Between-Day Reliability of Commonly Used IMU Features during a Fatiguing Run and the Effect of Speed
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.1.1. Visit 1: Step-Ramp-Step Test
2.1.2. Visits 2–5: Experimental Trials
2.2. Data Processing
2.3. Feature Extraction
2.4. Statistical Analysis
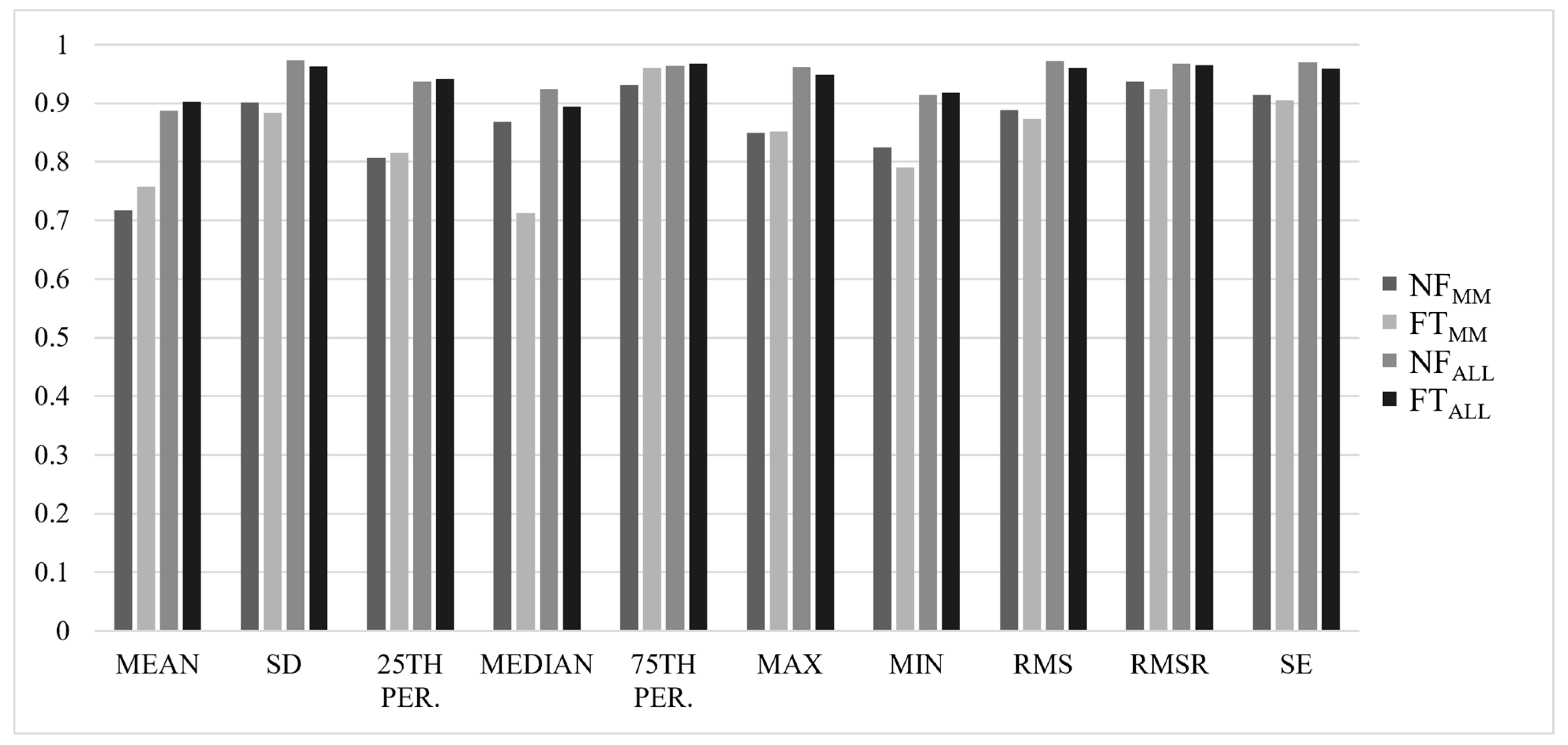
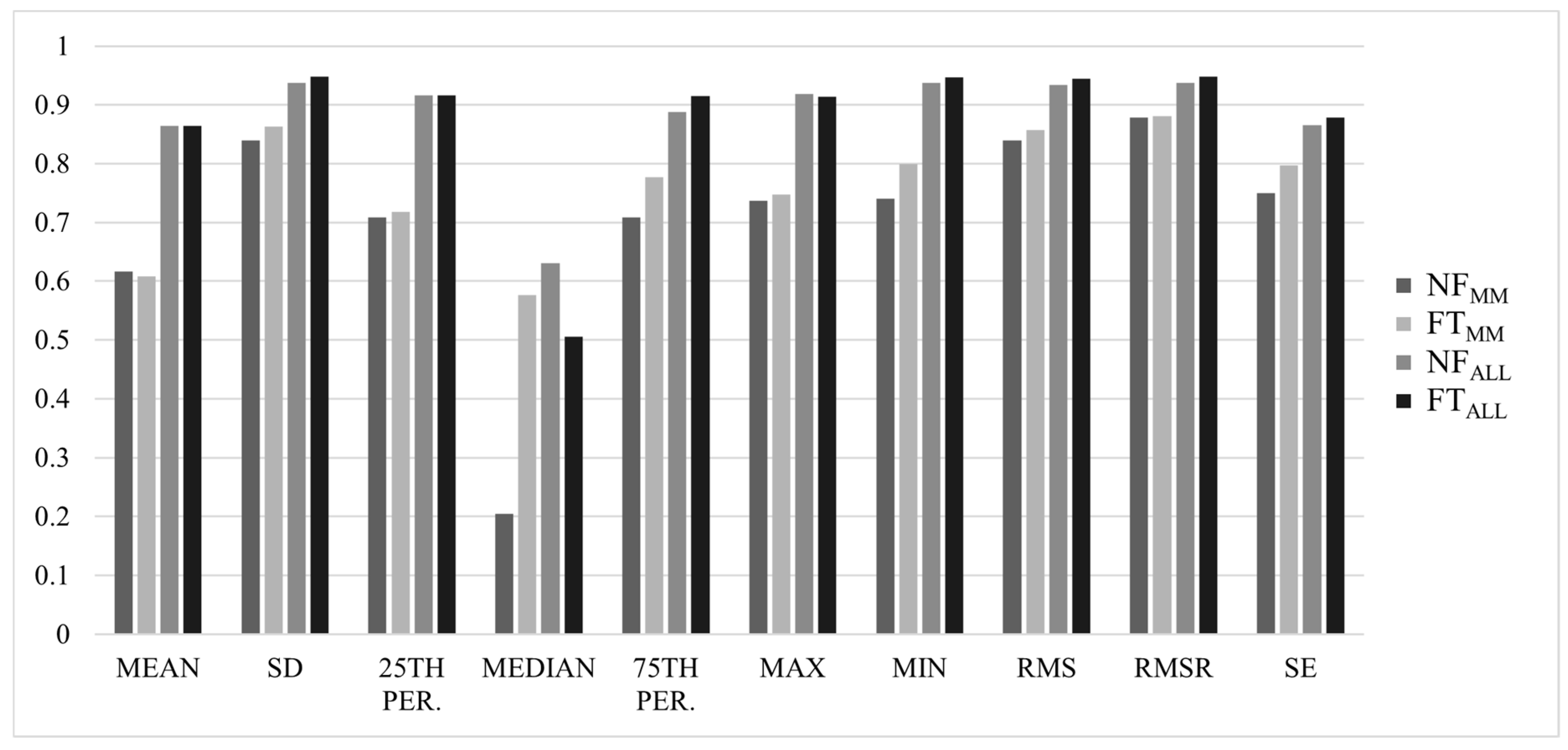
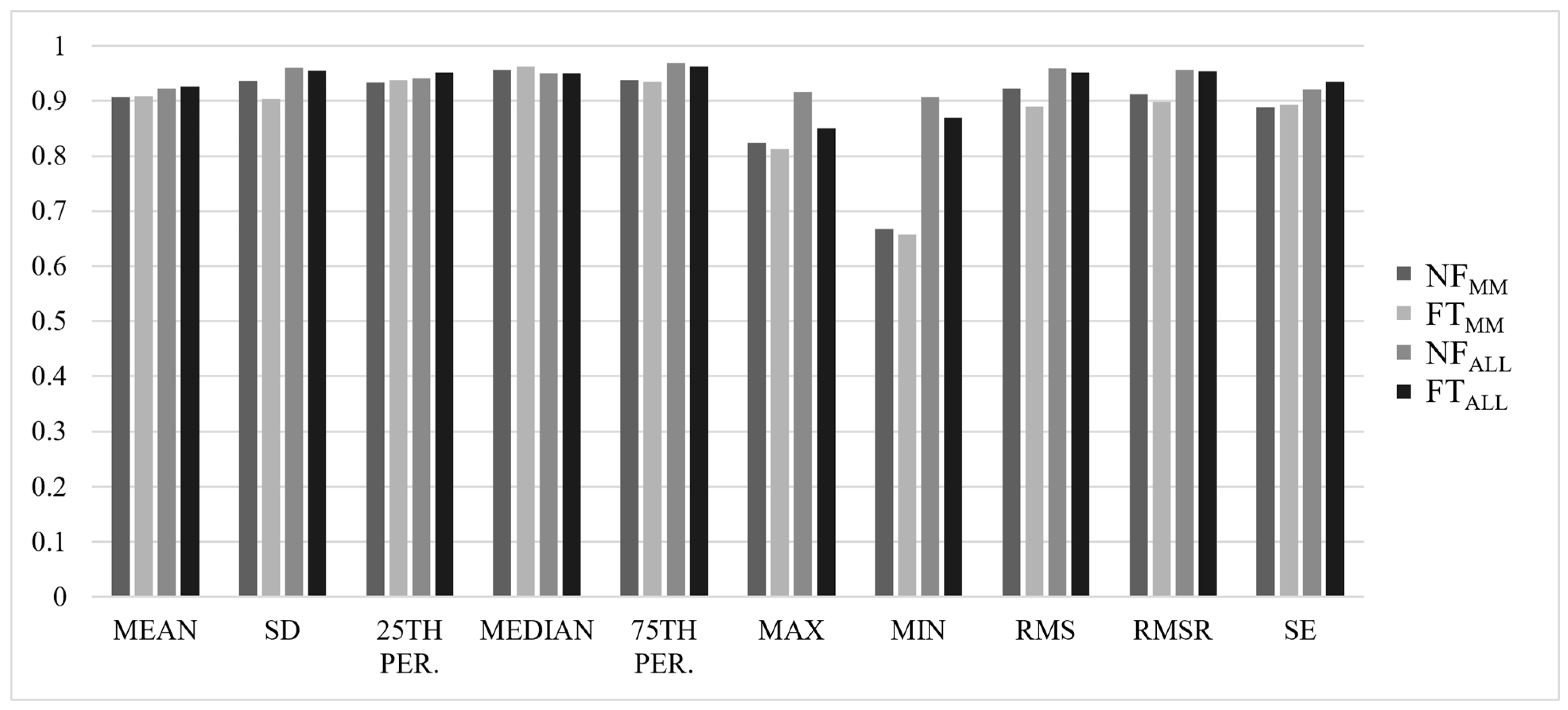
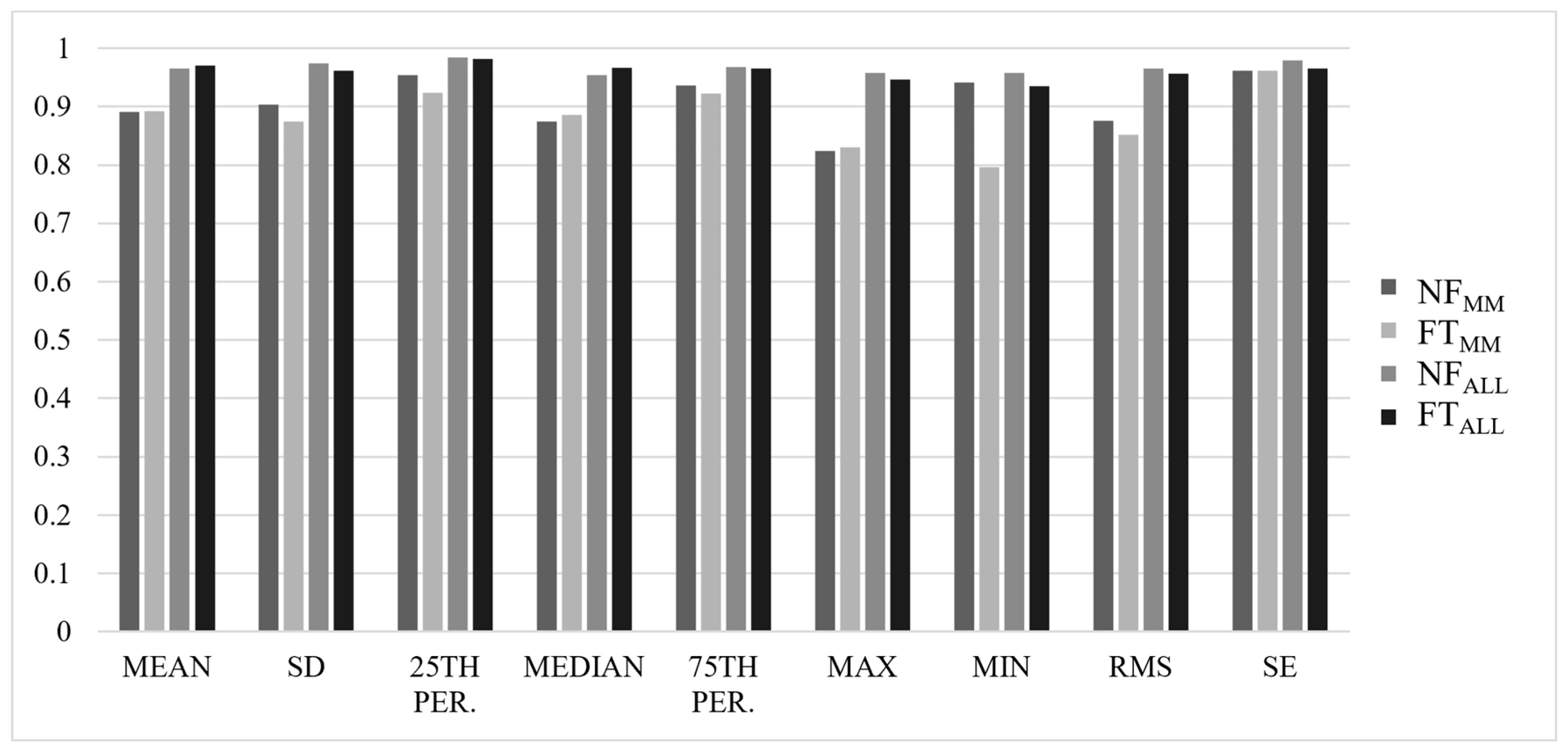
3. Results
3.1. Between-Trial Analysis
3.2. Reliability Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mannini, A.; Trojaniello, D.; Cereatti, A.; Sabatini, A. A Machine Learning Framework for Gait Classification Using Inertial Sensors: Application to Elderly, Post-Stroke and Huntington’s Disease Patients. Sensors 2016, 16, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.-C.; Hsu, Y.-L.; Yang, S.-C.; Lin, J.-C.; Wu, Z.-H. A Wearable Inertial Measurement System With Complementary Filter for Gait Analysis of Patients With Stroke or Parkinson’s Disease. IEEE Access 2016, 4, 8442–8453. [Google Scholar] [CrossRef]
- van den Tillaar, R.; Bhandurge, S.; Stewart, T. Detection of Different Throw Types and Ball Velocity with IMUs and Machine Learning in Team Handball. In Proceedings of the International Society of Biomechanics in Sport annual conference. International Society of Biomechanics in Sport (ISBS), Virtual Conference, Virtual, 21–25 July 2020; pp. 184–187. [Google Scholar]
- Allard, P.; Martinez, R.; Deguire, S.; Tremblay, J. In-Season Session Training Load Relative to Match Load in Professional Ice Hockey. J. Strength Cond. Res. 2022, 36, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Kwapisz, J.R.; Weiss, G.; Moore, S.A. Activity recognition using cell phone accelerometers. ACM SIGKDD Explor. Newsl. 2011, 12, 74–82. [Google Scholar] [CrossRef]
- Preece, S.J.; Goulermas, J.Y.; Kenney, L.P.J.; Howard, D. A Comparison of Feature Extraction Methods for the Classification of Dynamic Activities From Accelerometer Data. IEEE Trans. Biomed. Eng. 2009, 56, 871–879. [Google Scholar] [CrossRef]
- Wundersitz, D.W.T.; Gastin, P.B.; Richter, C.; Robertson, S.J.; Netto, K.J. Validity of a Trunk-Mounted Accel-erometer to Assess Peak Accelerations during Walking, Jogging and Running. Eur. J. Sport Sci. 2015, 15, 382–390. [Google Scholar] [CrossRef]
