Preliminary Evaluation of the Effect of Mechanotactile Feedback Location on Myoelectric Prosthesis Performance Using a Sensorized Prosthetic Hand
Abstract
:1. Introduction
2. Materials and Methods
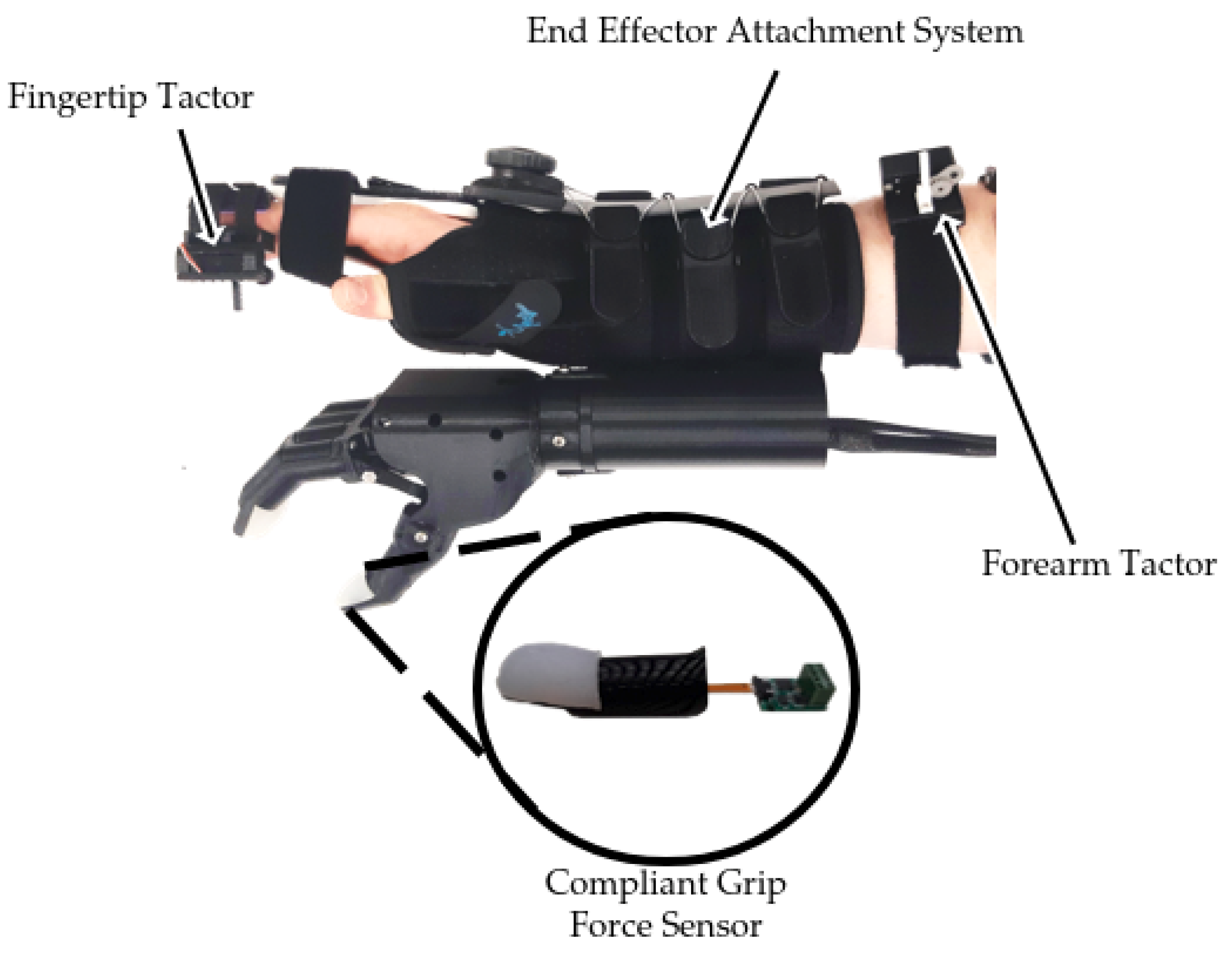
2.1. Modular Simulated Prosthesis
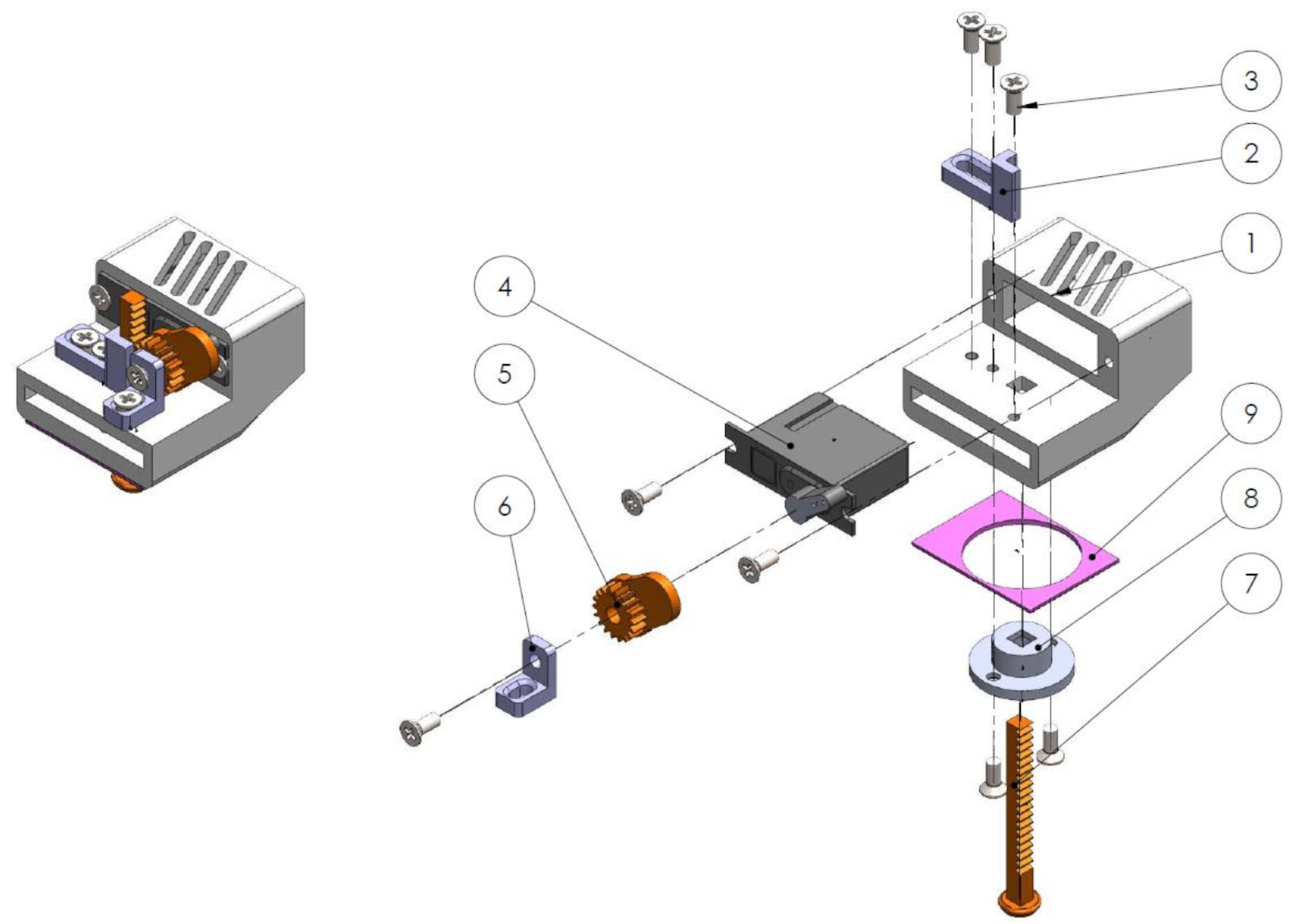
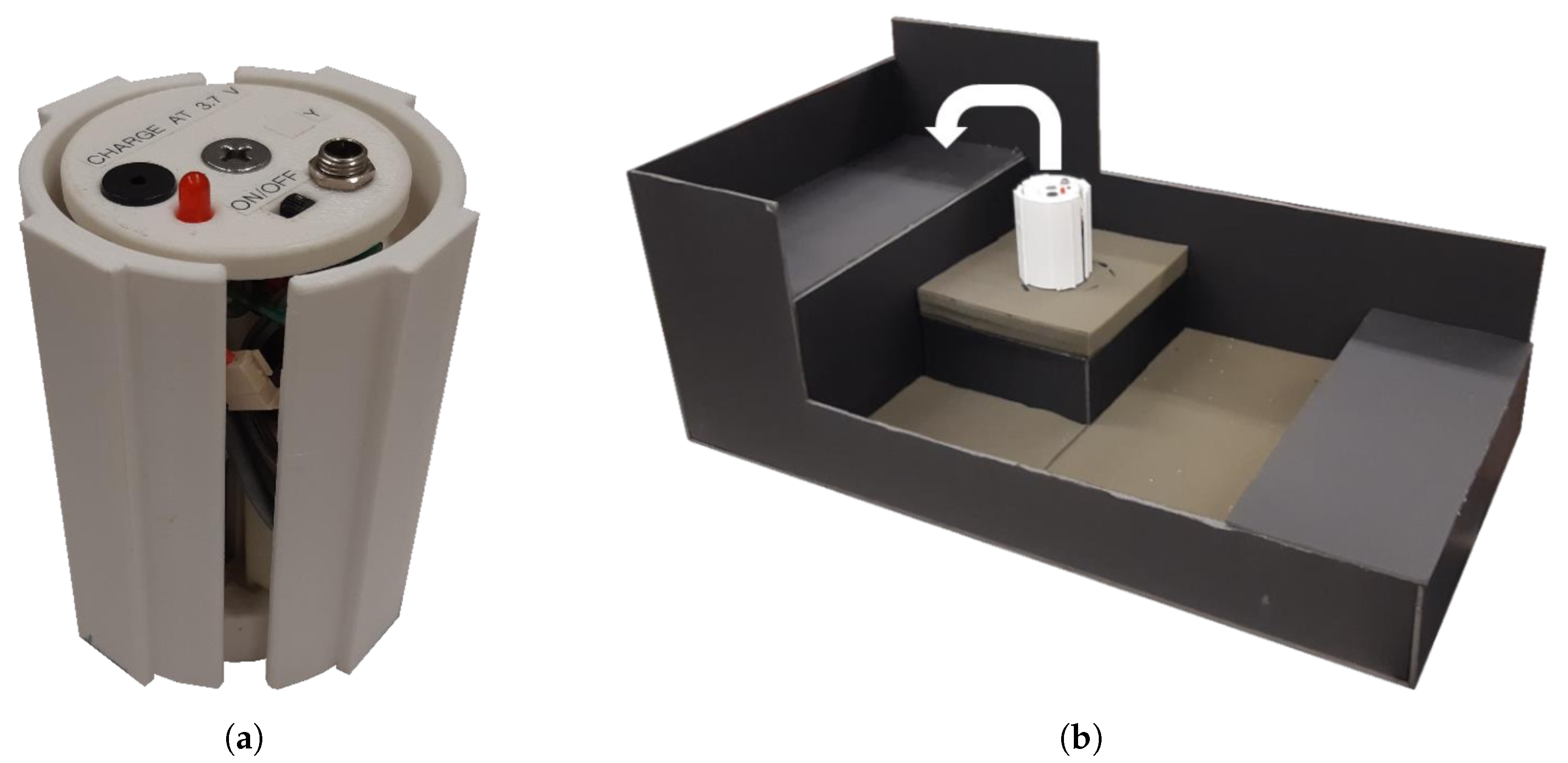
Mechanotactile Tactor Design
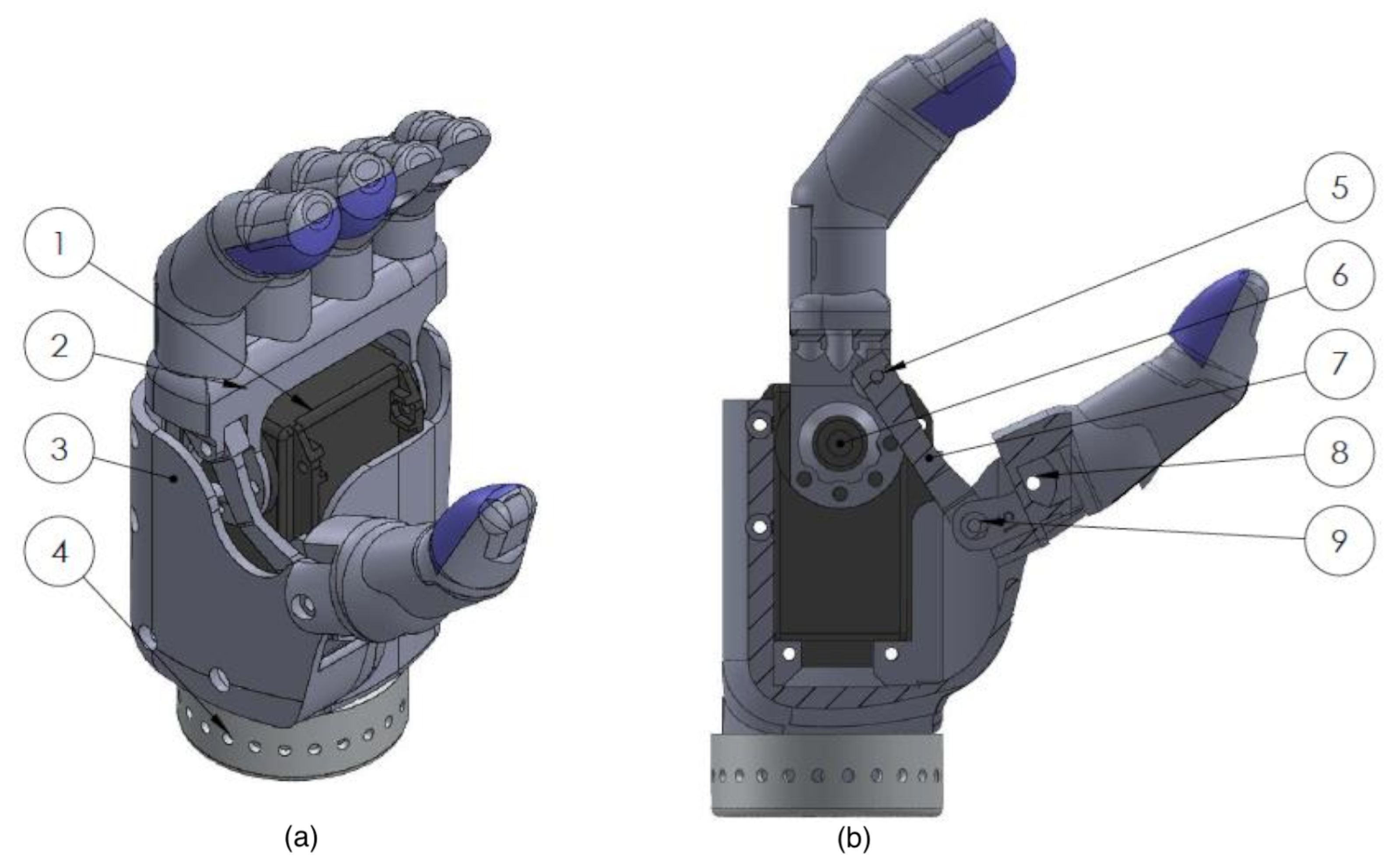
2.2. End-Effector Design
2.3. Hand Restraint Mechanism
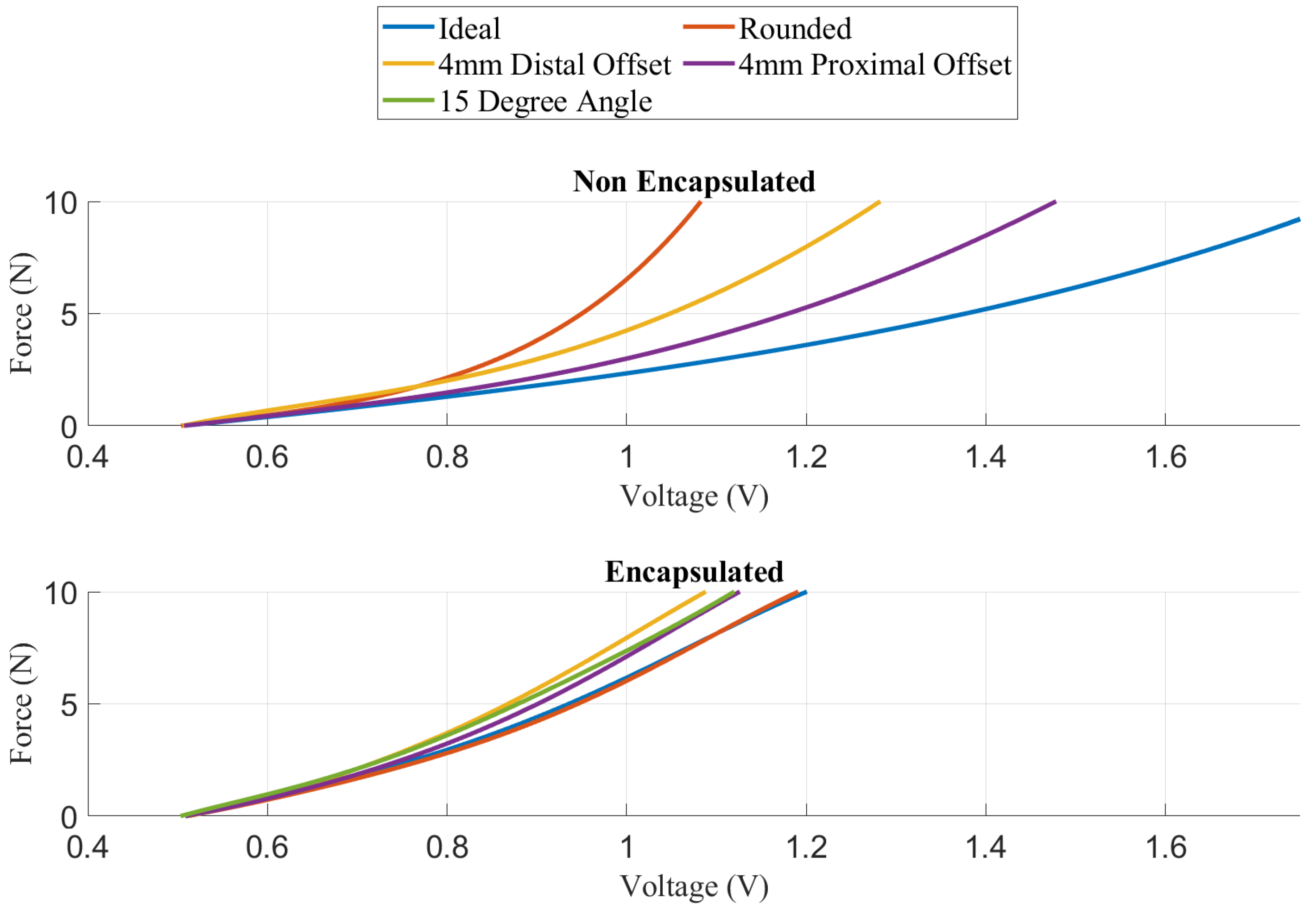
2.4. Fragile Object Simulator Device
2.5. Study Participants
2.6. Experimental Setup
2.7. Experimental Protocol
2.7.1. Outcome Measures
Success Rate
Maximum Grasp
Completion Time and Grasp Time
Hand Aperture Adjustments
2.7.2. Statistical Analysis
3. Results
3.1. Within-Participant Results
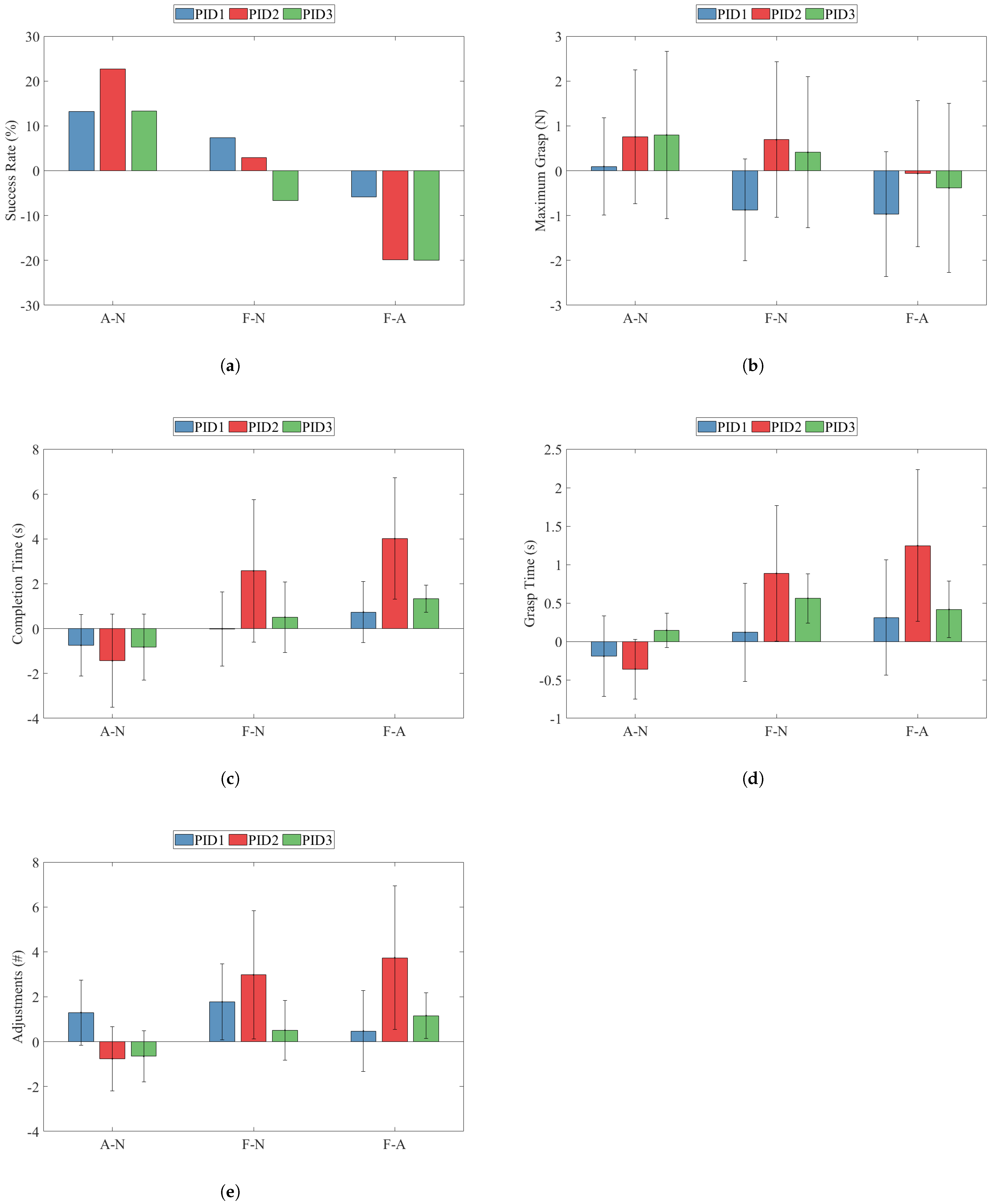
3.1.1. Success Rate
3.1.2. Maximum Grasp
3.1.3. Completion Time
3.1.4. Grasp Time
3.1.5. Adjustments
3.2. Between-Participant Power Analysis
4. Discussion
4.1. Success Rate
4.2. Maximum Grasp Force
4.3. Completion Time and Grasp Time
4.4. Adjustments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biddiss, E.; Beaton, D.; Chau, T. Consumer design priorities for upper limb prosthetics. Disabil. Rehabil. Assist. Technol. 2007, 2, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Asghari Oskoei, M.; Hu, H. Myoelectric control systems—A survey. Biomed. Signal Process. Control 2007, 2, 275–294. [Google Scholar] [CrossRef]
- Scheme, E.; Englehart, K. Electromyogram pattern recognition for control of powered upper-limb prostheses: State of the art and challenges for clinical use. J. Rehabil. Res. Dev. 2011, 48, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.; Phinyomark, A.; Scheme, E. Current Trends and Confounding Factors in Myoelectric Control: Limb Position and Contraction Intensity. Sensors 2020, 20, 1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biddiss, E.; Chau, T. Upper-Limb Prosthetics: Critical Factors in Device Abandonment. Am. J. Phys. Med. Rehabil. 2007, 86, 977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biddiss, E.A.; Chau, T.T. Upper limb prosthesis use and abandonment: A survey of the last 25 years. Prosthetics Orthot. Int. 2007, 31, 236–257. [Google Scholar] [CrossRef]
