One-Step Co-Electrodeposition of Copper Nanoparticles-Chitosan Film-Carbon Nanoparticles-Multiwalled Carbon Nanotubes Composite for Electroanalysis of Indole-3-Acetic Acid and Salicylic Acid
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Apparatus
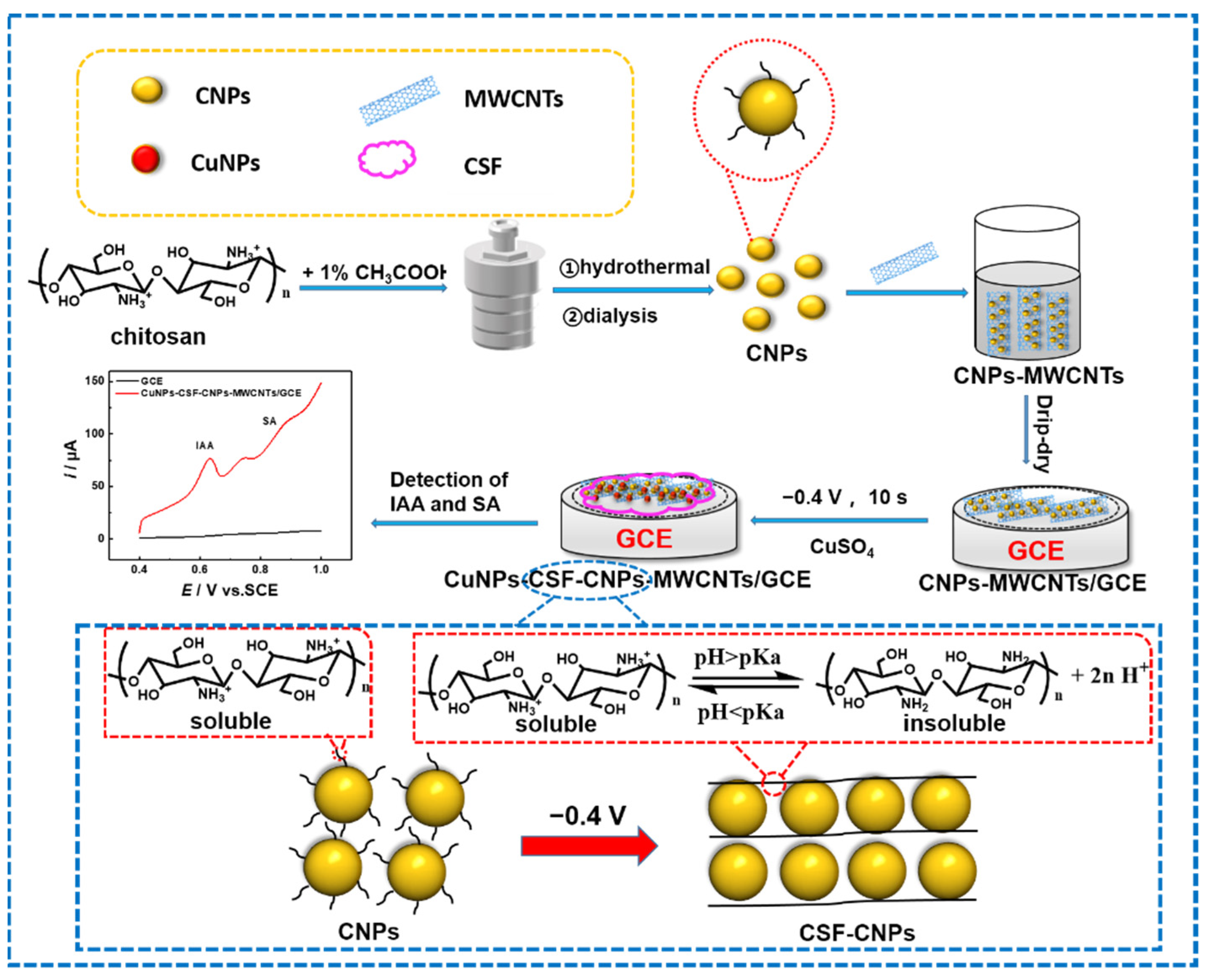
2.2. Procedures
3. Results and Discussion
3.1. Characterization of CuNPs-CSF-CNPs-MWCNTs Composite
3.2. Optimization of Experimental Conditions
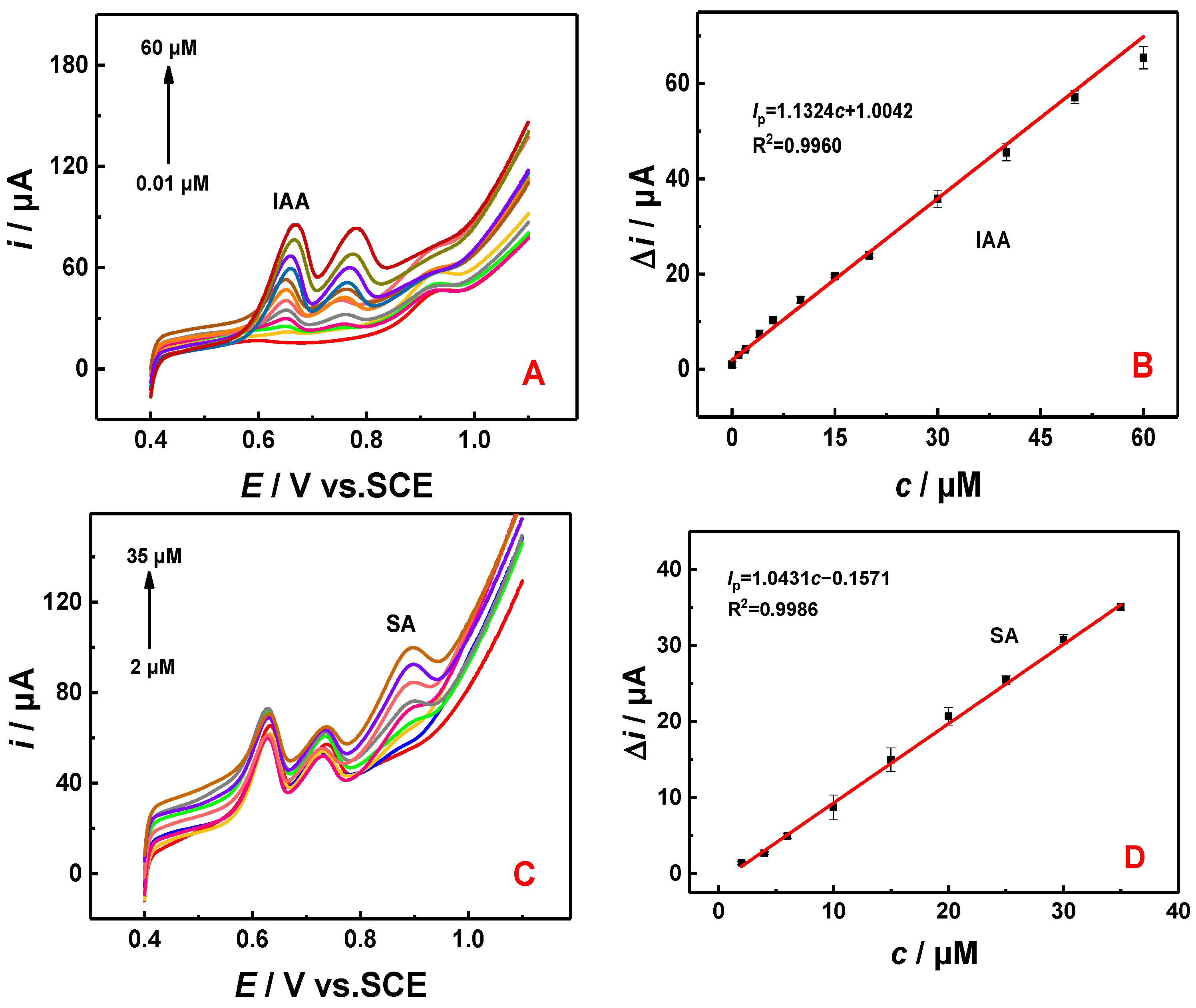
3.3. Kinetic Behavior of IAA and SA Detection
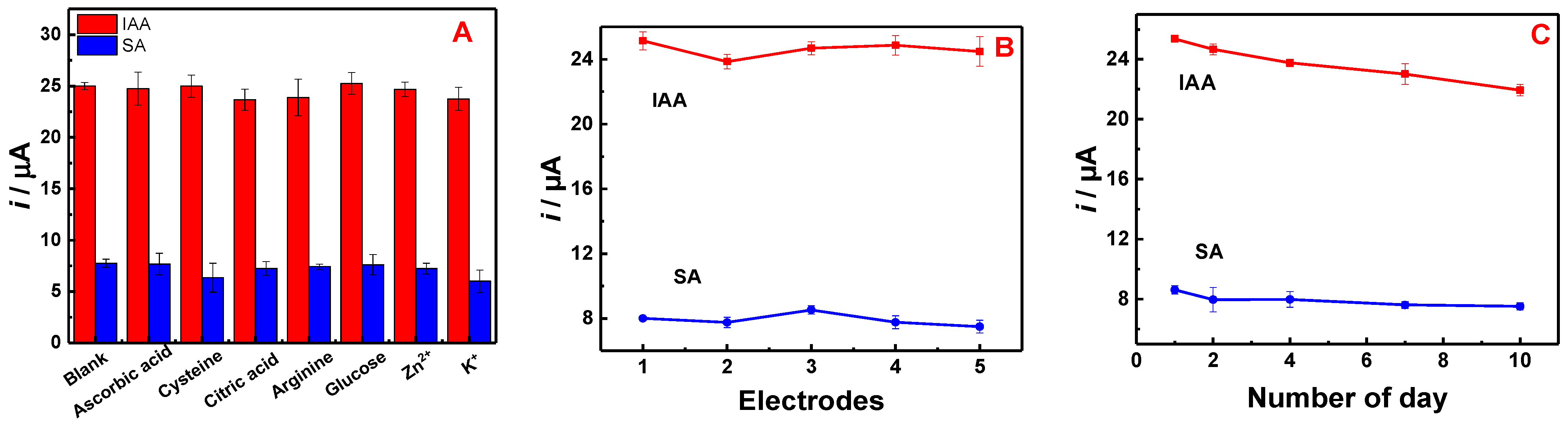
3.4. Detection of IAA and SA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, S.; Bai, L.; Wen, Y.; Li, M.; Yan, D.; Zhang, R.; Chen, K. Water-dispersed carboxymethyl cellulose-montmorillonite-single walled carbon nanotube composite with enhanced sensing performance for simultaneous voltammetric determination of two trace phytohormones. J. Solid State Electrochem. 2015, 19, 2023–2037. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Hassan, M.J.; Li, Z.; Peng, Y. Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Dai, J.; Ge, Q. Responses of autumn phenology to climate change and the correlations of plant hormone regulation. Sci. Rep. 2020, 10, 9039. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040–104059. [Google Scholar] [CrossRef]
- Qi, B.; Wu, C.; Liang, H.; Cui, K.; Fahad, S.; Wang, M.; Liu, B.; Nie, L.; Huang, J.; Tang, H. Optimized High-Performance Liquid Chromatography Method for Determining Nine Cytokinins, Indole-3-acetic Acid and Abscisic Acid. Sustainability 2021, 13, 6889. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, X.; Wu, T.; Zhao, M.; Liu, H. Rapid and sensitive detection of auxins and flavonoids in plant samples by high-performance liquid chromatography coupled with tandem mass spectrometry. J. Sep. Sci. 2015, 35, 2559–2566. [Google Scholar] [CrossRef]
- Wei, C.; Zhou, H.; Chen, C.; Li, Z.; Zhou, J. On-Line Monitoring 1H-Indole-3-Acetic Acid in Plant Tissues Using Molecular Imprinting Monolayer Techniques on a Surface Plasmon Resonance Sensor. Anal. Lett. 2011, 44, 2911–2921. [Google Scholar] [CrossRef]
