Contactless Electrocatheter Tracing within Human Body via Magnetic Sensing: A Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
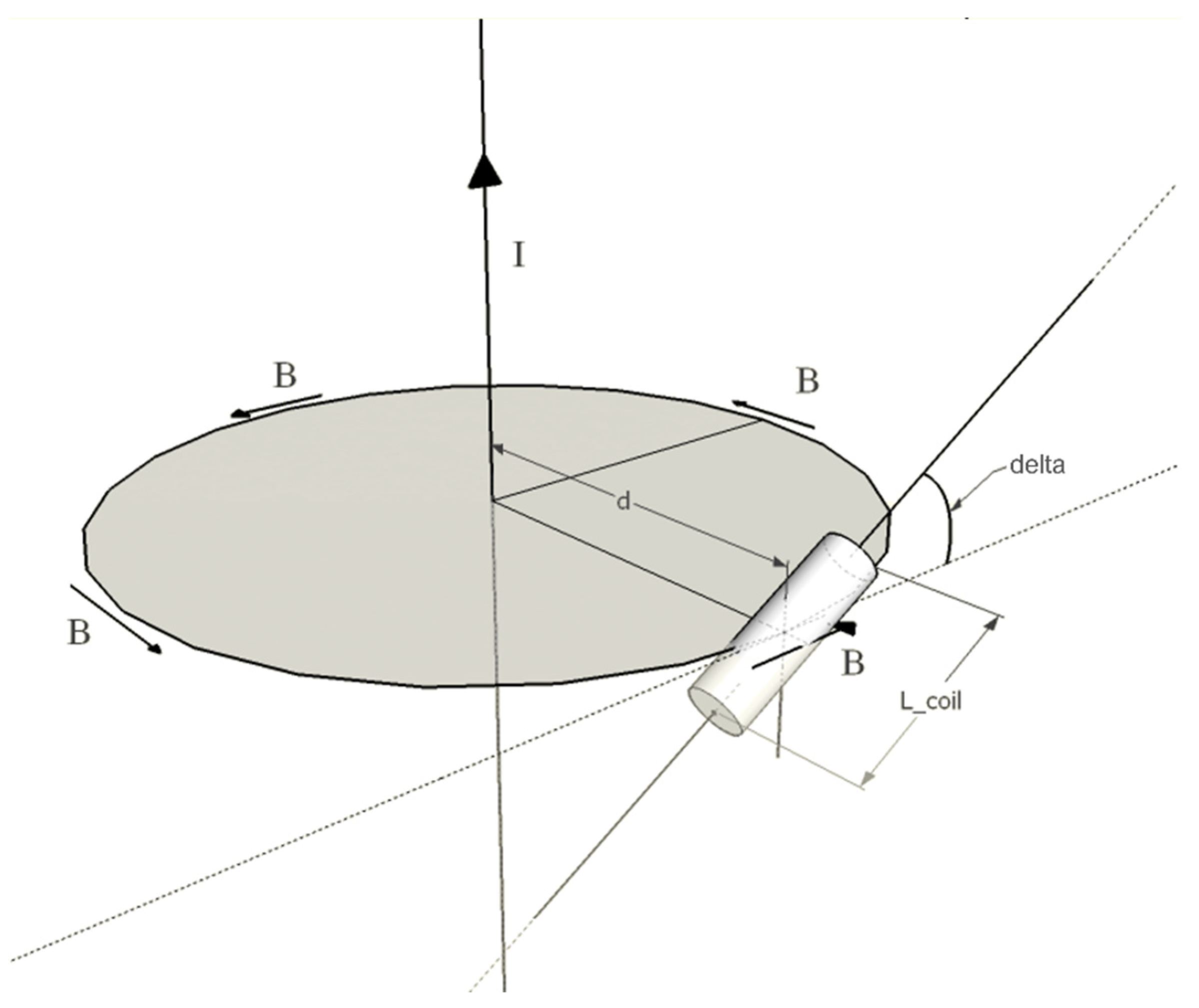
2.1. Principles of Operation
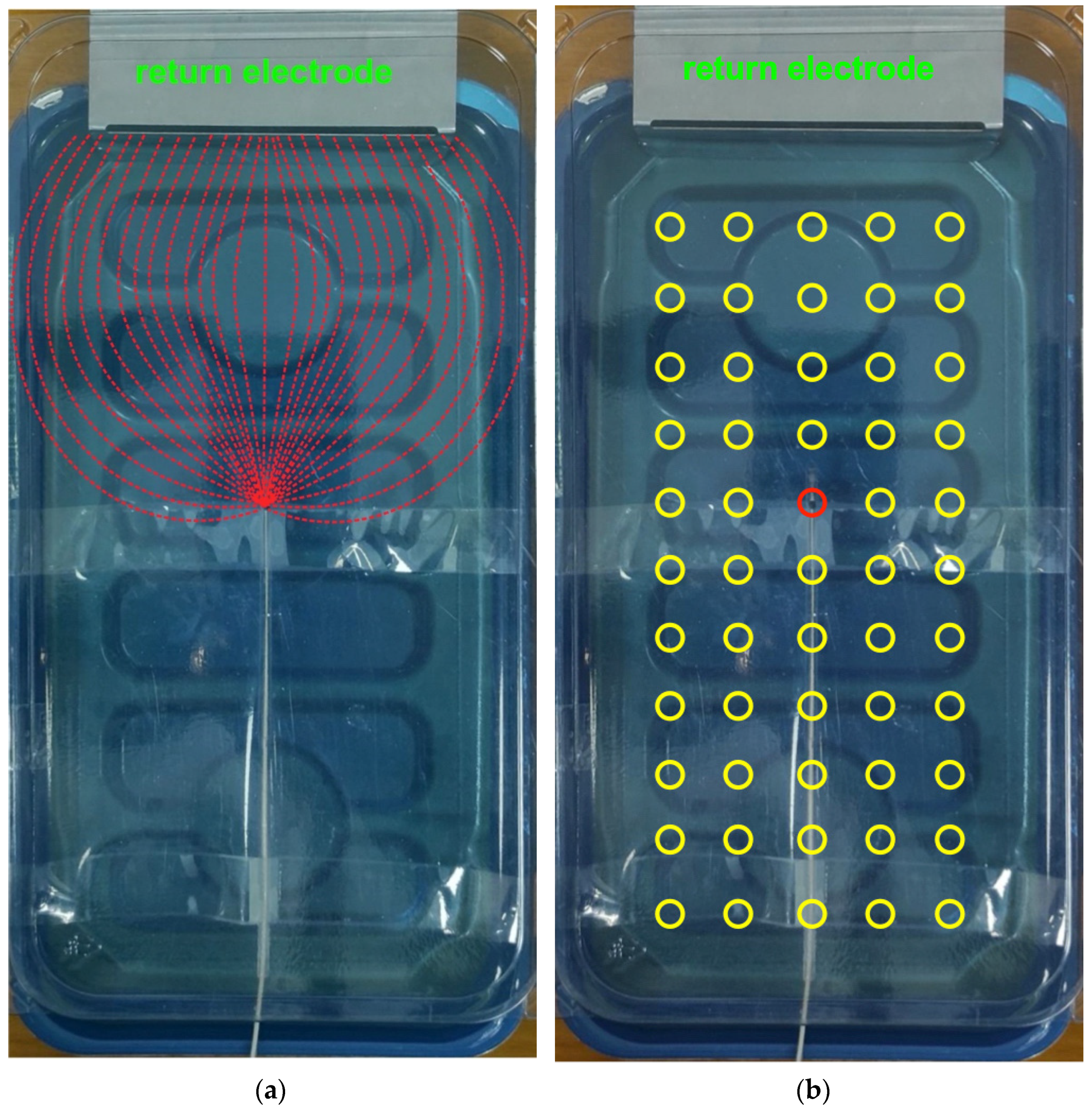
2.2. Experimental Setup
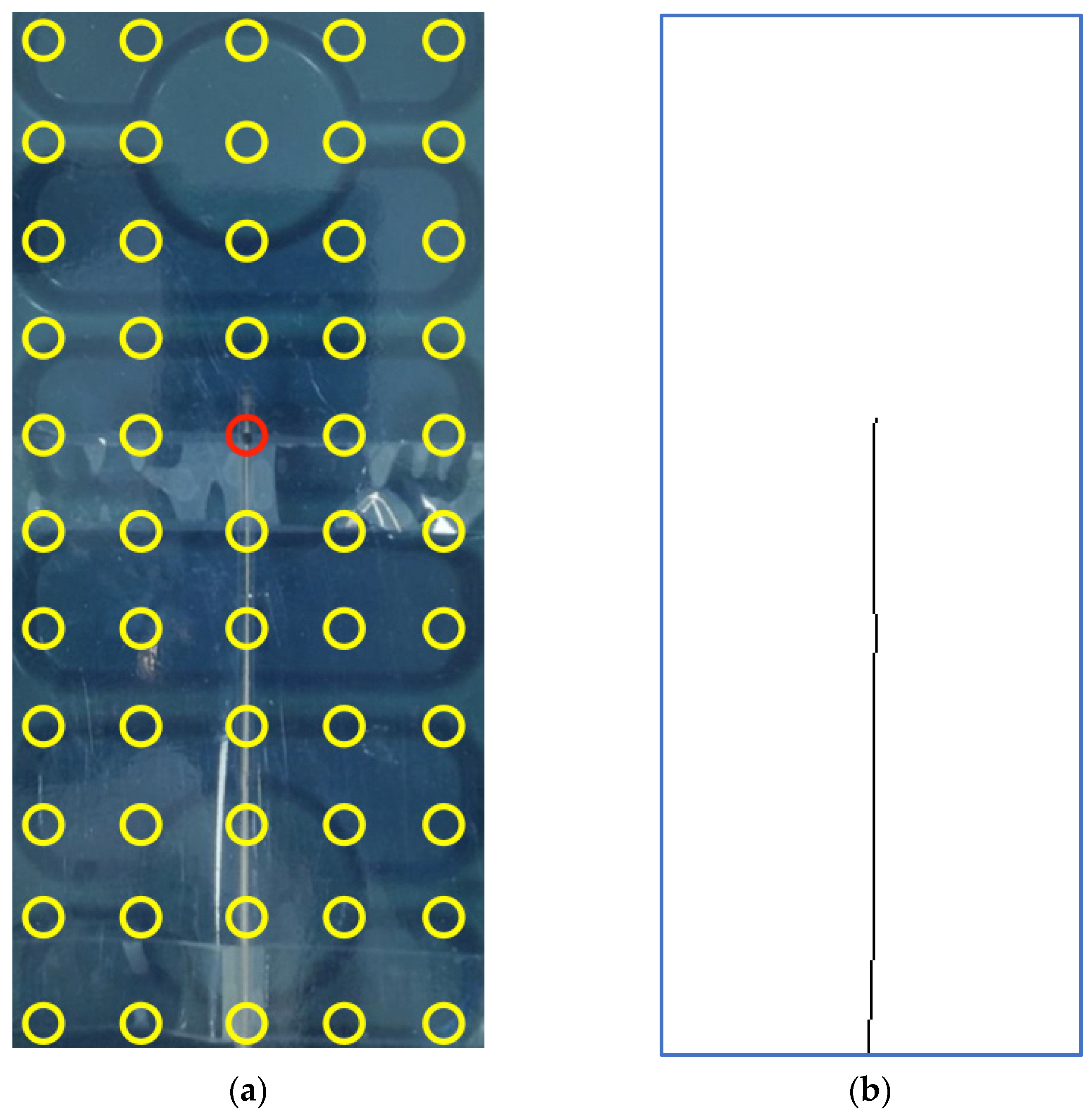
2.3. Test on Copper Wire in Air
2.4. Test on Electrocatheter in Saline Solution
2.5. Data Processing
2.6. Theoretical Evaluation of Tissue Heating Effects
3. Results
3.1. Copper Wire in Air
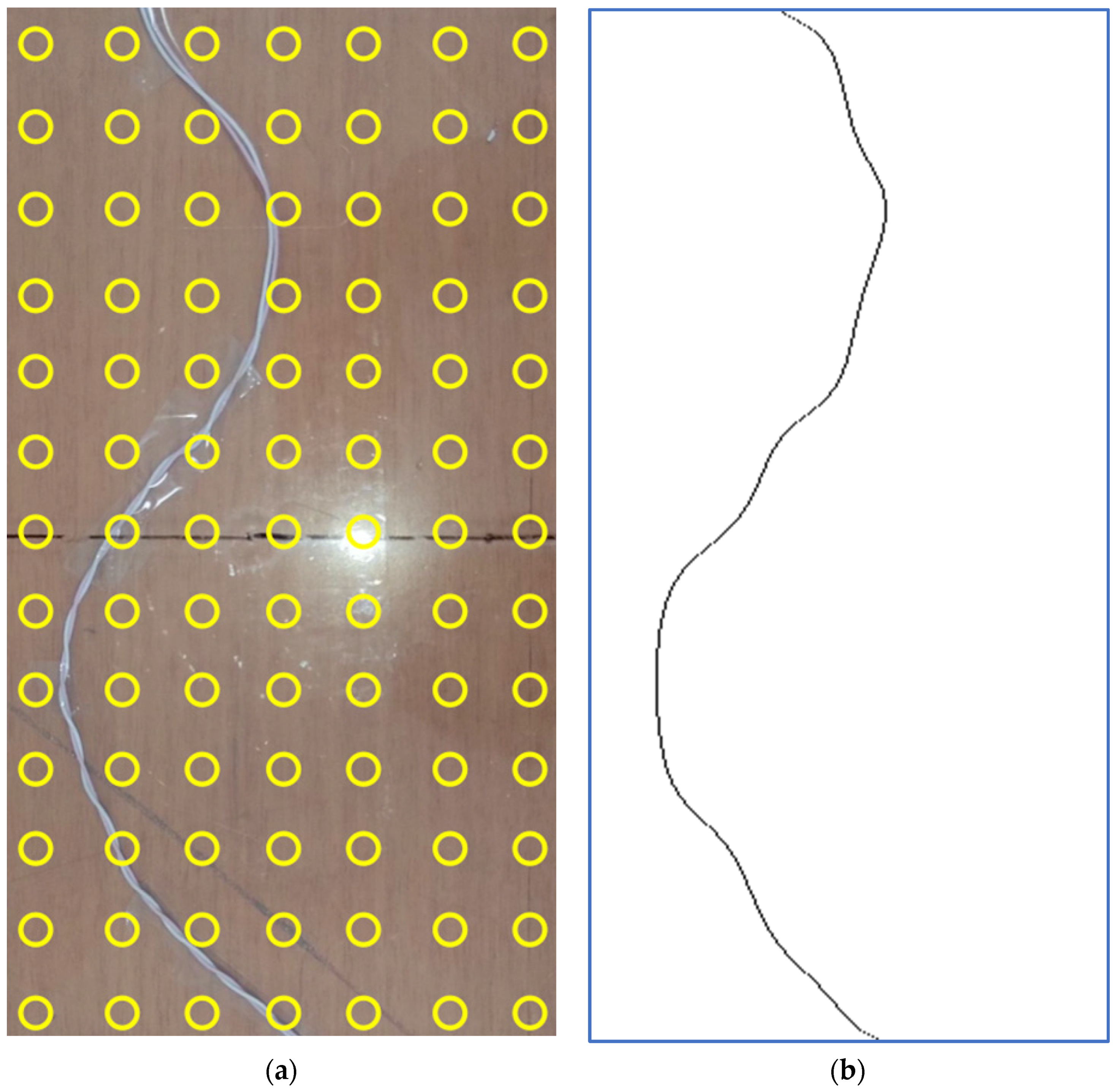
3.2. Electrocatheter in Saline Solution
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haghjoo, M. Techniques of Permanent Pacemaker Implantation. In Current Issues and Recent Advances in Pacemaker Therapy; Roka, A., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Al-Khatib, S.M.; Friedman, P.; Ellenbogen, K.A. Defibrillators: Selecting the Right Device for the Right Patient. Circulation 2016, 134, 1390–1404. [Google Scholar] [CrossRef] [PubMed]
- Tsalafoutas, I.A.; Spanodimos, S.G.; Maniatis, P.N.; Fournarakis, G.M.; Koulentianos, E.D.; Tsigas, D.L. Radiation doses to patients and cardiologists from permanent cardiac pacemaker implantation procedures. Pacing Clin. Electrophysiol. 2005, 28, 910–916. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, J.D.; Ottervanger, J.P.; Delnoy PP, H.; Lagerweij, M.; Knollema, S.; Slump, C.H.; Jager, P.L. Impact of new X-ray technology on patient dose in pacemaker and implantable cardioverter defibrillator (ICD) implantations. J. Interv. Card Electrophysiol. 2017, 48, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picano, E.; Piccaluga, E.; Padovani, R.; Antonio Traino, C.; Grazia Andreassi, M.; Guagliumi, G. Risks Related To Fluoroscopy Radiation Associated With Electrophysiology Procedures. J. Atr. Fibrillation 2014, 7, 1044. [Google Scholar] [CrossRef]
- Nair, G.M.; Nery, P.B.; Redpath, C.J.; Sadek, M.M.; Birnie, D.H. Radiation safety and ergonomics in the electrophysiology laboratory: Update on recent advances. Curr. Opin. Cardiol. 2016, 31, 11–22. [Google Scholar] [CrossRef]
- Cesarelli, M.; Bifulco, P.; Cerciello, T.; Romano, M.; Paura, L. X-ray fluoroscopy noise modeling for filter design. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Casella, M.; Dello Russo, A.; Russo, E.; Catto, V.; Pizzamiglio, F.; Zucchetti, M.; Majocchi, B.; Riva, S.; Vettor, G.; Dessanai, M.A.; et al. X-Ray Exposure in Cardiac Electrophysiology: A Retrospective Analysis in 8150 Patients Over 7 Years of Activity in a Modern, Large-Volume Laboratory. J. Am. Heart Assoc. 2018, 7, e008233. [Google Scholar] [CrossRef] [Green Version]