- Benson, L.C.; Clermont, C.A.; Osis, S.T.; Kobsar, D.; Ferber, R. Classifying running speed conditions using a single wearable sensor: Optimal segmentation and feature extraction methods. J. Biomech. 2018, 71, 94–99. [Google Scholar] [CrossRef]
- Strohrmann, C.; Harms, H.; Kappeler-Setz, C.; Troster, G. Monitoring Kinematic Changes With Fatigue in Running Using Body-Worn Sensors. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 983–990. [Google Scholar] [CrossRef]
- Kobsar, D.; Ferber, R. Wearable Sensor Data to Track Subject-Specific Movement Patterns Related to Clinical Outcomes Using a Machine Learning Approach. Sensors 2018, 18, 2828. [Google Scholar] [CrossRef] [Green Version]
- Op De Beéck, T.; Meert, W.; Schütte, K.; Vanwanseele, B.; Davis, J. Fatigue Prediction in Outdoor Runners Via Machine Learning and Sensor Fusion. In Proceedings of the 24th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining ACM, London, UK, 19 July 2018; pp. 606–615. [Google Scholar]
- Schütte, K.H.; Maas, E.A.; Exadaktylos, V.; Berckmans, D.; Venter, R.; Vanwanseele, B. Wireless Tri-Axial Trunk Accelerometry Detects Deviations in Dynamic Center of Mass Motion Due to Running-Induced Fatigue. PLoS ONE 2015, 10, e0141957. [Google Scholar] [CrossRef] [Green Version]
- Clermont, C.A.; Benson, L.C.; Edwards, W.B.; Hettinga, B.A.; Ferber, R. New Considerations for Wearable Technology Data: Changes in Running Biomechanics During a Marathon. J. Appl. Biomech. 2019, 35, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Hulteen, R.M.; Smith, J.J.; Morgan, P.J.; Barnett, L.M.; Hallal, P.C.; Colyvas, K.; Lubans, D.R. Global Partici-pation in Sport and Leisure-Time Physical Activities: A Systematic Review and Meta-Analysis. Prev. Med. 2017, 95, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Videbæk, S.; Bueno, A.M.; Nielsen, R.O.; Rasmussen, S. Incidence of Running-Related Injuries Per 1000 h of running in Different Types of Runners: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1017–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Gent, R.N.; Siem, D.; van Middelkoop, M.; van Os, A.G.; Bierma-Zeinstra, S.M.A.; Koes, B.W. Incidence