Mechanocardiography in the Detection of Acute ST Elevation Myocardial Infarction: The MECHANO-STEMI Study
Abstract
:1. Introduction
2. Background and Motivation
2.1. Background
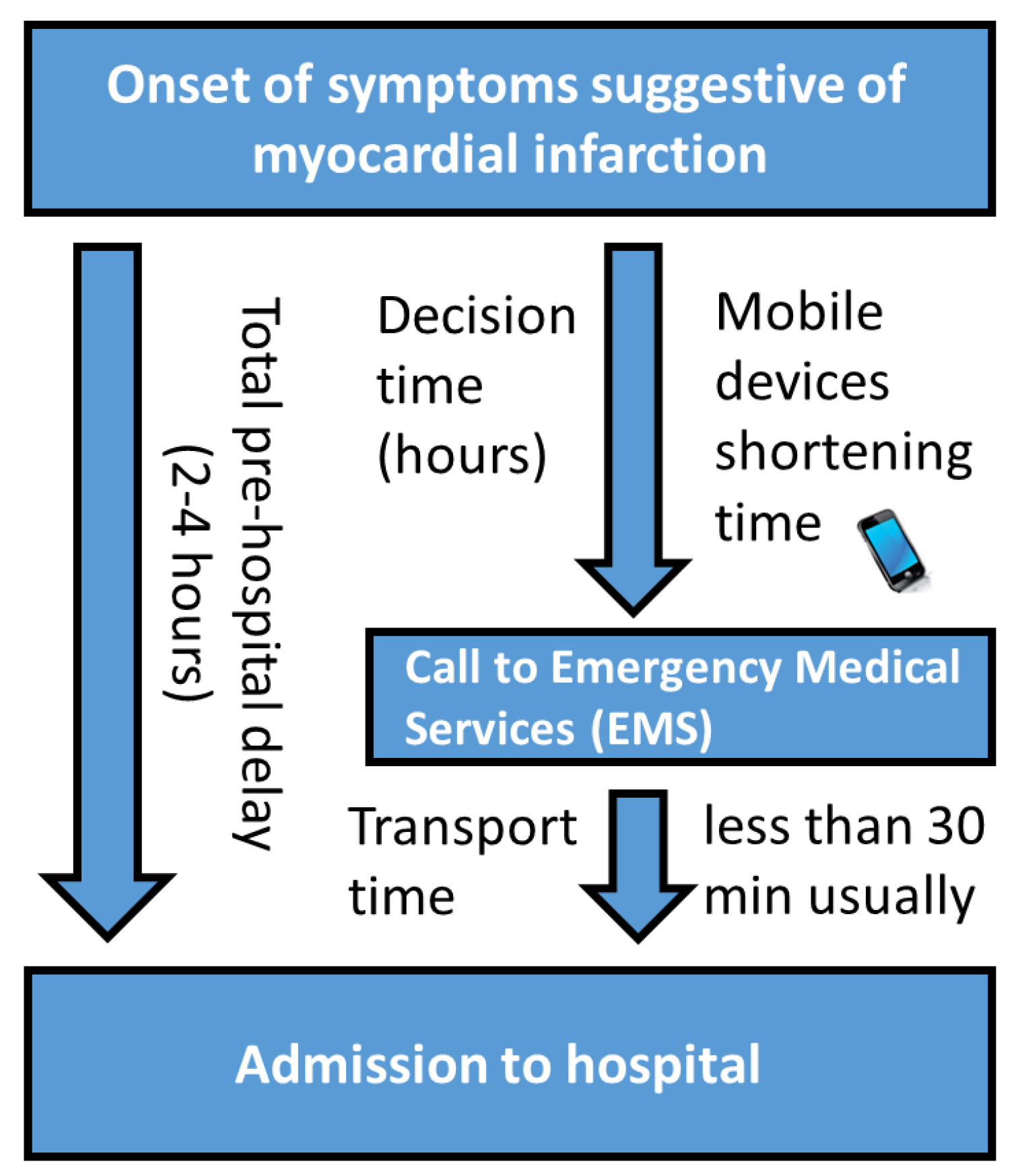
2.2. Motivation
3. Materials and Methods
3.1. Patients
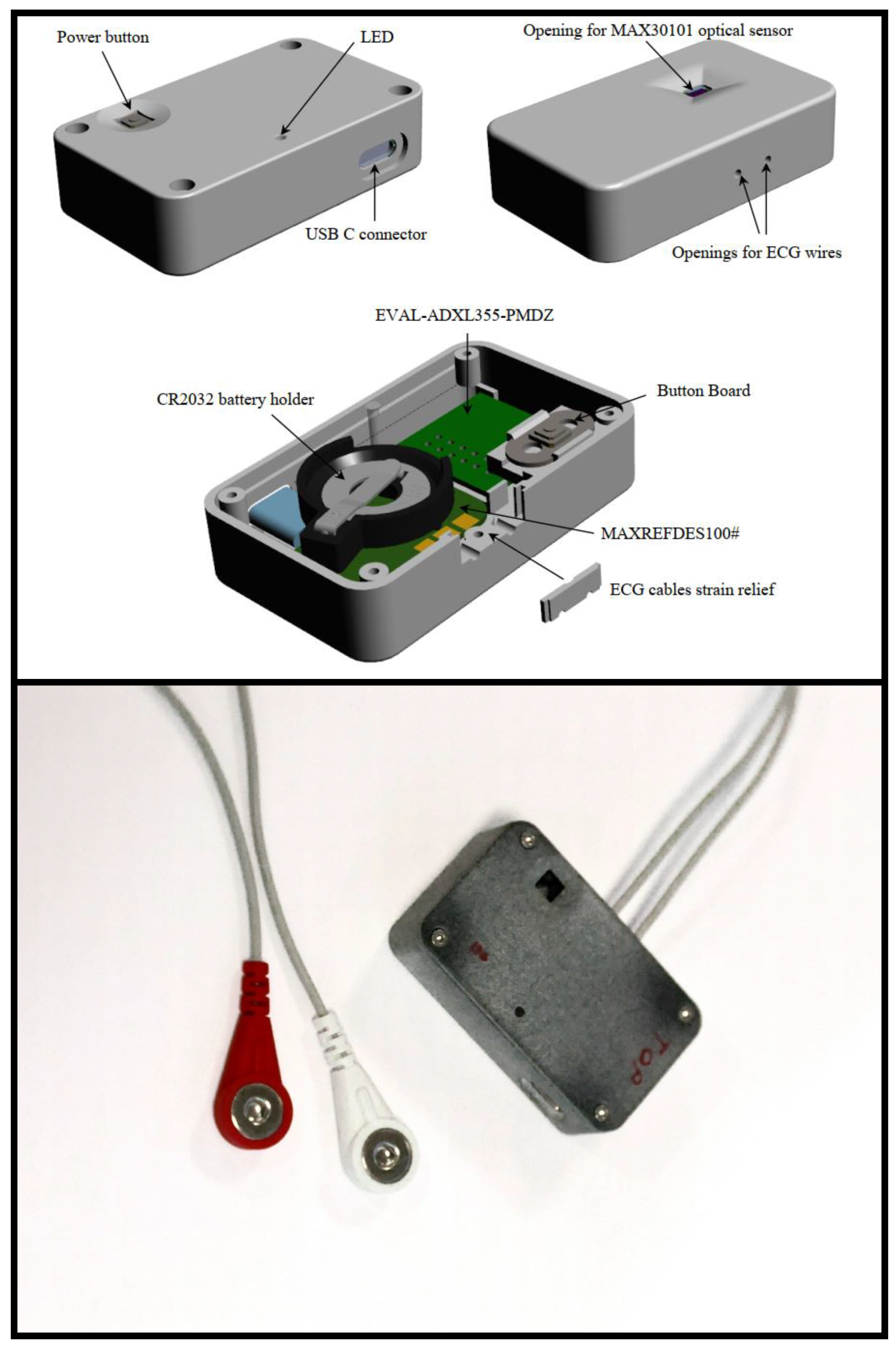
3.2. Data Acquisition
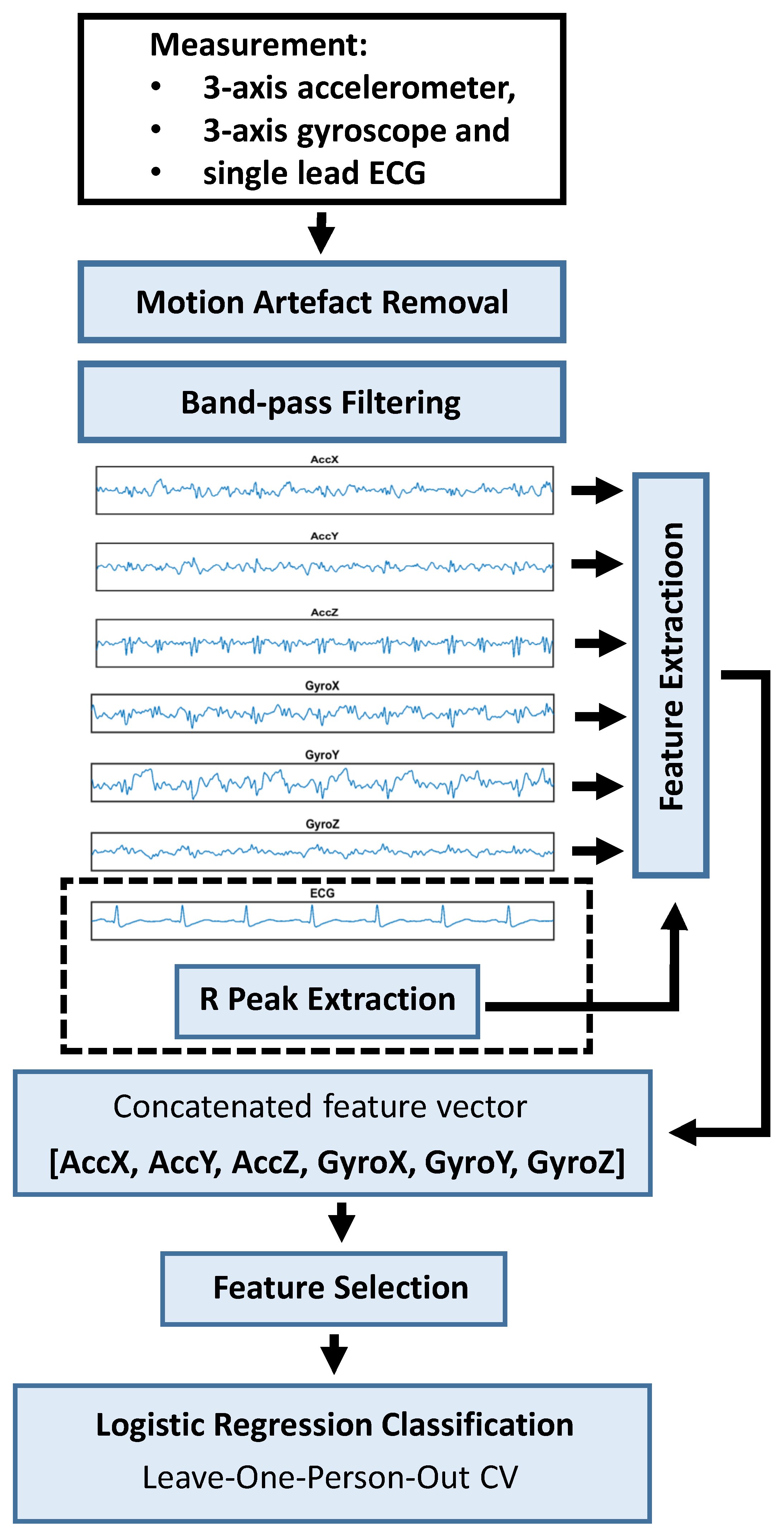
3.3. Motion Artefact Removal
3.4. ML Pipeline
3.4.1. Feature Extraction
3.4.2. Feature Selection and Classification
4. Results
4.1. Classification Results
4.2. Feature Analysis
| Study | Modality | Wearable | Method | Patients | Duration of | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| Device | (STEMI/Control) | Recordings | |||||
| Heden et al., (1997) [12] | 12-lead ECG | No | ANN | 1120/10,452 | NA | 46.2% | 95.4% |
| Heden et al., (1997) [12] | 12-lead ECG | No | Rule-based | 1120/10,452 | NA | 30.7% | 95.4% |
| Haraldsson et al., (2004) [13] | 12-lead ECG | No | Bayesian ANN | 1119/1119 | NA | 63.3% | 85.0% |
| Haraldsson et al., (2004) [13] | 12-lead ECG | No | Hermite functions | 1119/1119 | NA | 61.5% | 85.0% |
| Haraldsson et al., (2004) [13] | 8-lead ECG | No | Bayesian ANN | 1119/1119 | NA | 56.3% | 85.0% |
| Haraldsson et al., (2004) [13] | 8-lead ECG | No | Hermite functions | 1119/1119 | NA | 59.3% | 85.0% |
| Green et al., (2006) [14] | 12-lead ECG | No | ANN ensemble | 130/504 | NA | 95.0% | 41.4% |
| Green et al., (2006) [14] | 12-lead ECG | No | Logistic regression | 130/504 | NA | 95.0% | 33.7% |
| Olsson et al., (2006) [15] * | 12-lead ECG | No | Feed-forward ANN | 736/3264 | NA | 95.0% | 88.0% |
| Tripathy et al., (2019) [16] | 12-lead ECG | No | Fourier-Bessel DNN | 100/52 | 24 h | 99.9% | 99.6% |
| Van Heuverswyn et al., (2019) [17] | 3-lead ECG | Yes | Rule-based (ST-seg.) | 59 (5011 rec.) | NA | 87–100% | 96.0% |
| Spaccarotella et al., (2020) [18] | 1-lead ECG x 9 | Yes | Human expert | 54/19 | 5.8 min | 93.0% | 95% |