- Peerdeman, B.; Boere, D.; Witteveen, H.; Huis in ’t Veld, R.; Hermens, H.; Stramigioli, S.; Rietman, H.; Veltink, P.; Misra, S. Myoelectric forearm prostheses: State of the art from a user-centered perspective. J. Rehabil. Res. Dev. 2011, 48, 719–738. [Google Scholar] [CrossRef] [Green Version]
- Antfolk, C.; D’Alonzo, M.; Rosén, B.; Lundborg, G.; Sebelius, F.; Cipriani, C. Sensory feedback in upper limb prosthetics. Expert Rev. Med. Devices 2013, 10, 45–54. [Google Scholar] [CrossRef]
- Schofield, J.S.; Evans, K.R.; Carey, J.P.; Hebert, J.S. Applications of sensory feedback in motorized upper extremity prosthesis: A review. Expert Rev. Med. Devices 2014, 11, 499–511. [Google Scholar] [CrossRef]
- Shehata, A.W.; Rehani, M.; Jassat, Z.E.; Hebert, J.S. Mechanotactile Sensory Feedback Improves Embodiment of a Prosthetic Hand during Active Use. Front. Neurosci. 2020, 14, 263. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, A.; Ramon, J.L.; Morell, V.; Garcia, G.J.; Pomares, J.; Jara, C.A.; Ubeda, A. Evaluation of Optimal Vibrotactile Feedback for Force-Controlled Upper Limb Myoelectric Prostheses. Sensors 2019, 19, 5209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sensinger, J.W.; Dosen, S. A Review of Sensory Feedback in Upper-Limb Prostheses From the Perspective of Human Motor Control. Front. Neurosci. 2020, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Stephens-Fripp, B.; Alici, G.; Mutlu, R. A Review of Non-Invasive Sensory Feedback Methods for Transradial Prosthetic Hands. IEEE Access 2018, 6, 6878–6899. [Google Scholar] [CrossRef]
- Raspopovic, S.; Valle, G.; Petrini, F.M. Sensory feedback for limb prostheses in amputees. Nat. Mater. 2021, 20, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, A.; Wijk, U.; Antfolk, C.; Bjorkamn-Burtscher, I.; Rosen, B. Sensory qualities of the phantom hand map in the residual forearm of amputees. J. Rehabil. Med. 2016, 48, 365–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuiken, T.A.; Barlow, A.K.; Hargrove, L.J.; Dumanian, G.A. Targeted Muscle Reinnervation for the Upper and Lower Extremity. Tech. Orthop. 2017, 32, 109–116. [Google Scholar] [CrossRef]