- Cao, J.; Wang, M.; Han, D.; Qiao, F.; Yan, H. Attapulgite/hydrophilic molecularly imprinted monolithic resin composite for the selective recognition and sensitive determination of plant growth regulators in cucumbers. Food Chem. 2019, 297, 124974–124981. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Li, Y.; Yang, Y.; Yang, D.; Yin, S.; Liu, L.; Sun, C. Simultaneous determination of seven plant growth regulators in melons and fruits by modified QuEChERS coupled with capillary electrophoresis. Food Anal. Methods 2018, 11, 2788–2798. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Sun, N.; Xu, M.H.; Lu, Y.-J.; Qiu, B.; Cheng, M.-J.; Wong, W.-L.; Chow, C.-F. Molecular Interaction Kinetics and Mechanism Study of Phytohormones and Plant Protein with Fluorescence and Synchronous Fluorescence Techniques. ChemistrySelect 2017, 2, 3993–4000. [Google Scholar] [CrossRef]
- Su, Z.; Xu, X.; Cheng, Y.; Tan, Y.; Xiao, L.; Tang, D.; Jiang, H.; Qin, X.; Wang, H. Chemical pre-reduction and electro-reduction guided preparation of a porous graphene bionanocomposite for indole-3-acetic acid detection. Nanoscale 2019, 11, 962–967. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Chakaravarthy, S.; Hernandez-Rangel, A.; Prokhorov, E.; Luna-Bárcenas, G.; Esparza, R.; Meyyappan, M. Chitosan supported silver nanowires as a platform for direct electrochemistry and highly sensitive electrochemical glucose biosensing. RSC Adv. 2016, 6, 20102–20108. [Google Scholar] [CrossRef]
- Zhou, Q.; Xie, Q.; Fu, Y.; Su, Z.; Jia, A.X.; Yao, S. Electrodeposition of Carbon Nanotubes− Chitosan− Glucose Oxidase Biosensing Composite Films Triggered by Reduction of p-Benzoquinone or H2O2. J. Phys. Chem. B 2007, 111, 11276–11284. [Google Scholar] [CrossRef]
- Wu, L.-Q.; Gadre, A.P.; Yi, H.; Kastantin, M.J.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F.; Ghodssi, R. Voltage-dependent assembly of the polysaccharide chitosan onto an electrode surface. Langmuir 2002, 18, 8620–8625. [Google Scholar] [CrossRef]
