A Machine Learning Model for Predicting Sit-to-Stand Trajectories of People with and without Stroke: Towards Adaptive Robotic Assistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
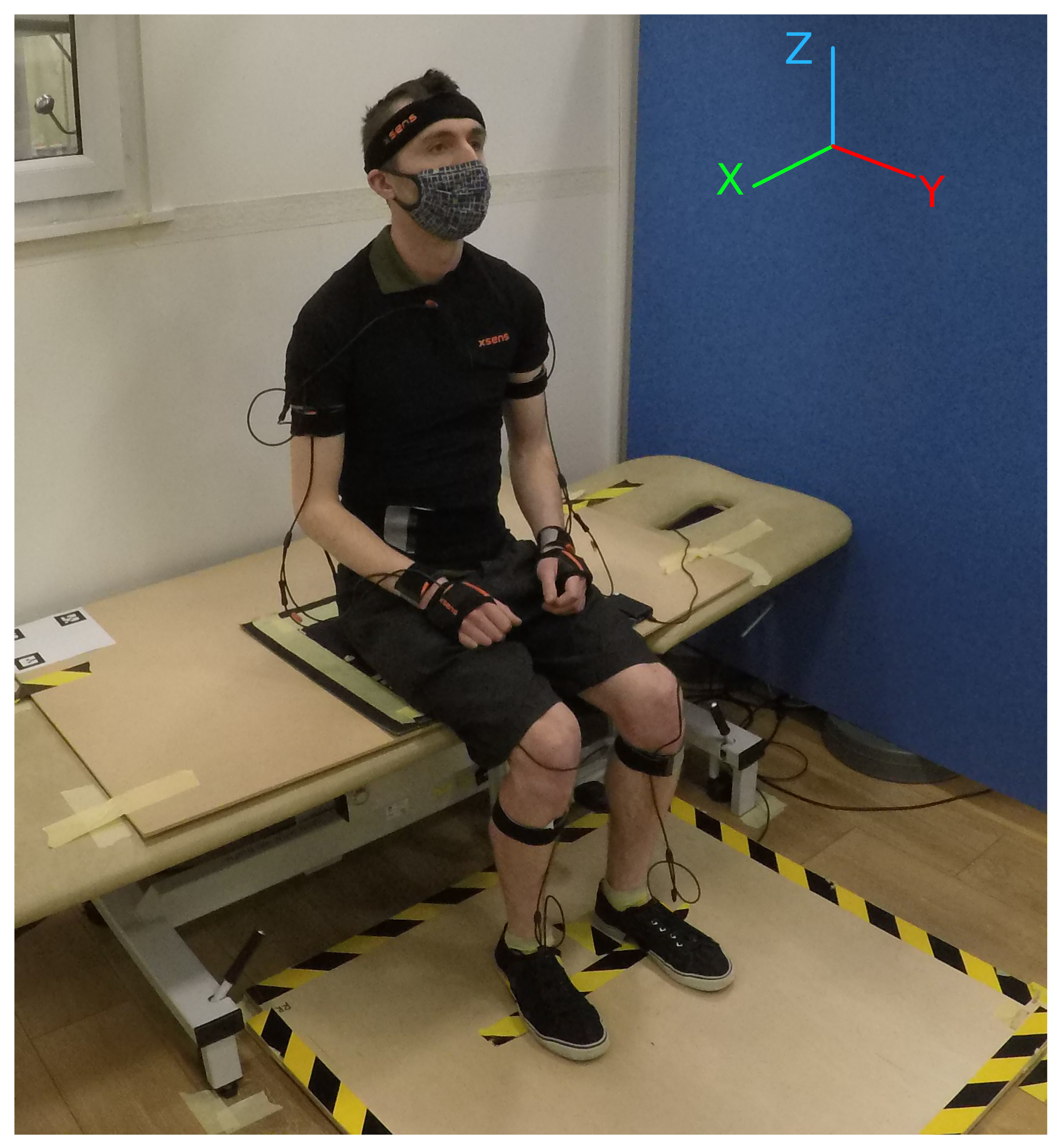
2.1.1. Experimental Setup
2.1.2. Experimental Protocol
2.2. STSTS Trajectory Prediction
3. Results
3.1. STSTS Dataset Analysis
3.1.1. Balance Board
3.1.2. Seating Mat
3.2. STSTS Trajectory Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| STS | Sit to stand |
| STSTS | Sit to stand to sit |
| Centre of pressure | |
| k-nearest neighbours | |
| LR | Linear regression |
| IMU | Inertial measurement unit |
| BMI | Body mass index |
| Coefficient of determination | |
| z position of the participant’s right shoulder | |
| z position of the participant’s left shoulder | |
| Midshoulder z coordinate | |
| True z position of the participant’s midshoulder at time 0 | |
| Predicted z position of the participant’s midshoulder created from algorithm | |
| Initial predicted trajectory created from algorithm | |
| Predicted trajectory with start position adjusted by participant’s true start position | |
| Final predicted trajectory with end point adjusted through LR | |
| Predicted end-point trajectory from algorithm | |
| Predicted end-point of midshoulder trajectory from LR | |
| position of the front left balance board sensor | |
| position of the front right balance board sensor | |
| position of the rear left balance board sensor | |
| position of the rear right balance board sensor | |
| Pressure reading on the front left balance board sensor | |
| Pressure reading on the front right balance board sensor | |
| Pressure reading on the rear left balance board sensor | |
| Pressure reading on the rear right balance board sensor |
References
- Chemerinski, E.; Robinson, R.G. The neuropsychiatry of stroke. Psychosomatics 2000, 41, 5–14. [Google Scholar] [CrossRef]
- King, D.; Wittenberg, R.; Patel, A.; Quayyum, Z.; Berdunov, V.; Knapp, M. The future incidence, prevalence and costs of stroke in the UK. Age Ageing 2020, 49, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Santos, C.; Frizera, A.; Ceres, R. A review of the functionalities of smart walkers. Med. Eng. Phys. 2015, 37, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Shomin, M.; Forlizzi, J.; Hollis, R. Sit-to-stand assistance with a balancing mobile robot. In Proceedings of the 2015 IEEE International Conference on Robotics and Automation (ICRA), Seattle, WA, USA, 26–30 May 2015; pp. 3795–3800. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged: The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Dall, P.M.; Kerr, A. Frequency of the sit to stand task: An observational study of free-living adults. Appl. Ergon. 2010, 41, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Nyberg, L.; Gustafson, Y. Patient falls in stroke rehabilitation: A challenge to rehabilitation strategies. Stroke 1995, 26, 838–842. [Google Scholar] [CrossRef]