and Determinants of Lower Extremity Running Injuries in Long Distance Runners: A Systematic Review. Br. J. Sports Med. 2007, 41, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Lopes, A.D.; Hespanhol, L.C., Jr.; Yeung, S.S.; Costa, L.O.P. What Are the Main Running-Related Musculoskeletal Injuries? Sports Med. 2012, 42, 891–905. [Google Scholar] [CrossRef]
- Knobloch, K.; Yoon, U.; Vogt, P.M. Acute and Overuse Injuries Correlated to Hours of Training in Master Running Athletes. Foot Ankle Int. 2008, 29, 671–676. [Google Scholar] [CrossRef]
- Voloshin, A.S.; Mizrahi, J.; Verbitsky, O.; Isakov, E. Dynamic loading on the human musculoskeletal system—Effect of fatigue. Clin. Biomech. 1998, 13, 515–520. [Google Scholar] [CrossRef]
- Mizrahi, J.; Verbitsky, O.; Isakov, E.; Daily, D. Effect of fatigue on leg kinematics and impact acceleration in long distance running. Hum. Mov. Sci. 2000, 19, 139–151. [Google Scholar] [CrossRef]
- Christina, K.A.; White, S.C.; Gilchrist, L.A. Effect of Localized Muscle Fatigue on Vertical Ground Reaction Forces and Ankle Joint Motion during Running. Hum. Mov. Sci. 2001, 20, 257–276. [Google Scholar] [CrossRef]
- Grimston, S.K.; Zernicke, R.F. Exercise-Related Stress Responses in Bone. J. Appl. Biomech. 1993, 9, 2–14. [Google Scholar] [CrossRef]
- Hreljac, A. Etiology, Prevention, and Early Intervention of Overuse Injuries in Runners: A Biomechanical Perspective. Phys. Med. Rehabil. Clin. N. Am. 2005, 16, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Derrick, T.R.; Dereu, D.; McLean, S.P. Impacts and kinematic adjustments during an exhaustive run. Med. Sci. Sports Exerc. 2002, 34, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Clansey, A.C.; Hanlon, M.; Wallace, E.S.; Lake, M.J. Effects of Fatigue on Running Mechanics Associated with Tibial Stress Fracture Risk. Med. Sci. Sports Exerc. 2012, 44, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, T.; Hamacher, D.; Braumann, K.-M.; Zech, A.; Hollander, K. Analysis of running stability during 5000 m running. Eur. J. Sport Sci. 2019, 19, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Meardon, S.A.; Hamill, J.; Derrick, T.R. Running injury and stride time variability over a prolonged run. Gait Posture 2011, 33, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Le Bris, R.; Billat, V.; Auvinet, B.; Chaleil, D.; Hamard, L.; Barrey, E. Effect of Fatigue on Stride Pattern Continu-ously Measured by an Accelerometric Gait Recorder in Middle Distance Runners. J. Sports Med. Phys. Fit. 2006, 46, 227–231. [Google Scholar]