| Ours ** | MCG (1-lead ECG) | Yes | Logistic regression | 41/49 | 15 min | 85.7% | 73.9% |
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salomaa, V.; Ketonen, M.; Koukkunen, H.; Immonen-Räihä, P.; Jerkkola, T.; Kärjä-Koskenkari, P.; Mähönen, M.; Niemelä, M.; Kuulasmaa, K.; Palomäki, P.; et al. Decline in out-of-hospital coronary heart disease deaths has contributed the main part to the overall decline in coronary heart disease mortality rates among persons 35 to 64 years of age in Finland: The FINAMI Study. Circulation 2003, 108, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Karam, N.; Bataille, S.; Marijon, E.; Tafflet, M.; Benamer, H.; Caussin, C.; Garot, P.; Juliard, J.; Pires, V.; Boche, T.; et al. Incidence, mortality, and outcome-predictors of sudden cardiac arrest complicating myocardial infarction prior to hospital admission. Circ. Cardiovasc. Interv. 2019, 12, e007081. [Google Scholar] [CrossRef] [PubMed]
- Tadi, M.J.; Lehtonen, E.; Saraste, A.; Tuominen, J.; Koskinen, J.; Teräs, M.; Airaksinen, J.; Pänkäälä, M.; Koivisto, T. Gyrocardiography: A new non-invasive monitoring method for the assessment of cardiac mechanics and the estimation of hemodynamic variables. Sci. Rep. 2017, 7, 6823. [Google Scholar] [CrossRef] [PubMed]
- Gurev, V.; Tavakolian, K.; Constantino, J.; Kaminska, B.; Blaber, A.P.; Trayanova, N.A. Mechanisms underlying isovolumic contraction and ejection peaks in seismocardiogram morphology. J. Med. Biol. Eng. 2012, 32, 103–110. [Google Scholar] [CrossRef]
- Sieciński, S.; Kostka, P.S.; Tkacz, E.J. Gyrocardiography: A review of the definition, history, waveform description, and applications. Sensors 2020, 20, 6675. [Google Scholar] [CrossRef]
- Jaakkola, J.; Jaakkola, S.; Lahdenoja, O.; Hurnanen, T.; Koivisto, T.; Pänkäälä, M.; Knuutila, T.; Kiviniemi, T.O.; Vasankari, T.; Airaksinen, K.E.J. Mobile phone detection of atrial fibrillation with mechanocardiography: The MODE-AF Study. Circulation 2018, 137, 1524–1527. [Google Scholar] [CrossRef]