- Uranga, J.; Puertas, A.; Etxabide, A.; Dueñas, M.T.; Guerrero, P.; De La Caba, K. Citric acid-incorporated fish gelatin/chitosan composite films. Food Hydrocoll. 2019, 86, 95–103. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and Chitosan Coating Nanoparticles for the Treatment of Brain Disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, S.; Jaiswal, A.K. A review on nanomaterials and nanohybrids based bio-nanocomposites for food packaging. Food Chem. 2022, 376, 131912–131927. [Google Scholar] [CrossRef]
- Ou, Y.; Tsen, W.C.; Gong, C.; Wang, J.; Liu, H.; Zheng, G.; Qin, C.; Wen, S. Chitosan-based composite membranes containing chitosan-coated carbon nanotubes for polymer electrolyte membranes. Polym. Adv. Technol. 2018, 29, 612–622. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z. A sensitive electrochemical sensor manufactured from multi-wall carbon nanotubes-polyethylenimine nanocomposite for malachite green detection. J. Alloys Compd. 2022, 897, 163216–163226. [Google Scholar] [CrossRef]
- Alawady, A.R.; Alshahrani, A.A.; Aouak, T.A.; Alandis, N.M. Polysulfone membranes with CNTs/Chitosan biopolymer nanocomposite as selective layer for remarkable heavy metal ions rejection capacity. Chem. Eng. J. 2020, 388, 124267–124276. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Wang, C.; Hou, P.; Dong, H.; Luo, B.; Li, A. A multifunctional ratiometric electrochemical sensor for combined determination of indole-3-acetic acid and salicylic acid. RSC Adv. 2020, 10, 3115–3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Liu, X.; Gao, L.; Lu, Y.; Li, Y.; Pan, Z.; Bao, N.; Gu, H. Simultaneous electrochemical determination of indole-3-acetic acid and salicylic acid in pea roots using a multiwalled carbon nanotube modified electrode. Anal. Lett. 2015, 48, 1578–1592. [Google Scholar] [CrossRef]