- Hebert, J.S.; Olson, J.L.; Morhart, M.J.; Dawson, M.R.; Marasco, P.D.; Kuiken, T.A.; Chan, K.M. Novel Targeted Sensory Reinnervation Technique to Restore Functional Hand Sensation After Transhumeral Amputation. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 765–773. [Google Scholar] [CrossRef]
- Marasco, P.D.; Hebert, J.S.; Sensinger, J.W.; Beckler, D.T.; Thumser, Z.C.; Shehata, A.W.; Williams, H.E.; Wilson, K.R. Neurorobotic fusion of prosthetic touch, kinesthesia, and movement in bionic upper limbs promotes intrinsic brain behaviors. Sci. Robot. 2021, 6, eabf3368. [Google Scholar] [CrossRef]
- Pasluosta, C.; Kiele, P.; Stieglitz, T. Paradigms for restoration of somatosensory feedback via stimulation of the peripheral nervous system. Clin. Neurophysiol. 2018, 129, 851–862. [Google Scholar] [CrossRef]
- Yildiz, K.A.; Shin, A.Y.; Kaufman, K.R. Interfaces with the peripheral nervous system for the control of a neuroprosthetic limb: A review. J. Neuroeng. Rehabil. 2020, 17, 43. [Google Scholar] [CrossRef]
- Tan, D.W.; Schiefer, M.A.; Keith, M.W.; Anderson, J.R.; Tyler, J.; Tyler, D.J. A neural interface provides long-term stable natural touch perception. Sci. Transl. Med. 2014, 6, 257ra138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, G.S.; Lawrence, S.M.; Hutchinson, D.T.; Horch, K.W. Residual function in peripheral nerve stumps of amputees: Implications for neural control of artificial limbs. J. Hand Surg. 2004, 29, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Valle, G.; Mazzoni, A.; Iberite, F.; D’Anna, E.; Strauss, I.; Granata, G.; Controzzi, M.; Clemente, F.; Rognini, G.; Cipriani, C.; et al. Biomimetic Intraneural Sensory Feedback Enhances Sensation Naturalness, Tactile Sensitivity, and Manual Dexterity in a Bidirectional Prosthesis. Neuron 2018, 100, 37–45.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, J.A.; Page, D.M.; Davis, T.S.; Duncan, C.C.; Hutchinson, D.T.; Rieth, L.; Clark, G.A. Long-term performance of Utah Slanted Electrode Arrays and intramuscular electromyographic leads implanted chronically in human arm nerves and muscles. J. Neural Eng. 2020, 17, 056042. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, E.; Valle, G.; Mazzoni, A.; Strauss, I.; Iberite, F.; Patton, J.; Petrini, F.M.; Raspopovic, S.; Granata, G.; Iorio, R.D.; et al. A closed-loop hand prosthesis with simultaneous intraneural tactile and position feedback. Sci. Robot. 2019, 4, eaau8892. [Google Scholar] [CrossRef]