- Marotta, L.; Buurke, J.; van Beijnum, B.-J.; Reenalda, J. Towards Machine Learning-Based Detection of Running-Induced Fatigue in Real-World Scenarios: Evaluation of IMU Sensor Configurations to Reduce Intrusiveness. Sensors 2021, 21, 3451. [Google Scholar] [CrossRef]
- Buckley, C.; O’Reilly, M.A.; Whelan, D.; Farrell, A.V.; Clark, L.; Longo, V.; Gilchrist, M.D.; Caulfield, B. Binary Classification of Running Fatigue Using a Single Inertial Measurement Unit. In Proceedings of the 14th International Conference on Wearable and Implantable Body Sensor Networks (BSN) IEEE, Eindhoven, The Netherlands, 9–12 May 2017; pp. 197–201. [Google Scholar]
- Tang, S.; Loh, W.P. Classification predictive models of running- and cycling-induced fatigue. AIP Conf. Proc. 2019, 2138, 050031. [Google Scholar] [CrossRef]
- Lindsay, T.R.; Yaggie, J.A.; McGregor, S.J. A wireless accelerometer node for reliable and valid measurement of lumbar accelerations during treadmill running. Sports Biomech. 2016, 15, 11–22. [Google Scholar] [CrossRef]
- Kawabata, M.; Goto, K.; Fukusaki, C.; Sasaki, K.; Hihara, E.; Mizushina, T.; Ishii, N. Acceleration patterns in the lower and upper trunk during running. J. Sports Sci. 2013, 31, 1841–1853. [Google Scholar] [CrossRef]
- McGregor, S.J.; Busa, M.A.; Yaggie, J.A.; Bollt, E.M. High Resolution MEMS Accelerometers to Estimate VO2 and Compare Running Mechanics between Highly Trained Inter-Collegiate and Untrained Runners. PLoS ONE 2009, 4, e7355. [Google Scholar] [CrossRef] [PubMed]
- Beneke, R.; von Duvillard, S.P. Determination of Maximal Lactate Steady State Response in Selected Sports Events. Med. Sci. Sports Exerc. 1996, 28, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Billat, V.L.; Sirvent, P.; Py, G.; Koralsztein, J.-P.; Mercier, J. The Concept of Maximal Lactate Steady State: A Bridge Between Biochemistry, Physiology and Sport Science. Sports Med. 2003, 33, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Faude, O.; Kindermann, W.; Meyer, T. Lactate Threshold Concepts: How Valid Are They? Sports Med. 2009, 39, 469–490. [Google Scholar] [CrossRef]
