2.2. Experimental Setup
The experimental setup (
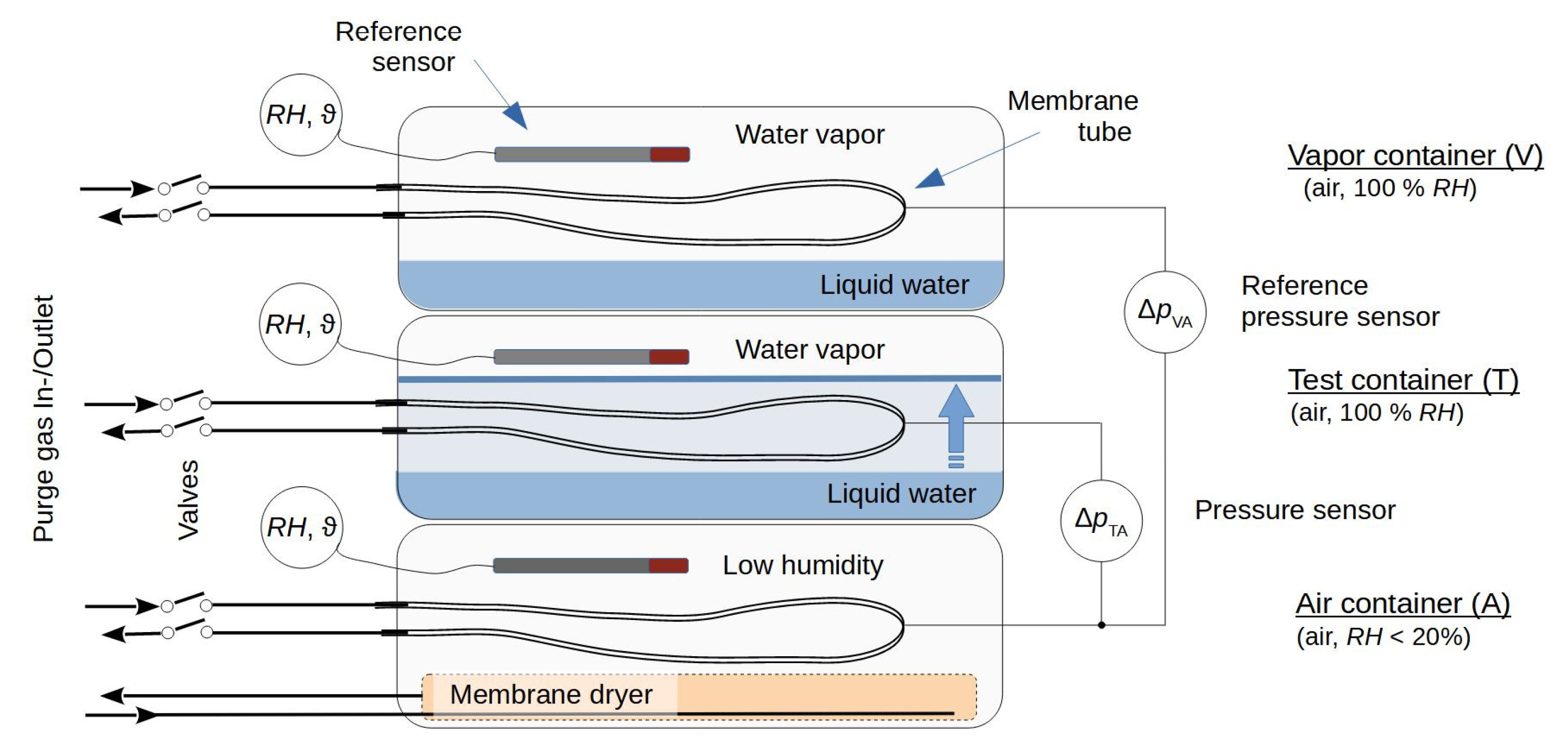
Figure 1) consists of three closed 5.6 l plastic containers stacked on top of each other. The air container (“A”) at the bottom was prepared to hold relatively dry air around a membrane tube, and a vapor container (“V”) was placed at the top to adjust the vapor saturation around a second membrane tube. A test container (“T”), located between the other two containers, was prepared to allow the vapor-saturated air to be displaced by liquid around a third membrane tube during the experiment. The air container was equipped with a membrane dryer made from a PDMS tube (length: 4.5 m, inner diameter: 9 mm, outer diameter: 11 mm), which was connected upstream to dried compressor air and downstream to the air in the laboratory. The test and vapor containers were equipped with tubes to enable water to be added to them. To achieve pressure equilibration with the outer air pressure, the air-filled inner space of each container was connected via a syringe filter (pore size 0.2 µm, filter diameter 4 mm) to the ambient air. For better equilibration of the temperature between the containers, the stack of containers was covered with cloths.