- Davis, T.S.; Wark, H.A.C.; Hutchinson, D.T.; Warren, D.J.; O’Neill, K.; Scheinblum, T.; Clark, G.A.; Normann, R.A.; Greger, B. Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J. Neural Eng. 2016, 13, 036001. [Google Scholar] [CrossRef]
- Strauss, I.; Valle, G.; Artoni, F.; D’Anna, E.; Granata, G.; Di Iorio, R.; Guiraud, D.; Stieglitz, T.; Rossini, P.M.; Raspopovic, S.; et al. Characterization of multi-channel intraneural stimulation in transradial amputees. Sci. Rep. 2019, 9, 19258. [Google Scholar] [CrossRef]
- Horch, K.; Meek, S.; Taylor, T.G.; Hutchinson, D.T. Object Discrimination with an Artificial Hand Using Electrical Stimulation of Peripheral Tactile and Proprioceptive Pathways with Intrafascicular Electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 483–489. [Google Scholar] [CrossRef]
- Graczyk, E.L.; Resnik, L.; Schiefer, M.A.; Schmitt, M.S.; Tyler, D.J. Home Use of a Neural-connected Sensory Prosthesis Provides the Functional and Psychosocial Experience of Having a Hand Again. Sci. Rep. 2018, 8, 9866. [Google Scholar] [CrossRef] [Green Version]
- Raspopovic, S.; Capogrosso, M.; Petrini, F.M.; Bonizzato, M.; Rigosa, J.; Pino, G.D.; Carpaneto, J.; Controzzi, M.; Boretius, T.; Fernandez, E.; et al. Restoring Natural Sensory Feedback in Real-Time Bidirectional Hand Prostheses. Sci. Transl. Med. 2014, 6, 222ra19. [Google Scholar] [CrossRef]
- George, J.A.; Kluger, D.T.; Davis, T.S.; Wendelken, S.M.; Okorokova, E.V.; He, Q.; Duncan, C.C.; Hutchinson, D.T.; Thumser, Z.C.; Beckler, D.T.; et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot. 2019, 4, 2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Catalan, M.; Mastinu, E.; Sassu, P.; Aszmann, O.; Brånemark, R. Self-Contained Neuromusculoskeletal Arm Prostheses. N. Engl. J. Med. 2020, 382, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, C.; Zaccone, F.; Micera, S.; Carrozza, M.C. On the Shared Control of an EMG-Controlled Prosthetic Hand: Analysis of User—Prosthesis Interaction. IEEE Trans. Robot. 2008, 24, 170–184. [Google Scholar] [CrossRef]