- Poole, D.C.; Jones, A.M. Oxygen Uptake Kinetics. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 933–996. [Google Scholar]
- Gerlach, K.E.; White, S.C.; Burton, H.W.; Dorn, J.M.; Leddy, J.J.; Horvath, P.J. Kinetic Changes with Fatigue and Relationship to Injury in Female Runners. Med. Sci. Sports Exerc. 2005, 37, 657–663. [Google Scholar] [CrossRef]
- Scharhag-Rosenberger, F.; Meyer, T.; Gäßler, N.; Faude, O.; Kindermann, W. Exercise at given percentages of VO2max: Heterogeneous metabolic responses between individuals. J. Sci. Med. Sport 2010, 13, 74–79. [Google Scholar] [CrossRef]
- Iannetta, D.; Inglis, E.C.; Pogliaghi, S.; Murias, J.M.; Keir, D.A. A ‘Step–Ramp–Step’ Protocol to Identify the Maximal Metabolic Steady State. Med. Sci. Sports Exerc. 2020, 52, 2011–2019. [Google Scholar] [CrossRef]
- Borg, G.A.V. Pyschophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Jones, A.M.; Doust, J.H. A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J. Sports Sci. 1996, 14, 321–327. [Google Scholar] [CrossRef]
- Tundo, M.D.; Lemaire, E.; Baddour, N. Correcting Smartphone Orientation for Accelerometer-Based Analysis. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications (MeMeA) IEEE, Gatineau, QC, USA, 4–5 May 2013; pp. 58–62. [Google Scholar]
- Kobsar, D.; Osis, S.T.; Phinyomark, A.; Boyd, J.E.; Ferber, R. Reliability of gait analysis using wearable sensors in patients with knee osteoarthritis. J. Biomech. 2016, 49, 3977–3982. [Google Scholar] [CrossRef]
- Pirttikangas, S.; Fujinami, K.; Nakajima, T. Feature Selection and Activity Recognition from Wearable Sensors; International Symposium on Ubiquitious Computing Systems; Springer: Berlin, Germany, 2006; pp. 516–527. [Google Scholar]
- Pla, G.A.; Hollville, E.; Schütte, K.; Vanwanseele, B. The Use of a Single Trunk-Mounted Accelerometer to Detect Changes in Center of Mass Motion Linked to Lower-Leg Overuse Injuries: A Prospective Study. Sensors 2021, 21, 7385. [Google Scholar] [CrossRef]