The membrane tube in the test container was placed between horizontally arranged plastic grids that were used to define its position as the water level rose above or fell below these grids. The membrane tubes in the air and vapor containers were set up in the same way using similar plastic grids. The grids had wide meshes of size 60 mm × 7 mm and a web thickness of 3 mm to ensure that most of the tube surface was not in contact with these supports. Two different membrane tube sets were used to construct the cells, as follows:
Set 1: (silicone peroxide, Fisher Bioblock Scientific, Illkirch, France),
Set 2: (platinum cross-linked silicone, Versilic SPX-50, Saint Gobain Performance Plastics),
where is the inner volume, are the inner and outer radii, and is the length of the membrane tube.
Gas-tight polyurethane tubes (, Festo, Esslingen, Germany) were used to connect the membrane tubes with pinch valves. These valves (108P8NO12- 01B, Bio-Chem Fluidics, Inc., Boonton, NJ, USA) were used to enable the purging of the membrane tubes with dried compressor air that escapes downstream from the purge gas outlet into the air. The pressure sensors were connected with C-flex tubing (, Saint Gobain Performance Plastics). The inner volumes of the tubing, fittings, and pressure sensors added a dead volume of about mm3 to the inner volume of each chamber.
Pressure sensors of type AMS 5812-0000-D-B (range ±5.17 hPa, precision ±2% of full scale, Amsys GmbH & Co, Mainz, Germany) were used to measure the pressure difference between the chambers in the test and air containers and the pressure difference between the vapor and air containers.
Dried air from a compressor () was used as the purge gas for all chambers. The airflow was adjusted using a mass flow controller (MFC 8710, range 0–5 l/min for air, Bürkert Fluid Control Systems, Ingelfingen, Germany) and controlled by a glass tube dipped into an open water-filled bottle. In this way, a pressure buildup of 20 hPa upstream from the cells was created.
Each cyclic measurement consisted of 110 s for purging the chambers, about 6 s for the consecutive pressure measurement, and a pause of about 4 s, resulting in a total sampling time of 120 s. The initialization of the measurement step caused pressure equilibration within the chambers after the pinch valves were closed. To minimize the influence of this relaxation process on the measurement signal, an offset time of 0.1 s was applied between the closing of the pinch valves and the start of pressure registration. To control the cyclic measurement (i.e., to switch the pinch valves and register the pressure differences
and
), the actuator unit described in [
25] was used. The differential pressure changes
and
were calculated from the measured pressure–time curves
and
(for more detailed information, see [
25]), where a correction of these pressure changes to the inner volume
of the membrane tubes was carried out by multiplication with (
, and stored on a laptop.
Sensors for temperature and relative humidity (EE060, E+E Elektronik, Engerwitzdorf, Austria) were placed in the upper part of each container. The measurement range for humidity ranged from zero to full saturation, with a precision of ±2.5% of the measured value. The temperature range was between −40 and 60 °C, with a precision of ±0.3 K. The outer air pressure was measured using an HCA-BARO series air pressure sensor (range 600–1100 hPa, accuracy ±1% full-scale span, First Sensor, Berlin, Germany). The measurements were recorded on a PC using DASYLab 10.0 (
dasylab.com).
2.3. Theory
To investigate the response of the MHS, depending on the aggregate state of water, the relationship between the pressure change
measured within the membrane tube and the steady-state flow
[mol/s] of the gas components of air through its tubular wall must be considered. A pressure change
develops in the measurement step, close to the steady-state gas flow, and depends on the change in the mole number
of gas in the inner void of the membrane tube
. With respect to the initial gas pressure
[hPa] in the chamber and the molar volume
[L/mol], the relationship between the change in the mole number and the change in pressure follows according to the ideal gas law as:
Based on the assumptions of (i) the adsorption–desorption equilibria for the gas components in the air and the surfaces of the membrane, (ii) the applicability of the solution-diffusion model for gas transport through a non-porous membrane, and (iii) the validity of the superposition principle, the steady-state diffusive flow
through the wall of the membrane can be expressed as the weighted sum [
37] of the differences in the outer
and inner
partial pressures [hPa] of the ambient gas components (“
”):
where
are the permeabilities (given in [m
2/s] in Table 1 of [
25]),
is the universal gas constant, and
[K] is the absolute temperature. The partial pressures
depend on the mole fractions
of the components of the air and its pressure
in the surroundings of the membrane tube. The partial pressures
in the purged membrane tube depend on the mole fractions
of the purge gas and its mean pressure
. To purge a gas through the chamber, its inlet pressure
must be higher than the outlet pressure
. Assuming a linear pressure drop along the tubular membrane and neglecting the pressure drops in the valves, fittings, and connecting tubes of the chamber, then for the air as purge gas escaping into the surroundings (
), the mean pressure in the membrane tube is equal to
.