- Amsuess, S.; Vujaklija, I.; Goebel, P.; Roche, A.D.; Graimann, B.; Aszmann, O.C.; Farina, D. Context-Dependent Upper Limb Prosthesis Control for Natural and Robust Use. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 744–753. [Google Scholar] [CrossRef]
- Johansen, D.; Cipriani, C.; Popović, D.B.; Struijk, L.N.S.A. Control of a Robotic Hand Using a Tongue Control System—A Prosthesis Application. IEEE Trans. Biomed. Eng. 2016, 63, 1368–1376. [Google Scholar] [CrossRef]
- Amsuess, S.; Goebel, P.; Graimann, B.; Farina, D. Extending mode switching to multiple degrees of freedom in hand prosthesis control is not efficient. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 658–661. [Google Scholar] [CrossRef]
- Farrell, T.R.; Weir, R.F. The Optimal Controller Delay for Myoelectric Prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 111–118. [Google Scholar] [CrossRef]
- Matrone, G.; Cipriani, C.; Carrozza, M.C.; Magenes, G. Two-channel real-time EMG control of a dexterous hand prosthesis. In Proceedings of the 5th International IEEE/EMBS Conference on Neural Engineering, Cancun, Mexico, 27 April–1 May 2011; pp. 554–557. [Google Scholar] [CrossRef]
- White, M.M.; Zhang, W.; Winslow, A.T.; Zahabi, M.; Zhang, F.; Huang, H.; Kaber, D.B. Usability Comparison of Conventional Direct Control Versus Pattern Recognition Control of Transradial Prostheses. IEEE Trans. Hum.-Mach. Syst. 2017, 47, 1146–1157. [Google Scholar] [CrossRef]
- Brenneis, D.J.A.; Dawson, M.R.; Tanikawa, H.; Hebert, J.S.; Carey, J.P.; Pilarski, P.M. The Effect of an Automatically Levelling Wrist Control System. In Proceedings of the 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; pp. 816–823. [Google Scholar] [CrossRef]
- Bouwsema, H.; van der Sluis, C.K.; Bongers, R.M. Changes in performance over time while learning to use a myoelectric prosthesis. J. Neuroeng. Rehabil. 2014, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, S.B.; Bianchi, M.; Bicchi, A.; Santello, M. Influence of force feedback on grasp force modulation in prosthetic applications: A preliminary study. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5439–5442. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, C.; Segil, J.L.; Clemente, F.; Weir, R.F.; Edin, B. Humans can integrate feedback of discrete events in their sensorimotor control of a robotic hand. Exp. Brain Res. 2014, 232, 3421–3429. [Google Scholar] [CrossRef] [Green Version]
- Williams, H.E.; Boser, Q.A.; Pilarski, P.M.; Chapman, C.S.; Vette, A.H.; Hebert, J.S. Hand Function Kinematics when using a Simulated Myoelectric Prosthesis. In Proceedings of the 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; pp. 169–174. [Google Scholar] [CrossRef]
- Wells, E.D.; Carpenter, S.; Dawson, M.R.; Shehata, A.W.; Carey, J.P.; Hebert, J.S. Development of a Modular Simulated Prosthesis and Evaluation of a Compliant Grip Force Sensor. In Proceedings of the Myoelectric Controls and Upper Limb Prosthetics Symposium, Fredericton, NB, Canada, 7–12 August 2020; pp. 179–182. [Google Scholar]
- Schoepp, K.R.; Dawson, M.R.; Schofield, J.S.; Carey, J.P.; Hebert, J.S. Design and Integration of an Inexpensive Wearable Mechanotactile Feedback System for Myoelectric Prostheses. IEEE J. Transl. Eng. Health Med. 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Wijk, U.; Svensson, P.; Antfolk, C.; Carlsson, I.K.; Björkman, A.; Rosén, B. Touch on predefined areas on the forearm can be associated with specific fingers: Towards a new principle for sensory feedback in hand prostheses. J. Rehabil. Med. 2019, 51, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, E. E.H. Weber on the Tactile Senses, 1st ed.; Psychology Press: London, UK, 1996. [Google Scholar] [CrossRef]
- Weinstein, S. Intensive, extensive aspects of tactile sensitivity as a function of body part, sex and laterality. In The Skin Senses; Charles C Thomas: Springfield, IL, USA, 1968; Volume 1, pp. 195–222. [Google Scholar]
- De Nunzio, A.M.; Dosen, S.; Lemling, S.; Markovic, M.; Schweisfurth, M.A.; Ge, N.; Graimann, B.; Falla, D.; Farina, D. Tactile feedback is an effective instrument for the training of grasping with a prosthesis at low- and medium-force levels. Exp. Brain Res. 2017, 235, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.R.; Williams, H.E.; Murgatroyd, G.S.; Hebert, J.S.; Pilarski, P.M. BrachIOPlexus: Myoelectric Training Software for Clinical and Research Applications. In Proceedings of the Myoelectric Controls and Upper Limb Prosthetics Symposium, Fredericton, NB, Canada, 7–12 August 2020; pp. 90–93. [Google Scholar]
