Noninvasive Blood Glucose Monitoring Systems Using Near-Infrared Technology—A Review
Abstract
:1. Introduction
2. Noninvasive Glucose Sensing Methods
2.1. Infrared (IR) Spectroscopy
2.1.1. Mid-Infrared (MIR) Spectroscopy
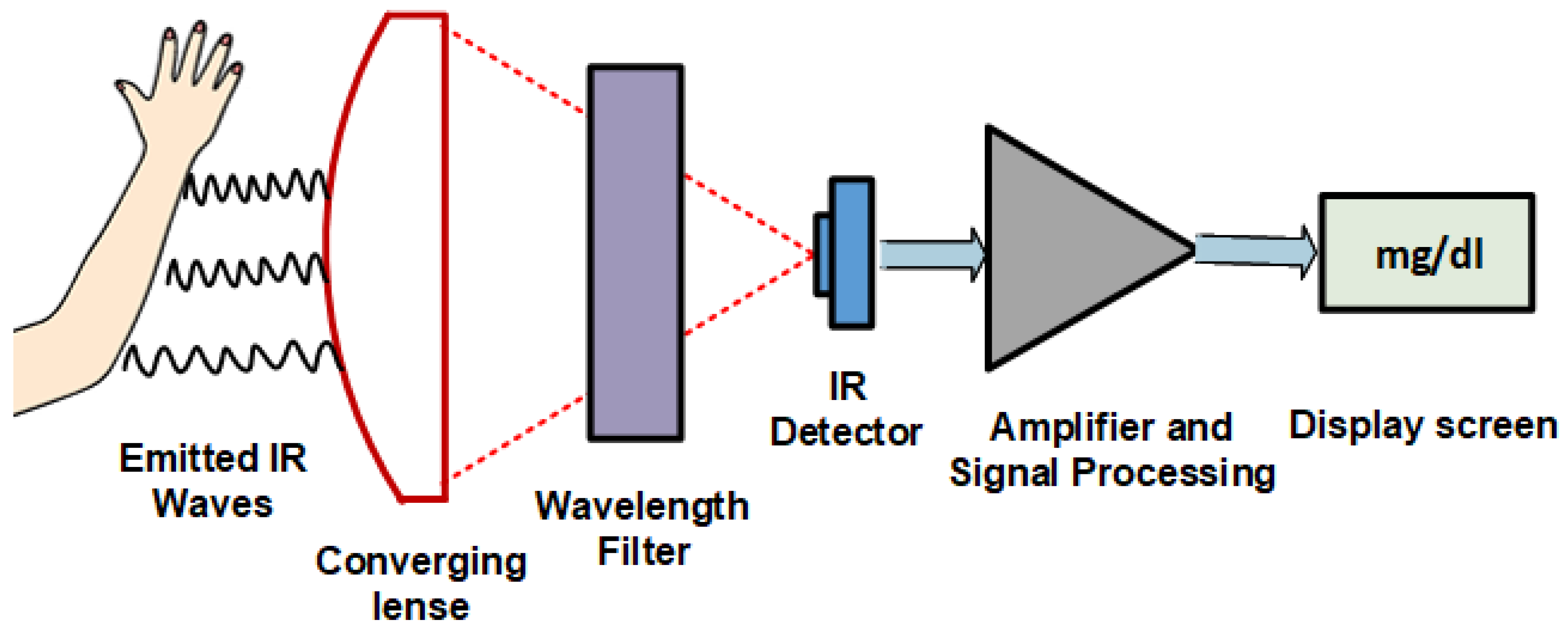
2.1.2. Near Infrared (NIR) Spectroscopy
2.2. Raman Spectroscopy
2.3. Thermal Emission Spectroscopy (TES)
2.4. Microwave Spectroscopy (MWS)
2.5. Metabolic Heat Conformation (MHC)
2.6. Photoacoustic Spectroscopy (PAS)
2.7. Occlusion Spectroscopy (OP)
2.8. Optical Polarimetry (OP)
2.9. Optical Coherence Tomography (OCT)
2.10. Bio-Impedance Spectroscopy
2.11. Electromagnetic Sensing
2.12. Noninvasive Enzymatic Technology
3. NIR Spectrometry