- Janssen, W.G.; Bussmann, H.B.; Stam, H.J. Determinants of the sit-to-stand movement: A review. Phys. Ther. 2002, 82, 866–879. [Google Scholar] [CrossRef]
- Batchelor, F.A.; Mackintosh, S.F.; Said, C.M.; Hill, K.D. Falls after stroke. Int. J. Stroke 2012, 7, 482–490. [Google Scholar] [CrossRef]
- Noble, N.L.; Sweeney, N.L. Barriers to the Use of Assistive Devices in Patient Handling. Workplace Health Saf. 2018, 66, 41–48. [Google Scholar] [CrossRef]
- Kamali, K.; Akbari, A.A.; Akbarzadeh, A. Trajectory generation and control of a knee exoskeleton based on dynamic movement primitives for sit-to-stand assistance. Adv. Robot. 2016, 30, 846–860. [Google Scholar] [CrossRef]
- Shepherd, M.K.; Rouse, E.J. Design and validation of a torque-controllable knee exoskeleton for sit-to-stand assistance. IEEE/ASME Trans. Mechatron. 2017, 22, 1695–1704. [Google Scholar] [CrossRef]
- Squeri, V.; Basteris, A.; Sanguineti, V. Adaptive regulation of assistance ‘as needed’ in robot-assisted motor skill learning and neuro-rehabilitation. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011; pp. 1–6. [Google Scholar] [CrossRef]
- Chaparro-Rico, B.D.M.; Cafolla, D.; Ceccarelli, M.; Castillo-Castaneda, E. Experimental characterization of NURSE, a device for arm motion guidance. J. Healthc. Eng. 2018, 2018, 303282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaparro-Rico, B.D.; Cafolla, D. Test-retest, inter-rater and intra-rater reliability for spatiotemporal gait parameters using SANE (an eaSy gAit aNalysis systEm) as measuring instrument. Appl. Sci. 2020, 10, 5781. [Google Scholar] [CrossRef]
- Pfister, A.; West, A.M.; Bronner, S.; Noah, J.A. Comparative abilities of Microsoft Kinect and Vicon 3D motion capture for gait analysis. J. Med. Eng. Technol. 2014, 38, 274–280. [Google Scholar] [CrossRef]
- Obdržálek, Š.; Kurillo, G.; Ofli, F.; Bajcsy, R.; Seto, E.; Jimison, H.; Pavel, M. Accuracy and robustness of Kinect pose estimation in the context of coaching of elderly population. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 1188–1193. [Google Scholar]
- Arborelius, U.P.; Wretenberg, P.; Lindberg, F. The effects of armrests and high seat heights on lower-limb joint load and muscular activity during sitting and rising. Ergonomics 1992, 35, 1377–1391. [Google Scholar] [CrossRef]
- Munro, B.J.; Steele, J.R.; Bashford, G.M.; Ryan, M.; Britten, N. A kinematic and kinetic analysis of the sit-to-stand transfer using an ejector chair: Implications for elderly rheumatoid arthritic patients. J. Biomech. 1997, 31, 263–271. [Google Scholar] [CrossRef]