- Eichenlaub, M.; Astheimer, K.; Minners, J.; Blum, T.; Restle, C.; Maring, C.; Schweitzer, S.; Thiel, U.; Neumann, F.J.; Arentz, T.; et al. Evaluation of a new ultralow-dose radiation protocol for electrophysiological device implantation: A near-zero fluoroscopy approach for device implantation. Heart Rhythm 2020, 17, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Andreozzi, E.; Fratini, A.; Esposito, D.; Cesarelli, M.; Bifulco, P. Toward a priori noise characterization for real-time edge-aware denoising in fluoroscopic devices. Biomed. Eng. Online 2021, 20, 36. [Google Scholar] [CrossRef]
- Sarno, A.; Andreozzi, E.; De Caro, D.; Di Meo, G.; Strollo, A.G.M.; Cesarelli, M.; Bifulco, P. Real-time algorithm for Poissonian noise reduction in low-dose fluoroscopy: Performance evaluation. Biomed. Eng. Online 2019, 18, 94. [Google Scholar] [CrossRef] [Green Version]
- Castellano, G.; De Caro, D.; Esposito, D.; Bifulco, P.; Napoli, E.; Petra, N.; Andreozzi, E.; Cesarelli, M.; Strollo, A.G.M. An FPGA-Oriented Algorithm for Real-Time Filtering of Poisson Noise in Video Streams, with Application to X-ray Fluoroscopy. Circuits Syst. Signal Process. 2019, 38, 3269–3294. [Google Scholar] [CrossRef]