- Morra, S.; Hossein, A.; Rabineau, J.; Gorlier, D.; Racape, J.; van de Borne, P. Assessment of left ventricular twist by 3D ballistocardiography and seismocardiography compared with 2D STI echocardiography in a context of enhanced inotropism in healthy subjects. Sci. Rep. 2021, 11, 683. [Google Scholar] [CrossRef]
- Morra, S.; Pitisci, L.; Su, F.; Hossein, A.; Rabineau, J.; Racape, J.; Gorlier, D.; Herpain, A.; Migeotte, P.; Creteur, J.; et al. Quantification of Cardiac Kinetic Energy and Its Changes During Transmural Myocardial Infarction Assessed by Multi-Dimensional Seismocardiography. Front. Cardiovasc. Med. 2021, 8, 603319. [Google Scholar] [CrossRef]
- Becker, M.; Roehl, A.B.; Siekmann, U.; Koch, A.; de la Fuente, M.; Roissant, R.; Radermacher, K.; Marx, N.; Hein, M. Simplified detection of myocardial ischemia by seismocardiography. Differentiation between causes of altered myocardial function. Herz 2014, 39, 586–592. [Google Scholar]
- Inan, O.T.; Pouyan, M.B.; Javaid, A.Q.; Dowling, S.; Etemadi, M.; Dorier, A.; Heller, J.A.; Bicen, A.O.; Roy, S.; Marco, T.D.; et al. Novel Wearable Seismocardiography and Machine Learning Algorithms Can Assess Clinical Status of Heart Failure Patients. Circ. Heart Fail. 2018, 11, e004313. [Google Scholar] [CrossRef]
- Iftikhar, Z.; Lahdenoja, O.; Tadi, M.J.; Hurnanen, T.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; Koivisto, T.; Pänkäälä, M. Multiclass classifier based cardiovascular condition detection using smartphone mechanocardiography. Sci. Rep. 2018, 8, 9344. [Google Scholar] [CrossRef]
- Hedén, B.; Öhlin, H.; Rittner, R.; Edenbrandt, L. Acute myocardial infarction detected in the 12-lead ECG by artificial neural networks. Circulation 1997, 96, 1798–1802. [Google Scholar] [CrossRef]