- Ellis, M.; Seedhom, B.; Wright, V. Forces in the knee joint whilst rising from a seated position. J. Biomed. Eng. 1984, 6, 113–120. [Google Scholar] [CrossRef]
- Hurley, S.T.; Rutherford, D.J.; Hubley-Kozey, C. The effect of age and seat height on sit-to-stand transfer biomechanics and muscle activation. Phys. Occup. Ther. Geriatr. 2016, 34, 169–185. [Google Scholar] [CrossRef]
- Cheng, P.T.; Wu, S.H.; Liaw, M.Y.; Wong, A.M.; Tang, F.T. Symmetrical body-weight distribution training in stroke patients and its effect on fall prevention. Arch. Phys. Med. Rehabil. 2001, 82, 1650–1654. [Google Scholar] [CrossRef]
- Marques, D.L.; Neiva, H.P.; Pires, I.M.; Marinho, D.A.; Marques, M.C. Accelerometer data from the performance of sit-to-stand test by elderly people. Data Brief 2020, 33, 106328. [Google Scholar] [CrossRef]
- Yamako, G.; Chosa, E.; Totoribe, K.; Fukao, Y.; Deng, G. Quantification of the sit-to-stand movement for monitoring age-related motor deterioration using the Nintendo Wii Balance Board. PLoS ONE 2017, 12, e0188165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, P.D.; Danforth, S.M.; Fu, X.Y.; Moore, T.Y.; Vasudevan, R. Characterizing the limits of human stability during motion: Perturbative experiment validates a model-based approach for the Sit-to-Stand task. R. Soc. Open Sci. 2020, 7, 191410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolaghassi, R.; Al-Hares, M.K.; Sirlantzis, K. Systematic Review of Intelligent Algorithms in Gait Analysis and Prediction for Lower Limb Robotic Systems. IEEE Access 2021, 9, 113788–113812. [Google Scholar] [CrossRef]
- Chen, H.B.; Wei, T.S.; Chang, L.W. Postural influence on Stand-to-Sit leg load sharing strategies and sitting impact forces in stroke patients. Gait Posture 2010, 32, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Kuželički, J.; Žefran, M.; Burger, H.; Bajd, T. Synthesis of standing-up trajectories using dynamic optimization. Gait Posture 2005, 21, 1–11. [Google Scholar] [CrossRef]
- Geravand, M.; Korondi, P.Z.; Werner, C.; Hauer, K.; Peer, A. Human sit-to-stand transfer modeling towards intuitive and biologically-inspired robot assistance. Auton. Robots 2017, 41, 575–592. [Google Scholar] [CrossRef] [Green Version]
- Dudani, S.A. The distance-weighted k-nearest-neighbor rule. IEEE Trans. Syst. Man Cybern. 1976, 4, 325–327. [Google Scholar] [CrossRef]
- Dean, C.M.; Richards, C.L.; Malouin, F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: A randomized, controlled pilot trial. Arch. Phys. Med. Rehabil. 2000, 81, 409–417. [Google Scholar] [CrossRef]
- Dean, C.M.; Channon, E.F.; Hall, J.M. Sitting training early after stroke improves sitting ability and quality and carries over to standing up but not to walking: A randomised controlled trial. Aust. J. Physiother. 2007, 53, 97–102. [Google Scholar] [CrossRef]