Simplification: With respect to the permeability of water
and the ratios
, Equation (3) can be split into two terms representing the proportions of water
and the components of dry air
in the flow
:
The ambient air around the membrane tube contains vapor at the saturation vapor pressure
, according to Equation (1). For air as a purge gas at the same temperature and relative humidity
, the vapor pressure in the chamber is
, where
is the water activity in this air. The gas pressures on both sides of the membrane are determined by the air pressure, which is assumed to remain constant during measurement. Then, the positive difference in the water mole fractions between the two sides,
, describes the additional dilution of the gas components in the outer air by a proportion
. In terms of the mole fractions
of air in the chamber, the composition of the outer air is then
, where
is the Kronecker delta. Replacing the partial pressures in Equation (4) by the respective mole fractions enables the comparison of the contributions of the terms
and
:
According to Table 1 in [
25], the ratio
is 0.008 and
is 0.017. In terms of the mole fractions of the main components of dry air (N
2, O
2), the weighted sum in Equation (5) is about
. With a purge gas overpressure
of 20 hPa at the inlet of the chamber (see
Section 2.2), a vapor pressure
hPa at a temperature of 25 °C, an air pressure of 1013 hPa, and an
of 10% in the purge gas, the value of the ratio in Equation (5) is
. The term
can therefore be neglected, and the flow through the membrane can essentially be attributed to the difference in the partial pressures of the vapor:
Case 1: Diffusive depletion zone. A diffusive depletion zone of vapor can develop around the membrane tube in stagnant air as a result of the diffusive vapor transport towards its membrane surface, which acts as a sink due to the diffusive vapor flow through it. This zone can be described as a film with an outer radius
[m] around the membrane tube at which the vapor pressure approximates the saturation vapor pressure
. If
[m
2/s] is the diffusion coefficient of vapor for the air in this zone, the radial flow
can be expressed in an analogous way to Equation (6), as follows:
In the steady state, the flow through the film is the same as that through the membrane, and hence:
Combining Equations (2) and (6) gives the pressure change within a cell regardless of any additional outer film layer. This condition was adjusted experimentally in the test container, as shown in
Figure 1, where the membrane tube was surrounded by a liquid (“liq”) water layer. The pressure change
can therefore be denoted as
, and the experimental conditions in this container are considered to depend on the vapor pressure
and the temperature
. It follows that:
where (
considers the standard conditions (273.15 K, 1013.25 hPa) and
τ [s]:
is a time parameter that characterizes the response of the cell. The water permeability of the membrane can be obtained for this case from the measurement in the test container and can be estimated using Equations (9) and (10).
Combining Equations (2) and (8) and considering the experimental conditions (vapor pressure
and temperature
) for the vapor container shown in
Figure 1 gives a pressure change
, measured with a membrane tube that is surrounded by air (“gas”) and situated in a stagnant gaseous film forming the diffusive depletion zone:
The pressure changes in Equations (9) and (11) can be expressed independently of the varying outer conditions as:
The flow
through the film in Equation (7) is equal to that given by Equation (8). Thus, for the cell installed in the vapor container holds:
The same left-side term results in
when comparing the pressure changes in Equations (9), (11) and (12). Hence, Equations (13) and (14) enable the estimation of the water activity
at the outer surface (“
s”) of the membrane tube in the vapor container, as follows:
From Equation (14), the film thickness
can be determined as:
Case 2: Preferred absorption of water from a liquid. An alternative to
Case 1 is that the sorption behavior of the hydrophobic membrane material may change in the case of direct contact with liquid water with respect to its sorption behavior for the single water molecules of the gaseous surroundings that touch the membrane surface. If the stagnant gaseous film can be neglected around the membrane tube in the vapor container, Equations (11) and (12) give
and the permeability in Equation (10) is determined by the pressure change in the vapor container.
The changed absorption efficiency (i.e., the changed density of the water molecules in the membrane surface of a liquid water-environment with respect to a saturated vapor environment) can be expressed in an analogous way to the water activity
at the outer membrane surface (
Case 1) by an equivalent parameter
representing the water activity of the boundary layer (“
b”) of the membrane in the test container:
A comparison of Equations (17) and (18) enables the calculation of this equivalent based on simultaneous measurements in the test and vapor containers:
If , the density of the water molecules between the chains of polymer in the membrane surface can then be described in terms of the equivalent of an over- or under-saturated vapor.
As shown in
Figure 1, the evolutions of the pressure differences
and
between the various containers were measured. These measurements indicate a transition from a simple pressure change
within a single chamber to a differential pressure change
between the respective chamber pairs. Transforming
in Equations (9) and (11) for this measurement arrangement gives
, with water activity
in the air container, and the ratio
of the conditions, under which the water permeation takes place inside the respective container pairs. For the same container temperatures, these ratios approximate
. Then, the (possibly changing) vapor pressure of the purge gas no longer influences the measurement value; instead, it is determined by the
in the air container, which then acts as a common reference point for the cells in the test and vapor containers. In this case, the measured pressure changes depend on the vapor pressure differences between the respective container pairs (T, A) or (V, A):