- Haraldsson, H.; Edenbrandt, L.; Ohlsson, M. Detecting acute myocardial infarction in the 12-lead ECG using Hermite expansions and neural networks. Artif. Intell. Med. 2004, 32, 127–136. [Google Scholar] [CrossRef]
- Green, M.; Björk, J.; Forberg, J.; Ekelund, U.; Edenbrandt, L.; Ohlsson, M. Comparison between neural networks and multiple logistic regression to predict acute coronary syndrome in the emergency room. Artif. Intell. Med. 2006, 38, 305–318. [Google Scholar] [CrossRef]
- Olsson, S.E.; Ohlsson, M.; Ohlin, H.; Dzaferagic, S.; Nilsson, M.L.; Sandkull, P.; Edenbrandt, L. Decision support for the initial triage of patients with acute coronary syndromes. Clin. Phys. Func. Imaging 2006, 26, 151–156. [Google Scholar] [CrossRef]
- Tripathy, R.K.; Bhattacharyya, A.; Pachori, R.B. A novel approach for detection of myocardial infarction from ECG signals of multiple electrodes. IEEE Sens. J. 2019, 19, 4509–4517. [Google Scholar] [CrossRef]
- Heuverswyn, F.V.; Buyzere, M.D.; Coeman, M.; Pooter, J.D.; Drieghe, B.; Duytschaever, M.; Gevaert, S.; Kayaert, P.; Vandekerckhove, Y.; Voet, J.; et al. Feasibility and performance of a device for automatic self-detection of symptomatic acute coronary artery occlusion in outpatients with coronary artery disease: A multicentre observational study. Lancet Digit. Health 2019, 1, e90–e99. [Google Scholar] [CrossRef]
- Spaccarotella, C.A.M.; Polimeni, A.; Migliarino, S.; Principe, E.; Curcio, A.; Mongiardo, A.; Sorrentino, S.; Rosa, S.D.; Indolfi, C. Multichannel Electrocardiograms Obtained by a Smartwatch for the Diagnosis of ST-Segment Changes. JAMA Cardiol. 2020, 5, 1176–1180. [Google Scholar] [CrossRef]
- Gibson, C.M.; Holmes, D.; Mikdadi, G.; Presser, D.; Wohns, D.; Yee, M.K.; Kaplan, A.; Ciuffo, A.; Eberly, A.L.; Iteld, B.; et al. Implantable Cardiac Alert System for Early Recognition of ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 73, 1919–1927. [Google Scholar] [CrossRef]