- Wang, L.; Gopinath, S.C.; Anbu, P.; Rajapaksha, A.; Velusamy, P.; Pandian, K.; Arshad, M.K.; Lakshmipriya, T.; Lee, C.-G. Photovoltaic and antimicrobial potentials of electrodeposited copper nanoparticle. Biochem. Eng. J. 2019, 142, 115590–115598. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Zhao, Y. Purification and dispersibility of multi-walled carbon nanotubes in aqueous solution. Russ. J. Phys. Chem. A 2016, 90, 2619–2624. [Google Scholar] [CrossRef]
- Hu, S.; Chen, H.; Zhan, X.; Qin, X.; Kuang, Y.; Li, M.; Liang, Z.; Yang, J.; Su, Z. One-pot electrodeposition of metal organic frameworks composites accelerated by electroreduced graphene oxide and gold nanoparticles for rutin electroanalysis. J. Electroanal. Chem. 2021, 897, 115590–115598. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Zheng, M.; Hu, C.; Tan, S.; Xiao, Y.; Yang, Q.; Liu, Y. One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem. Commun. 2012, 48, 380–382. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, X.; Chen, G.; Ye, Y.; Xu, J.; Ouyang, G.; Zhu, F. Recent development in sample preparation techniques for plant hormone analysis. TrAC Trends Anal. Chem. 2019, 113, 224–233. [Google Scholar] [CrossRef]
- Shao, W.; Sun, Y.; Zangari, G. Electrodeposition of Cu-Ag Alloy Films at n-Si (001) and Polycrystalline Ru Substrates. Coatings 2021, 11, 1563. [Google Scholar] [CrossRef]
- Sooraj, M.; Nair, A.S.; Pillai, S.C.; Hinder, S.J.; Mathew, B. CuNPs decorated molecular imprinted polymer on MWCNT for the electrochemical detection of L-DOPA. Arab. J. Chem. 2020, 13, 2483–2495. [Google Scholar] [CrossRef]
- Gan, T.; Hu, C.; Chen, Z.; Hu, S. A disposable electrochemical sensor for the determination of indole-3-acetic acid based on poly(safranine T)-reduced graphene oxide nanocomposite. Talanta 2011, 85, 310–316. [Google Scholar] [CrossRef]
- Shang, W.; Shi, X.; Zhang, X.; Ma, C.; Wang, C. Growth and characterization of electro-deposited Cu2O and Cu thin films by amperometric I–T method on ITO/glass substrate. Appl. Phys. A 2007, 87, 129–135. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Laviron, E. Surface linear potential sweep voltammetry: Equation of the peaks for a reversible reaction when interactions between the adsorbed molecules are taken into account. J. Electroanal. Chem. Interfacial Electrochem. 1974, 52, 395–402. [Google Scholar] [CrossRef]