- Gepstein, L.; Hayam, G.; Ben-Haim, S.A. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart. In vitro and in vivo accuracy results. Circulation 1997, 95, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Shpun, S.; Gepstein, L.; Hayam, G.; Ben-Haim, S.A. Guidance of radiofrequency endocardial ablation with real-time three-dimensional magnetic navigation system. Circulation 1997, 96, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Granell, R.; Ferrero, A.; Morell-Cabedo, S.; Martinez-Brotons, A.; Bertomeu, V.; Llacer, A.; García-Civera, R. Implantation of single-lead atrioventricular permanent pacemakers guided by electroanatomic navigation without the use of fluoroscopy. Europace 2008, 10, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.R.; Saini, A.; Moore, J.; Huizar, J.F.; Tan, A.Y.; Ellenbogen, K.A.; Kaszala, K. Fluoroscopy reduction during device implantation by using three-dimensional navigation. A single-center experience. J. Cardiovasc. Electrophysiol. 2019, 30, 2027–2033. [Google Scholar] [CrossRef]
- Guo, P.; Qiu, J.; Wang, Y.; Chen, G.; Proietti, R.; Fadhle, A.S.; Zhao, C.; Wen Wang, D. Zero-fluoroscopy permanent pacemaker implantation using Ensite NavX system: Clinical viability or fanciful technique? Pacing Clin. Electrophysiol. 2018, 41, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Wang, Y.; Chen, G.; Zhao, C.; Wang, D.W. Progress in zero-fluoroscopy implantation of cardiac electronic device. Pacing Clin. Electrophysiol. 2020, 43, 609–617. [Google Scholar] [CrossRef]