- Myerburg, R.J.; Kessler, K.M.; Castellanos, A. Sudden cardiac death: Structure, function, and time-dependence of risk. Circulation 1992, 85 (Suppl. I), I2–I10. [Google Scholar]
- Kannel, W.B.; Doyle, J.T.; McNamara, P.M.; Quickenton, P.; Gordon, T. Precursors of sudden coronary death. Factors related to the incidence of sudden death. Circulation 1975, 51, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, K.E.J. Autonomic mechanisms and sudden death during abrupt coronary occlusion. Ann. Med. 1999, 31, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Kaplinsky, E.; Ogawa, S.; Balke, C.W.; Dreifus, L.S. Two periods of early ventricular arrhythmia in the canine acute myocardial infarction model. Circulation 1979, 60, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From Vulnerable Plaque to Vulnerable Patient: A Call for New Definitions and Risk Assessment Strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Saczynski, J.S.; Yarzebski, J.; Lessard, D.; Spencer, F.A.; Gurwitz, J.H.; Gore, J.M.; Goldberg, R.J. Trends in prehospital delay in patients with acute myocardial infarction (from the Worcester Heart Attack Study). Am. J. Cardiol. 2008, 102, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Inan, O.T.; Migeotte, P.F.; Park, K.S.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G.K.; et al. Ballistocardiography and Seismocardiography: A Review of Recent Advances. IEEE J. Biomed. Health Inform. 2015, 19, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Shandhi, M.M.H.; Semiz, B.; Hersek, S.; Goller, N.; Ayazi, F.; Inan, O.T. Performance Analysis of Gyroscope and Accelerometer Sensors for Seismocardiography-Based Wearable Pre-Ejection Period Estimation. IEEE J. Biomed. Health Inform. 2019, 23, 2365–2374. [Google Scholar] [CrossRef]
- Tadi, M.J.; Mehrang, S.; Kaisti, M.; Lahdenoja, O.; Hurnanen, T.; Jaakkola, J.; Jaakkola, S.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; et al. Comprehensive Analysis of Cardiogenic Vibrations for Automated Detection of Atrial Fibrillation Using Smartphone Mechanocardiograms. IEEE Sens. J. 2019, 19, 2230–2242. [Google Scholar] [CrossRef]