- Yardım, Y.; Erez, M.E. Electrochemical behavior and electroanalytical determination of indole-3-acetic acid phytohormone on a boron-doped diamond electrode. Electroanalysis 2011, 23, 667–673. [Google Scholar] [CrossRef]
- Zhu, Y.; Guan, X.; Ji, H. Electrochemical solid phase micro-extraction and determination of salicylic acid from blood samples by cyclic voltammetry and differential pulse voltammetry. J. Solid State Electrochem. 2009, 13, 1417–1423. [Google Scholar] [CrossRef]
- Tao, H.; Dryhurst, G. Electrochemical and peroxidase O2-mediated oxidation of indole-3-acetic acid at physiological pH. J. Electroanal. Chem. 1997, 432, 7–18. [Google Scholar]
- Cao, X.D.; Zhu, X.T.; He, S.D.; Xu, X.; Ye, Y. Electro-Oxidation and Simultaneous Determination of Indole-3-Acetic Acid and Salicylic Acid on Graphene Hydrogel Modified Electrode. Sensors 2019, 19, 5483. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Zhu, X.; He, S.; Xu, X.; Ye, Y. Gold nanoparticle-doped three-dimensional reduced graphene hydrogel modified electrodes for amperometric determination of indole-3-acetic acid and salicylic acid. Nanoscale 2019, 11, 10247–10256. [Google Scholar] [CrossRef]
- Sun, L.J.; Zhou, J.J.; Pan, J.L.; Liang, Y.-Y.; Fang, Z.-J.; Xie, Y.; Yang, H.; Gu, H.-Y.; Bao, N. Electrochemical mapping of indole-3-acetic acid and salicylic acid in whole pea seedlings under normal conditions and salinity. Sens. Actuators B-Chem. 2018, 276, 545–551. [Google Scholar] [CrossRef]
- Huo, X.L.; Zhu, C.C.; Jiang, H.; Yuan, Q.; Wang, J.-J.; Wang, J.-Y.; Pan, Z.-Q.; Chen, C.-L.; Wu, Z.-Q.; Bao, N. Rapid profiling of IAA and SA in tomato fruit during ripening using low-cost paper-based electroanalytical devices. Postharvest Biol. Technol. 2021, 180, 11635–11642. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Tang, L.; Fei, Y.; Pan, Y.; Sun, L. Paper-based electroanalytical devices for in situ determination of free 3-indoleacetic acid and salicylic acid in living Pyropia haitanensis thallus under various environmental stresses. J. Appl. Phycol. 2019, 32, 485–497. [Google Scholar] [CrossRef]