- Schütte, K.H.; Seerden, S.; Venter, R.; Vanwanseele, B. Influence of outdoor running fatigue and medial tibial stress syndrome on accelerometer-based loading and stability. Gait Posture 2018, 59, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological Time-Series Analysis Using Approximate Entropy and Sample Entropy. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; McGrath, D.; Stergiou, N. The Appropriate Use of Ap-proximate Entropy and Sample Entropy with Short Data Sets. Ann. Biomed. Eng. 2013, 41, 349–365. [Google Scholar] [CrossRef]
- Preatoni, E.; Ferrario, M.; Donà, G.; Hamill, J.; Rodano, R. Motor variability in sports: A non-linear analysis of race walking. J. Sports Sci. 2010, 28, 1327–1336. [Google Scholar] [CrossRef] [Green Version]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Sekine, M.; Tamura, T.; Yoshida, M.; Suda, Y.; Kimura, Y.; Miyoshi, H.; Kijima, Y.; Higashi, Y.; Fujimoto, T. A gait abnormality measure based on root mean square of trunk acceleration. J. Neuroeng. Rehabil. 2013, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Schütte, K.H.; Seerden, S.; Venter, R.; Vanwanseele, B. Fatigue-Related Asymmetry and Instability during a 3200-m Time-Trial Performance in Healthy Runners. In Proceedings of the 34 International Conference of Biomechanics in Sport, Tsukuba, Japan, 18–22 July 2016; p. 4. [Google Scholar]
- Winter, S.C.; Gordon, S.; Brice, S.; Lindsay, D.; Barrs, S. A Multifactorial Approach to Overuse Running Injuries: A 1-Year Prospective Study. Sports Health Multidiscip. Approach 2020, 12, 296–303. [Google Scholar] [CrossRef]
- Lipsitz, L.A.; Goldberger, A.L. Loss of “complexity” and aging. Potential applications of fractals and chaos theory to senescence. JAMA 1992, 267, 1806–1809. [Google Scholar] [CrossRef]
- Williams, K.R.; Snow, R.; Agruss, C. Changes in Distance Running Kinematics with Fatigue. Int. J. Sport Biomech. 1991, 7, 138–162. [Google Scholar] [CrossRef]
- Benson, L.C.; Ahamed, N.U.; Kobsar, D.; Ferber, R. New considerations for collecting biomechanical data using wearable sensors: Number of level runs to define a stable running pattern with a single IMU. J. Biomech. 