- Halvorsen, P.S.; Fleischer, L.A.; Espinoza, A.; Elle, O.J.; Hoff, L.; Skulstad, H.; Edvardsen, T.; Fosse, E. Detection of myocardial ischaemia by epicardial accelerometers in the pig. Br. J. Anaesth. 2009, 102, 29–37. [Google Scholar] [CrossRef]
- Theres, H.P.; Kaiser, D.R.; Nelson, S.D.; Glos, M.; Leuthold, T.; Baumann, G.; Sowelam, S.; Sheldon, T.J.; Stylos, L. Detection of acute myocardial ischemia during percutaneous transluminal coronary angioplasty by endocardial acceleration. PACE-Pacing Clin. Electrophysiol. 2004, 27, 621–625. [Google Scholar] [CrossRef]
- Harris, I.S.; Lee, E.; Yeghiazarians, Y.; Drew, B.J.; Michaels, A.D. Phonocardiographic timing of third and fourth heart sounds during acute myocardial infarction. J. Electrocardiol. 2006, 39, 305–309. [Google Scholar] [CrossRef]
- Siejko, K.Z.; Thakur, P.H.; Maile, K.; Patangay, A.; Olivari, M. Feasibility of heart sounds measurements from an accelerometer within an icd pulse generator. PACE-Pacing Clin. Electrophysiol. 2013, 36, 334–346. [Google Scholar] [CrossRef]
- Marcus, G.M.; Gerber, I.L.; McKeown, B.H.; Vessey, J.C.; Jordan, M.V.; Huddleston, M.; McCulloch, C.E.; Foster, E.; Chatterjee, K.; Michaels, A.D. Association between phonocardiographic third and fourth heart sounds and objective measures of left ventricular function. J. Am. Med. Assoc. 2005, 293, 2238–2244. [Google Scholar] [CrossRef]
- Morra, S.; Gauthey, A.; Hossein, A.; Rabineau, J.; Racape, J.; Gorlier, D.; Migeotte, P.; le Polain de Waroux, J.B.; van de Borne, P. Influence of sympathetic activation on myocardial contractility measured with ballistocardiography and seismocardiography during sustained end-expiratory apnea. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020, 319, 497–506. [Google Scholar] [CrossRef]


| STEMI Patients | Control Patients | p-Value | |
