobiech, P., & Jakubiak, S. (2022). The Influence of Air Humidity on the Output Signal from an Ionization Smoke Detector in the Presence of Soot Nanoparticles. Sensors, 22(10), 3639. https://doi.org/10.3390/s22103639