4. Blood Glucose Prediction Using NIR Techniques
4.1. NIR Spectrometry Analysis
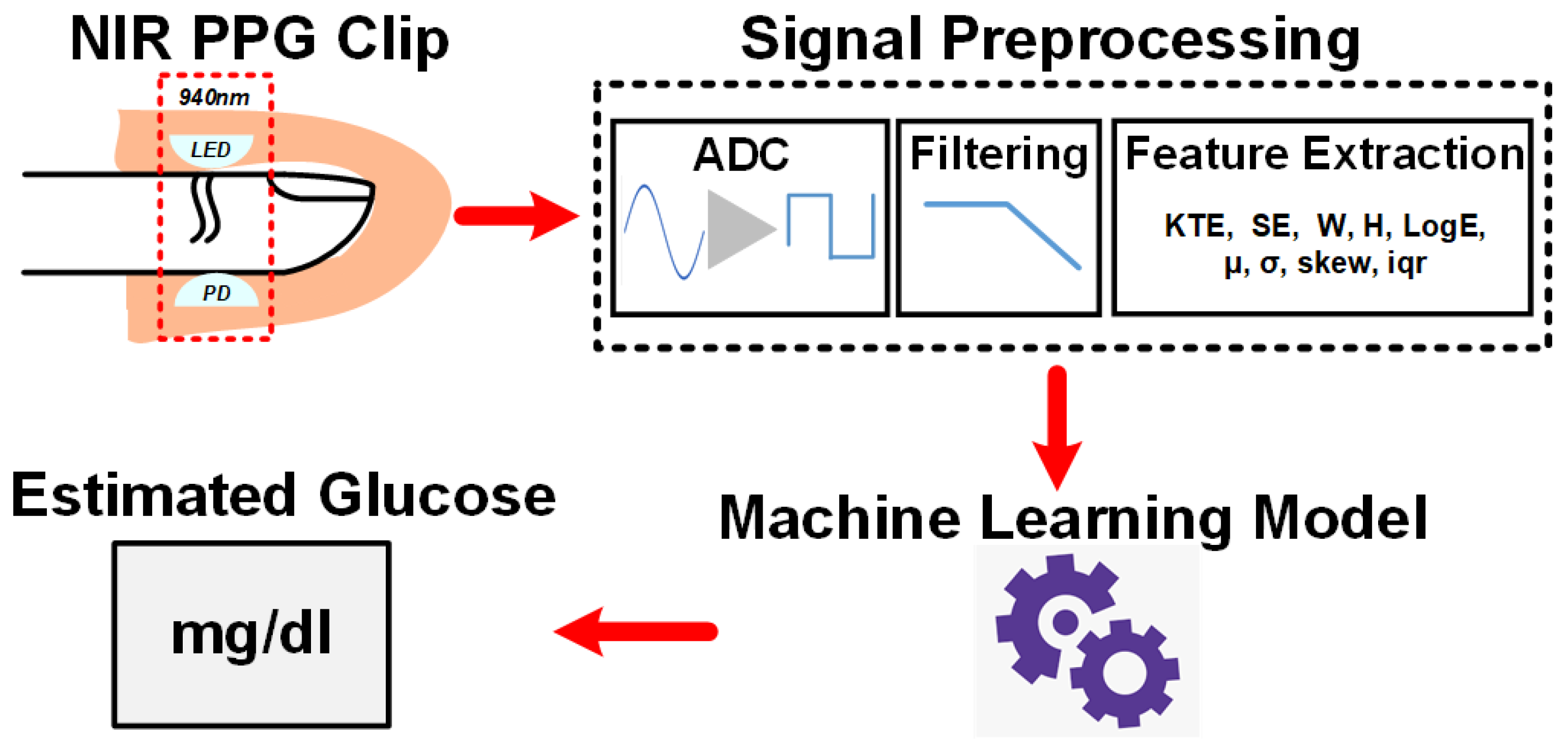
4.2. NIR PPG Signal Analysis with Machine Learning
4.3. Mathematical Details of PPG Features
- (1)
- Kaiser Teager Energy feature (KTE)
- (2)
- Logarithmic Energy feature (LogE)
- (3)
- Autoregressive model of PPG signal
- (4)
- Spectral entropy feature (SE)
- (5)
- Peak-to-peak interval (PPI)
- (6)
- Detrended Fluctuation Analysis (DFA)
- (7)
- Power Spectral Density (PSD)
- (8)
- Wavelet entropy (WE)
4.4. BGL Estimation Analysis and Comparision Table
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Noncommunicable Diseases. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 26 April 2022).