- Arias, P.; Robles-García, V.; Espinosa, N.; Corral, Y.; Cudeiro, J. Validity of the finger tapping test in Parkinson’s disease, elderly and young healthy subjects: Is there a role for central fatigue? Clin. Neurophysiol. 2012, 123, 2034–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Meek, S.G.; Jacobsen, S.C.; Goulding, P. Extended physiologic taction: Design and evaluation of a proportional force feedback system. J. Rehabil. Res. Dev. 1989, 26, 53–62. [Google Scholar]
- Clemente, F.; Dosen, S.; Lonini, L.; Markovic, M.; Farina, D.; Cipriani, C. Humans Can Integrate Augmented Reality Feedback in Their Sensorimotor Control of a Robotic Hand. IEEE Trans. Hum.-Mach. Syst. 2017, 47, 583–589. [Google Scholar] [CrossRef]
- Kim, K.; Colgate, J.E. Haptic Feedback Enhances Grip Force Control of sEMG-Controlled Prosthetic Hands in Targeted Reinnervation Amputees. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 798–805. [Google Scholar] [CrossRef]
- Pylatiuk, C.; Kargov, A.; Schulz, S. Design and Evaluation of a Low-Cost Force Feedback System for Myoelectric Prosthetic Hands. JPO J. Prosthetics Orthot. 2006, 18, 57–61. [Google Scholar] [CrossRef]
- Clemente, F.; D’Alonzo, M.; Controzzi, M.; Edin, B.B.; Cipriani, C. Non-Invasive, Temporally Discrete Feedback of Object Contact and Release Improves Grasp Control of Closed-Loop Myoelectric Transradial Prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 1314–1322. [Google Scholar] [CrossRef]
- Engels, L.F.; Shehata, A.W.; Scheme, E.J.; Sensinger, J.W.; Cipriani, C. When Less Is More – Discrete Tactile Feedback Dominates Continuous Audio Biofeedback in the Integrated Percept While Controlling a Myoelectric Prosthetic Hand. Front. Neurosci. 2019, 13, 587. [Google Scholar] [CrossRef]




| Mass (g) | 298 |
| Maximum Grip Aperture (mm) | 125 |
| Maximum Grip Strength (N) | 11 |
| Degrees of Freedom | 1 |
| Maximum Grip Speed (deg/s) | 180 |