2019, 85, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, N.U.; Benson, L.C.; Clermont, C.A.; Pohl, A.J.; Ferber, R. New Considerations for Collecting Biome-chanical Data Using Wearable Sensors: How Does Inclination Influence the Number of Runs Needed to Determine a Stable Running Gait Pattern? Sensors 2019, 19, 2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, L.C.; Clermont, C.A.; Ferber, R. New Considerations for Collecting Biomechanical Data Using Wearable Sensors: The Effect of Different Running Environments. Front. Bioeng. Biotechnol. 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, J.; Richards, J.; Taylor, P.J.; Edmundson, C.J.; Brooks, D.; Hobbs, S.J. Three-dimensional kinematic comparison of treadmill and overground running. Sports Biomech. 2013, 12, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Dierks, T.A.; Manal, K.T.; Hamill, J.; Davis, I.S. Proximal and Distal Influences on Hip and Knee Kinematics in Runners With Patellofemoral Pain During a Prolonged Run. J. Orthop. Sports Phys. Ther. 2008, 38, 448–456. [Google Scholar] [CrossRef]
- Benson, L.C.; O’Connor, K.M. The Effect of Exertion on Joint Kinematics and Kinetics During Running Using a Waveform Analysis Approach. J. Appl. Biomech. 2015, 31, 250–257. [Google Scholar] [CrossRef]
- Maas, E.; De Bie, J.; Vanfleteren, R.; Hoogkamer, W.; Vanwanseele, B. Novice runners show greater changes in kinematics with fatigue compared with competitive runners. Sports Biomech. 2017, 17, 350–360. [Google Scholar] [CrossRef]
- Burland, J.P.; Outerleys, J.B.; Lattermann, C.; Davis, I.S. Reliability of wearable sensors to assess impact metrics during sport-specific tasks. J. Sports Sci. 2021, 39, 406–411. [Google Scholar] [CrossRef]
| MLSS1 | MLSS2 | F | S | |
|---|---|---|---|---|
| Treadmill speed (m/s) | 3.35 ± 0.4 | 3.35 ± 0.4 | 3.53 ± 0.4 | 3.17 ± 0.4 |
| Number of strides used (NF) | 376.8 ± 119.2 | 390.5 ± 69.5 | 411.5 ± 127 | 395.5 ± 109.5 |
| Number of strides used (FT) | 375.5 ± 71.5 | 387.5 ± 53.5 | 366 ± 77 | 362.5 ± 61 |
| Trial time (min) * | 39.8 ± 6.6 @ | 37.7 ± 7.3 @ | 28.8 ± 6.9 | 42.0 ± 5.6 @ |
| RPENF | 11.5 ± 1.7 | 11.4 ± 1.8 | 12.5 ± 1.5 | 11.0 ± 1.5 |
| RPEFT * | 15.6 ± 1.6 #,^ | 15.3 ± 1.6 #,^ | 16.8 ± 1.3 †,‡,#,^ | 14.1 ± 1.7 ^ |
| ΔRPE | 4.1 ± 2.0 | 3.9 ± 2.0 | 4.3 ± 1.7 | 3.1 ± 1.6 |
| Axis | Feature | MM | ALL | ||
|---|---|---|---|---|---|