- IDF Diabetes Atlas. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 26 April 2022).
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2007, 30, S42–S47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Facchinetti, A. Continuous Glucose Monitoring Sensors for Diabetes Management: A Review of Technologies and Applications. Diabetes Metab. J. 2019, 43, 383–397. [Google Scholar] [CrossRef]
- Zisser, H.C.; Bailey, T.S.; Schwartz, S.; Ratner, R.E.; Wise, J. Accuracy of the SEVEN continuous glucose monitoring system: Comparison with frequently sampled venous glucose measurements. J. Diabetes Sci. Technol. 2009, 3, 1146–1154. [Google Scholar] [CrossRef]
- Weinstein, R.L.; Schwartz, S.L.; Brazg, R.L.; Bugler, J.R.; Peyser, T.A.; McGarraugh, G.V. Accuracy of the 5-day FreeStyle Navigator Continuous Glucose Monitoring System: Comparison with frequent laboratory reference measurements. Diabetes Care. 2007, 30, 1125–1130. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhao, X.-L.; Li, Z.-H.; Zhu, Z.-G.; Qian, S.-H.; Flewitt, A.J. Current and Emerging Technology for Continuous Glucose Monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef] [Green Version]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [Green Version]
- Facchinetti, A. Continuous Glucose Monitoring Sensors: Past, Present and Future Algorithmic Challenges. Sensors 2016, 16, 2093. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Bolla, A.S.; Priefer, R. Blood glucose monitoring- an overview of current and future non-invasive devices. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 739–751. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, S.; Jin, H.; Luo, Y.; Zheng, Z.; Gao, F.; Zheng, Y. Noninvasive Electromagnetic Wave Sensing of Glucose. Sensors 2019, 19, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Z.; Tang, F.; Ding, Y.; Li, S.; Wang, X. Non-invasive glucose continuous glucose monitoring using a multisensor based glucometer and time series analysis. Sci. Rep. 2017, 7, 12650. [Google Scholar] [CrossRef] [Green Version]
- Vrančić, C.; Fomichova, A.; Gretz, N.; Herrmann, C.; Neudecker, S.; Pucci, A.; Petrich, W. Continuous glucose monitoring by means of mid-infrared transmission laser spectroscopy in vitro. Analyst 2011, 136, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, R.; Kino, S.; Soyama, S.; Matsuura, Y. Noninvasive glucose monitoring using mid-infrared absorption spectroscopy based on a few wavenumbers. Biomed. Opt. Express 2018, 9, 289–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cozzolino, D. The Ability of Near Infrared (NIR) Spectroscopy to Predict Functional Properties in Foods: Challenges and Opportunities. Molecules 2021, 26, 6981. [Google Scholar] [CrossRef]
- Burns, D.A.; Ciurczak, E.W. Handbook of Near-Infrared Analysis: Practical Spectroscopy, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 349–369. [Google Scholar]
- Sakudo, A. Near-infrared spectroscopy for medical applications: Current status and future perspectives. Clin. Chim. Acta 2016, 455, 181–188. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar] [CrossRef]
- Cardoso, W.J.; Gomes, J.G.; Roque, J.V.; Barbosa, M.H.; Teófilo, R.F. Dehydration as a Tool to improve predictability of sugarcane juice carbohydrates using near-infrared spectroscopy based PLS models. Chemom. Intell. Lab. Syst. 2022, 220, 104459. [Google Scholar] [CrossRef]
- Rambla, F.J.; Garrigues, S.; de la Guardia, M. PLS-NIR determination of total sugar, glucose, fructose and sucrose in aqueous solutions of fruit juices. Anal. Chim. Acta 1997, 344, 41–53. [Google Scholar] [CrossRef]