| Maximum Current Draw (A) | 1.0 (@40% power) |
| Operating Voltage (V) | 12 |
| Cost ($ CAD) | 550 |
| Maximum Grasp | Completion Time | Grasp Time | Adjustments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A-N | F-N | F-A | A-N | F-N | F-A | A-N | F-N | F-A | A-N | F-N | F-A | |
| PID1 | 0.08 | −0.7 | −0.69 | −0.57 | −0.01 | 0.54 | −0.36 | 0.16 | 0.41 | 0.89 | 0.97 | 0.26 |
| PID2 | 0.51 | 0.39 | −0.04 | −0.7 | 0.77 | 1.49 | −0.94 | 0.88 | 1.27 | −0.53 | 0.92 | 1.17 |
| PID3 | 0.42 | 0.25 | −0.2 | −0.64 | 0.38 | 2.18 | 0.64 | 1.51 | 1.13 | −0.63 | 0.39 | 1.14 |
| Effect Size | Required Participants for Significance | |||||
|---|---|---|---|---|---|---|
| A-N | F-N | F-A | A-N | F-N | F-A | |
| Success Rate | 3.67 | 0.20 | −2.29 | 3 | >100 | 4 |
| Maximum Grasp | 1.70 | 0.11 | −1.26 | 5 | >100 | 8 |
| Completion Time | −3.24 | 0.92 | 1.42 | 4 | 12 | 7 |
| Grasp Time | −0.65 | 1.65 | 1.57 | 21 | 6 | 6 |
| Adjustments | −0.04 | 1.74 | 1.27 | >100 | 5 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wells, E.D.; Shehata, A.W.; Dawson, M.R.; Carey, J.P.; Hebert, J.S. Preliminary Evaluation of the Effect of Mechanotactile Feedback Location on Myoelectric Prosthesis Performance Using a Sensorized Prosthetic Hand. Sensors 2022, 22, 3892. https://doi.org/10.3390/s22103892
Wells ED, Shehata AW, Dawson MR, Carey JP, Hebert JS. Preliminary Evaluation of the Effect of Mechanotactile Feedback Location on Myoelectric Prosthesis Performance Using a Sensorized Prosthetic Hand. Sensors. 2022; 22(10):3892. https://doi.org/10.3390/s22103892
Chicago/Turabian StyleWells, Eric D., Ahmed W. Shehata, Michael R. Dawson, Jason P. Carey, and Jacqueline S. Hebert. 2022. "Preliminary Evaluation of the Effect of Mechanotactile Feedback Location on Myoelectric Prosthesis Performance Using a Sensorized Prosthetic Hand" Sensors 22, no. 10: 3892. https://doi.org/10.3390/s22103892
APA StyleWells, E. D., Shehata, A. W., Dawson, M. R., Carey, J. P., & Hebert, J. S. (2022). Preliminary Evaluation of the Effect of Mechanotactile Feedback Location on Myoelectric Prosthesis Performance Using a Sensorized Prosthetic Hand. Sensors, 22(10), 3892. https://doi.org/10.3390/s22103892