- Galli, M.; Cimolin, V.; Crivellini, M.; Campanini, I. Quantitative analysis of sit to stand movement: Experimental set-up definition and application to healthy and hemiplegic adults. Gait Posture 2008, 28, 80–85. [Google Scholar] [CrossRef]
- Schenkman, M.; Riley, P.; Pieper, C. Sit to stand from progressively lower seat heights—Alterations in angular velocity. Clin. Biomech. 1996, 11, 153–158. [Google Scholar] [CrossRef]
- Su, F.C.; Lai, K.; Hong, W. Rising from chair after total knee arthroplasty. Clin. Biomech. 1998, 13, 176–181. [Google Scholar] [CrossRef]
- Van Der Kruk, E.; Silverman, A.K.; Reilly, P.; Bull, A.M. Compensation due to age-related decline in sit-to-stand and sit-to-walk. J. Biomech. 2021, 122, 110411. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maroger, I.; Ramuzat, N.; Stasse, O.; Watier, B. Human Trajectory Prediction Model and its Coupling with a Walking Pattern Generator of a Humanoid Robot. IEEE Robot. Autom. Lett. 2021, 6, 6361–6369. [Google Scholar] [CrossRef]
- Gill, N.; Preece, S.J.; Young, S.; Bramah, C. Are the arms and head required to accurately estimate centre of mass motion during running? Gait Posture 2017, 51, 281–283. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Jiang, Z.; Guo, H.; Ni, X. Fall detection based on key points of human-skeleton using openpose. Symmetry 2020, 12, 744. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Introduction to Linear Regression Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Nagelkerke, N.J. A note on a general definition of the coefficient of determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- Bennett, T.; Ruiz Garate, V. STS Dataset. 2022. Available online: https://github.com/Assistive-Robotics-Lab/STS_Dataset (accessed on 26 May 2022).
- Kramer, O. K-nearest neighbors. In Dimensionality Reduction with Unsupervised Nearest Neighbors; Springer: New York, NY, USA, 2013; pp. 13–23. [Google Scholar]
- Komaris, D.S.; Govind, C.; Murphy, A.; Ewen, A.; Riches, P. Identification of movement strategies during the sit-to-walk movement in patients with knee osteoarthritis. J. Appl. Biomech. 2018, 34, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Cheng, P.T.; Liaw, M.Y.; Wong, M.K.; Tang, F.T.; Lee, M.Y.; Lin, P.S. The sit-to-stand movement in stroke patients and its correlation with falling. Arch. Phys. Med. Rehabil. 1998, 79, 1043–1046. [Google Scholar] [CrossRef]
- Engardt, M.; Olsson, E. Body weight-bearing while rising and sitting down in patients with stroke. Scand. J. Rehabil. Med. 1992, 24, 67–74. [Google Scholar] [PubMed]
- Dolecka, U.E.; Ownsworth, T.; Kuys, S.S. Comparison of sit-to-stand strategies used by older adults and people living with dementia. Arch. Gerontol. Geriatr. 2015, 60, 528–534. [Google Scholar] [CrossRef] [PubMed]







| Non-Stroke, n = 24 | Stroke, n = 6 | |