- Hua, W.; Liu, X.; Gu, M.; Niu, H.X.; Chen, X.; Tang, M.; Zhang, S. Novel Wide-Band Dielectric Imaging System Guided Lead Deployment for His Bundle Pacing: A Feasibility Study. Front. Cardiovasc. Med. 2021, 8, 712051. [Google Scholar] [CrossRef]
- De Santis, M.; Cacciotti, I. Wireless Implantable and Biodegradable Sensors for Postsurgery Monitoring: Current Status and Future Perspectives. Nanotechnology 2020, 31, 252001. [Google Scholar] [CrossRef]
- Fernandes, C.; Taurino, I. Biodegradable Molybdenum (Mo) and Tungsten (W) Devices: One Step Closer towards Fully-Transient Biomedical Implants. Sensors 2022, 22, 3062. [Google Scholar] [CrossRef]
- Boutry, C.M.; Chandrahalim, H.; Streit, P.; Schinhammer, M.; Hänzi, A.C.; Hierold, C. Towards Biodegradable Wireless Implants. Philos. Trans. A Math. Phys. Eng. Sci. 2012, 370, 2418–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, E.S.; Dervin, S.; Ganguly, P.; Dahiya, R. Biodegradable Materials for Sustainable Health Monitoring Devices. ACS Appl. Bio Mater. 2021, 4, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, E.; Gargiulo, G.D.; Fratini, A.; Esposito, D.; Bifulco, P. A Contactless Sensor for Pacemaker Pulse Detection: Design Hints and Performance Assessment. Sensors 2018, 18, 2715. [Google Scholar] [CrossRef] [Green Version]