- Simeone, M.L.F.; Parrella, R.A.; Schaffert, R.E.; Damasceno, C.M.; Leal, M.C.; Pasquini, C. Near infrared spectroscopy determination of sucrose, glucose and fructose in sweet sorghum juice. Microchem. J. 2017, 134, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Jintao, X.; Liming, Y.; Yufei, L.; Chunyan, L.; Han, C. Noninvasive and fast measurement of blood glucose in vivo by near infrared (NIR) spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liao, N.; Cheng, H.; Li, Y.; Bai, X.; Deng, C. Determination of NIR informative wavebands for transmission non-invasive blood glucose measurement using a Fourier transform spectrometer. AIP Adv. 2018, 8, 035216. [Google Scholar] [CrossRef]
- Yadav, J.; Rani, A.; Sing, V.; Murari, M.B. Near infrared LED based noninvasive blood glucose sensor. In Proceedings of the 2014 IEEE International Conference on Signal Processing and Integrated Networks (SPIN), Noida, India, 20–21 February 2014; pp. 591–594. [Google Scholar]
- Buda, R.; Addi, M.M. A portable non-invasive blood glucose monitoring device. In Proceedings of the 2014 IEEE Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 8–10 December 2014; pp. 964–969. [Google Scholar] [CrossRef]
- Hotmartua, R.; Pangestu, P.W.; Zakaria, H.; Irawan, Y.S. Non-invasive blood glucose detection using near-infrared sensor. In Proceedings of the 2014 IEEE Internation Conference on Electrical Engineering and Informatics, Denpasar, Indonesia, 10–11 August 2015; pp. 687–692. [Google Scholar]
- Haxha, S.; Jhoja, J. Optical Based Noninvasive Glucose Monitoring Sensor Prototype. IEEE Photonics J. 2016, 8, 6805911. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Kim, M.S.; Kim, O.-K.; Baik, H.-H.; Kim, J.-H. Near-Infrared Light Emitting Diode Based Non-Invasive Glucose Detection System. J. Nanosci. Nanotechnol. 2019, 19, 6187–6191. [Google Scholar] [CrossRef]
- Jain, P.; Maddila, R.; Joshi, A.M. A precise non-invasive blood glucose measurement system using NIR spectroscopy and Huber’s regression model. Opt. Quantum Electron. 2019, 51, 51. [Google Scholar] [CrossRef]
- Dai, J.; Ji, Z.; Du, Y.; Chen, S. In vivo noninvasive blood glucose detection using near-infrared spectrum based on the PSO-2ANN model. Technol. Health Care 2018, 26, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, A.M.; Jain, P.; Mohanty, S.P.; Agrawal, N. iGLU 2.0: A New Wearable for Accurate Non-Invasive Continuous Serum Glucose Measurement in IoMT Framework. IEEE Trans. Consum. Electron. 2020, 66, 327–335. [Google Scholar] [CrossRef]
- Elgendi, M. On the Analysis of Fingertip Photoplethysmogram Signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef]
- Saadeh, W.; Aslam, S.Z.; Hina, A.; Asghar, F. A 0.5V PPG-based heart rate and variability detection system. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Irace, C.; Carallo, C.; Scavelli, F.; De Franceschi, M.S.; Esposito, T.; Gnasso, A. Blood Viscosity in Subjects with Normoglycemia and Prediabetes. Diabetes Care 2014, 37, 488–492. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Budidha, K.; Kyriacou, P.A. Investigating the origin of photoplethysmography using a multiwavelength Monte Carlo model. Physiol. Meas. 2020, 41, 084001. [Google Scholar] [CrossRef]
- Monte-Moreno, E. Non-invasive estimate of blood glucose and blood pressure from a photoplethysmograph by means of machine learning techniques. Artif. Intell. Med. 2011, 53, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Rachim, V.P.; Chung, W.-Y. Wearable-band type visible-near infrared optical biosensor for non-invasive blood glucose monitoring. Sens. Actuators B Chem. 2019, 286, 173–180. [Google Scholar] [CrossRef]