|---|---|---|
| Gender Split (M/F) | 14/10 | 3/3 |
| Age (years) | 37.2 (±12.0) | 66.5 (±10.7) |
| Height (cm) | 175 (±8) | 170 (±5.3) |
| Weight (kg) | 74.7 (±14.9) | 87.0 (±18.0) |
| 100% Seat Height | 115% Seat Height | |||
|---|---|---|---|---|
| k | Sit-to-Stand | Stand-to-Sit | Sit-to-Stand | Stand-to-Sit |
| 2 | 0.854 ± 0.138 | 0.666 ± 0.448 | 0.719 ± 0.357 | 0.639 ± 0.397 |
| 3 | 0.864 ± 0.134 | 0.653 ± 0.376 | 0.762 ± 0.323 | 0.579 ± 0.443 |
| 4 | 0.832 ± 0.186 | 0.516 ± 0.570 | 0.784 ± 0.281 | 0.441 ± 0.644 |
| 5 | 0.830 ± 0.215 | 0.617 ± 0.453 | 0.799 ± 0.247 | 0.552 ± 0.543 |
| 100% Seat Height | 115% Seat Height | |||
|---|---|---|---|---|
| k | Sit-to-Stand | Stand-to-Sit | Sit-to-Stand | Stand-to-Sit |
| 2 | 0.861 ± 0.152 | 0.645 ± 0.316 | 0.755 ± 0.324 | 0.598 ± 0.356 |
| 3 | 0.854 ± 0.151 | 0.723 ± 0.261 | 0.754 ± 0.324 | 0.676 ± 0.314 |
| 4 | 0.833 ± 0.186 | 0.703 ± 0.294 | 0.759 ± 0.284 | 0.614 ± 0.358 |
| 5 | 0.852 ± 0.196 | 0.733 ± 0.266 | 0.787 ± 0.273 | 0.635 ± 0.332 |
| Participant | Sit-to-Stand, 100% | Stand-to-Sit, 100% | Sit-to-Stand, 115% | Stand-to-Sit, 115% |
|---|---|---|---|---|
| S1 | 0.112 | 0.372 | 0.495 | 0.287 |
| S2 | 0.929 | 0.474 | 0.894 | −0.007 |
| S3 | 0.989 | 0.966 | 0.991 | 0.967 |
| S4 | 0.843 | 0.387 | 0.674 | 0.759 |
| S5 | 0.708 | −0.509 | 0.365 | −1.82 |
| S6 | 0.823 | 0.966 | 0.917 | 0.9 |
| Average |
| Participant | Sit-to-Stand, 100% | Stand-to-Sit, 100% | Sit-to-Stand, 115% | Stand-to-Sit, 115% |
|---|---|---|---|---|
| S1 | 0.282 | 0.281 | 0.752 | 0.355 |
| S2 | 0.952 | 0.482 | 0.920 | 0.488 |
| S3 | 0.996 | 0.819 | 0.986 | 0.832 |
| S4 | 0.929 | 0.584 | 0.862 | 0.825 |
| S5 | 0.845 | 0.137 | 0.638 | −1.065 |
| S6 | 0.893 | 0.972 | 0.963 | 0.932 |
| Average |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennett, T.; Kumar, P.; Garate, V.R. A Machine Learning Model for Predicting Sit-to-Stand Trajectories of People with and without Stroke: Towards Adaptive Robotic Assistance. Sensors 2022, 22, 4789. https://doi.org/10.3390/s22134789
Bennett T, Kumar P, Garate VR. A Machine Learning Model for Predicting Sit-to-Stand Trajectories of People with and without Stroke: Towards Adaptive Robotic Assistance. Sensors. 2022; 22(13):4789. https://doi.org/10.3390/s22134789
Chicago/Turabian StyleBennett, Thomas, Praveen Kumar, and Virginia Ruiz Garate. 2022. "A Machine Learning Model for Predicting Sit-to-Stand Trajectories of People with and without Stroke: Towards Adaptive Robotic Assistance" Sensors 22, no. 13: 4789. https://doi.org/10.3390/s22134789
APA StyleBennett, T., Kumar, P., & Garate, V. R. (2022). A Machine Learning Model for Predicting Sit-to-Stand Trajectories of People with and without Stroke: Towards Adaptive Robotic Assistance. Sensors, 22(13), 4789. https://doi.org/10.3390/s22134789