- ISO 14117:2019(En); Active Implantable Medical Devices—Electromagnetic Compatibility—EMC Test Protocols for Implantable Cardiac Pacemakers, Implantable Cardioverter Defibrillators and Cardiac Resynchronization Devices. ISO: Geneva, Switzerland. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14117:ed-2:v1:en (accessed on 24 April 2022).
- Pennes, H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreozzi, E.; Esposito, D.; Bifulco, P. Contactless Electrocatheter Tracing within Human Body via Magnetic Sensing: A Feasibility Study. Sensors 2022, 22, 3880. https://doi.org/10.3390/s22103880
Andreozzi E, Esposito D, Bifulco P. Contactless Electrocatheter Tracing within Human Body via Magnetic Sensing: A Feasibility Study. Sensors. 2022; 22(10):3880. https://doi.org/10.3390/s22103880
Chicago/Turabian StyleAndreozzi, Emilio, Daniele Esposito, and Paolo Bifulco. 2022. "Contactless Electrocatheter Tracing within Human Body via Magnetic Sensing: A Feasibility Study" Sensors 22, no. 10: 3880. https://doi.org/10.3390/s22103880
APA StyleAndreozzi, E., Esposito, D., & Bifulco, P. (2022). Contactless Electrocatheter Tracing within Human Body via Magnetic Sensing: A Feasibility Study. Sensors, 22(10), 3880. https://doi.org/10.3390/s22103880