|---|---|---|---|
| n= 41 | n= 49 | ||
| Male | 25 (61.0) | 39 (79.6) | 0.088 |
| Weight, kg | 80.1 ± 18.6 | 84.8 ± 14.8 | 0.198 |
| Height, cm | 174.0 ± 10.5 | 174.1 ± 9.4 | 0.888 |
| Age, years | 66.8 ± 13.8 | 65.9 ± 10.3 | 0.874 |
| Syst. blood pressure, mmHg | 129.6 ± 27.2 | 140.1 ± 20.5 | 0.037 |
| Diast. blood pressure, mmHg | 71.3 ± 15.9 | 72.2 ± 10.7 | 0.401 |
| Heart rate, bpm | 77.3 ± 22.4 | 65.8 ± 10.8 | 0.026 |
| Maximum troponin T, ng/L | 4189 (5827) | N/A | - |
| Ejection fraction, mean,% | 37.8 ± 12.8 | 60.2 ± 7.3 | <0.001 |
| Body mass index, kg/m2 | 26.1 ± 4.5 | 28.0 ± 3.8 | 0.164 |
| Beta blocker | 11 (26.8) | 16 (32.7) | 0.712 |
| Coronary artery disease | 39 (95.1) | 39 (79.6) | 0.065 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koivisto, T.; Lahdenoja, O.; Hurnanen, T.; Vasankari, T.; Jaakkola, S.; Kiviniemi, T.; Airaksinen, K.E.J. Mechanocardiography in the Detection of Acute ST Elevation Myocardial Infarction: The MECHANO-STEMI Study. Sensors 2022, 22, 4384. https://doi.org/10.3390/s22124384
Koivisto T, Lahdenoja O, Hurnanen T, Vasankari T, Jaakkola S, Kiviniemi T, Airaksinen KEJ. Mechanocardiography in the Detection of Acute ST Elevation Myocardial Infarction: The MECHANO-STEMI Study. Sensors. 2022; 22(12):4384. https://doi.org/10.3390/s22124384
Chicago/Turabian StyleKoivisto, Tero, Olli Lahdenoja, Tero Hurnanen, Tuija Vasankari, Samuli Jaakkola, Tuomas Kiviniemi, and K. E. Juhani Airaksinen. 2022. "Mechanocardiography in the Detection of Acute ST Elevation Myocardial Infarction: The MECHANO-STEMI Study" Sensors 22, no. 12: 4384. https://doi.org/10.3390/s22124384
APA StyleKoivisto, T., Lahdenoja, O., Hurnanen, T., Vasankari, T., Jaakkola, S., Kiviniemi, T., & Airaksinen, K. E. J. (2022). Mechanocardiography in the Detection of Acute ST Elevation Myocardial Infarction: The MECHANO-STEMI Study. Sensors, 22(12), 4384. https://doi.org/10.3390/s22124384