| p Value | Direction of Change | p Value | Direction of Change | ||
| VT | Mean | 0.022 | - | 0.009 | - |
| St. deviation | 0.032 | + | 0.006 | + | |
| 25th percentile | 0.091 | 0.003 | - | ||
| Median | 0.014 | + | 0.001 | + | |
| 75th percentile | 0.741 | 0.479 | |||
| Max | 0.003 | + | 0.002 | + | |
| Min | 0.281 | 0.037 | - | ||
| RMS | 0.075 | 0.023 | + | ||
| RMSR | <0.001 | - | <0.001 | - | |
| SE | 0.001 | + | 0.001 | + | |
| ML | Mean | 0.098 | 0.115 | ||
| St. deviation | <0.001 | + | <0.001 | + | |
| 25th percentile | 0.152 | 0.025 | - | ||
| Median | 0.207 | 0.123 | |||
| 75th percentile | 0.492 | 0.212 | |||
| Max | <0.001 | + | <0.001 | + | |
| Min | 0.004 | - | <0.001 | - | |
| RMS | <0.001 | + | <0.001 | + | |
| RMSR | <0.001 | + | <0.001 | + | |
| SE | <0.001 | - | <0.001 | - | |
| AP | Mean | 0.001 | - | <0.001 | - |
| St. deviation | 0.007 | + | <0.001 | + | |
| 25th percentile | 0.002 | - | <0.001 | - | |
| Median | 0.699 | 0.241 | |||
| 75th percentile | 0.157 | 0.027 | + | ||
| Max | 0.123 | 0.514 | |||
| Min | 0.009 | - | <0.001 | - | |
| RMS | 0.008 | + | 0.001 | + | |
| RMSR | 0.078 | 0.028 | + | ||
| SE | 0.136 | 0.07 | |||
| RES | Mean | <0.001 | + | <0.001 | + |
| St. deviation | 0.016 | + | 0.009 | + | |
| 25th percentile | 0.001 | + | 0.06 | + | |
| Median | 0.169 | 0.005 | + | ||
| 75th percentile | 0.38 | 0.346 | |||
| Max | 0.003 | + | 0.004 | + | |
| Min | 0.136 | 0.601 | |||
| RMS | 0.001 | + | <0.001 | + | |
| SE | 0.017 | + | 0.01 | + | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimmick, H.L.; van Rassel, C.R.; MacInnis, M.J.; Ferber, R. Between-Day Reliability of Commonly Used IMU Features during a Fatiguing Run and the Effect of Speed. Sensors 2022, 22, 4129. https://doi.org/10.3390/s22114129
Dimmick HL, van Rassel CR, MacInnis MJ, Ferber R. Between-Day Reliability of Commonly Used IMU Features during a Fatiguing Run and the Effect of Speed. Sensors. 2022; 22(11):4129. https://doi.org/10.3390/s22114129
Chicago/Turabian StyleDimmick, Hannah L., Cody R. van Rassel, Martin J. MacInnis, and Reed Ferber. 2022. "Between-Day Reliability of Commonly Used IMU Features during a Fatiguing Run and the Effect of Speed" Sensors 22, no. 11: 4129. https://doi.org/10.3390/s22114129