- Li, M.; Kuang, Y.; Fan, Z.; Qin, X.; Hu, S.; Liang, Z.; Liu, Q.; Zhang, W.; Wang, B.; Su, Z. Simultaneous Electrochemical Sensing of Indole-3-Acetic Acid and Salicylic Acid on Poly (L-Proline) Nanoparticles–Carbon Dots–Multiwalled Carbon Nanotubes Composite-Modified Electrode. Sensors 2022, 22, 2222. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Li, X.; Qin, A.; He, C. A comparative study on the chitosan membranes prepared from glycine hydrochloride and acetic acid. Carbohydr. Polym. 2013, 91, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Wei, J.; Huang, S.; Wang, F.; Luo, X.; Sun, C.; Chen, D.; Wei, R.; Sha, L.; Liu, Y. A high performance Pb (ii) electrochemical sensor based on spherical CuS nanoparticle anchored gC3N4. Anal. Methods 2021, 13, 5617–5627. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Liu, Z.; Loh, K.P. A Graphene oxide and copper-centered metal organic framework composite as a tri-functional catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]









| Electrodes | Rs/Ω | Rct/Ω | C/F | W/J |
|---|---|---|---|---|
| GCE | 183.2 | 137.5 | 3.881 × 10−7 | 0.000624 |
| CuNPs/GCE | 141.2 | 52.66 | 2.363 × 10−7 | 0.000230 |
| CNPs/GCE | 145.7 | 602.9 | 6.489 × 10−7 | 0.000544 |
| MWCNTs/GCE | 123.9 | 29.24 | 2.321 × 10−7 | 0.003243 |
| CNPs-MWCNTs/GCE | 124.4 | 31.59 | 1.268 × 10−7 | 0.003287 |
| CuNPs-CSF-CNPs-MWCNTs/GCE | 126.6 | 28.86 | 1.766 × 10−7 | 0.001516 |
| Electrodes * | Detection Method | Detection Substance | Linear Range/μM | Detection Limit/μM | Ref. |
|---|---|---|---|---|---|
| GH/GCE | LSV | IAA SA | 0.6–10, 4–200 0.6–10, 4–200 | 1.42 2.8 | [38] |
| CB-MWCNT-Nafion/Fc/GCE | DPV | IAA SA | 25–1000 25–1000 | 1.99 3.3 | [22] |
| MWCNTs-CS/GCE | DPV | IAA SA | 0.67–48.82 0.67–48.82 | 0.1 0.1 | [23] |
| CMC-MMT-SWCNT/GCE | LSV | IAA SA | 0.005–0.3, 0.3–70 0.01–300 | 0.002 0.0063 | [1] |
| AuNPs-GH/GCE | i-t | IAA SA | 0.8–4, 4–128 0.8–8.4, 8.4–188 | 0.21 0.22 | [39] |
| CT | DPV | IAA SA | 1–100 1–100 | 0.1 0.1 | [40] |
| CCC/ITO | DPV | IAA SA | 10–100 10–100 | 3 2 | [41] |
| PADs | DPV | IAA SA | 1–60 1–60 | 0.1 0.1 | [42] |
| PPRONPs-CDs-MWCNTs/GCE | LSV | IAA SA | 0.05–25 0.2–40 | 0.007 0.1 | [43] |
| CuNPs-CSF-CNPs-MWCNTs/GCE | LSV | IAA SA | 0.01–50 4–30 | 0.0086 0.7 | This work |
| Sample | Analyte | Join (μM) | Detection (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Rape leaves | IAA SA | 10 5 | 9.20 5.45 | 92 109 | 2.43 2.98 |
| Tea leaves | IAA SA | 10 10 | 9.11 9.53 | 91.1 95.3 | 2.03 1.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, Y.; Li, M.; Hu, S.; Yang, L.; Liang, Z.; Wang, J.; Jiang, H.; Zhou, X.; Su, Z. One-Step Co-Electrodeposition of Copper Nanoparticles-Chitosan Film-Carbon Nanoparticles-Multiwalled Carbon Nanotubes Composite for Electroanalysis of Indole-3-Acetic Acid and Salicylic Acid. Sensors 2022, 22, 4476. https://doi.org/10.3390/s22124476
Kuang Y, Li M, Hu S, Yang L, Liang Z, Wang J, Jiang H, Zhou X, Su Z. One-Step Co-Electrodeposition of Copper Nanoparticles-Chitosan Film-Carbon Nanoparticles-Multiwalled Carbon Nanotubes Composite for Electroanalysis of Indole-3-Acetic Acid and Salicylic Acid. Sensors. 2022; 22(12):4476. https://doi.org/10.3390/s22124476
Chicago/Turabian StyleKuang, Yiwen, Mengxue Li, Shiyu Hu, Lu Yang, Zhanning Liang, Jiaqi Wang, Hongmei Jiang, Xiaoyun Zhou, and Zhaohong Su. 2022. "One-Step Co-Electrodeposition of Copper Nanoparticles-Chitosan Film-Carbon Nanoparticles-Multiwalled Carbon Nanotubes Composite for Electroanalysis of Indole-3-Acetic Acid and Salicylic Acid" Sensors 22, no. 12: 4476. https://doi.org/10.3390/s22124476
APA StyleKuang, Y., Li, M., Hu, S., Yang, L., Liang, Z., Wang, J., Jiang, H., Zhou, X., & Su, Z. (2022). One-Step Co-Electrodeposition of Copper Nanoparticles-Chitosan Film-Carbon Nanoparticles-Multiwalled Carbon Nanotubes Composite for Electroanalysis of Indole-3-Acetic Acid and Salicylic Acid. Sensors, 22(12), 4476. https://doi.org/10.3390/s22124476