- Ramasahayam, S.; Koppuravuri, S.H.; Arora, L.; Chowdhury, S.R. Noninvasive Blood Glucose Sensing Using Near Infra-Red Spectroscopy and Artificial Neural Networks Based on Inverse Delayed Function Model of Neuron. J. Med. Syst. 2015, 39, 166. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Yang, W.-T.; Lu, W.-R.; Chang, Y.-T.; Hsieh, T.-H.; Yang, F.-L. 90% Accuracy for Photoplethysmography-Based Non-Invasive Blood Glucose Prediction by Deep Learning with Cohort Arrangement and Quarterly Measured HbA1c. Sensors 2021, 21, 7815. [Google Scholar] [CrossRef]
- Habbu, S.; Dale, M.; Ghongade, R. Estimation of blood glucose by non-invasive method using photoplethysmography. Sadhana 2019, 44, 135. [Google Scholar] [CrossRef] [Green Version]
- Yadav, J.; Rani, A.; Singh, V.; Murari, B.M. Investigations on Multisensor-Based Noninvasive Blood Glucose Measurement System. J. Med. Devices 2017, 11, 031006. [Google Scholar] [CrossRef]
- Hina, A.; Saadeh, W. A Noninvasive Glucose Monitoring SoC Based on Single Wavelength Photoplethysmography. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 504–515. [Google Scholar] [CrossRef]
- Hina, A.; Nadeem, H.; Saadeh, W. A Single LED Photoplethysmography-based noninvasive glucose monitoring prototype system. In Proceedings of the 2019 IEEE International Symposium on Circuits and Systems (ISCAS), Sapporo, Japan, 26–29 May 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Hina, A.; Saadeh, W. A 186uW glucose monitoring SoC using near-infrared photoplethyysmography. In Proceedings of the IEEE Asian Solid-State Circuits Conference (ASSCC), Hiroshima, Japan, 9–11 November 2020; pp. 1–4. [Google Scholar]
- Hina, A.S.; Minto, S.; Saadeh, W. A 208 μW PPG-based glucose monitoring SoC using ensembled boosted trees. In Proceedings of the IEEE 20th IEEE Interregional NEWCAS Conference, Québec, QC, Canada, 19–22 June 2022. [Google Scholar]
- Saleh, G.; Alkaabi, F.; Al-Hajhouj, N.; Al-Towailib, F.; Al-Hamza, S. Design of non-invasive glucose meter using near-infrared technique. J. Med. Eng. Technol. 2018, 42, 140–147. [Google Scholar] [CrossRef]
- Srichan, C.; Srichan, W.; Danvirutai, P.; Ritsongmuang, C.; Sharma, A.; Anutrakulchai, S. Non-invasively accuracy enhanced blood glucose sensor using shallow dense neural networks with NIR monitoring and medical features. Sci. Rep. 2022, 12, 1769. [Google Scholar] [CrossRef]
- Alsunaidi, B.; Althobaiti, M.; Tamal, M.; Albaker, W.; Al-Naib, I. A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring. Sensors 2021, 21, 6820. [Google Scholar] [CrossRef]
- Enejder, A.M.K.; Scecina, T.G.; Oh, J.; Hunter, M.; Shih, W.-C.; Sasic, S.; Horowitz, G.L.; Feld, M.S. Raman spectroscopy for noninvasive glucose measurements. J. Biomed. Opt. 2005, 10, 031114. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Paidi, S.; Valdez, T.A.; Zhang, C.; Spegazzini, N.; Dasari, R.R.; Barman, I. Noninvasive Monitoring of Blood Glucose with Raman Spectroscopy. Acc. Chem. Res. 2017, 50, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchert, J.M. Thermal emission spectroscopy as a tool for noninvasive blood glucose measurements. Proc. SPIE 2004, 5566, 100–111. [Google Scholar] [CrossRef]
- Choi, H.; Naylon, J.; Luzio, S.; Beutler, J.; Birchall, J.; Martin, C.; Porch, A. Design and In Vitro Interference Test of Microwave Noninvasive Blood Glucose Monitoring Sensor. IEEE Trans. Microw. Theory Tech. 2015, 63, 3016–3025. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Kim, C.-S.; Choi, B.-C.; Ham, K.-Y. The correlation of the complex dielectric constant and blood glucose at low frequency. Biosens. Bioelectron. 2003, 19, 321–324. [Google Scholar] [CrossRef]
- Hayashi, Y.; Livshits, L.; Caduff, A.; Feldman, Y. Dielectric spectroscopy study of specific glucose influence on human erythrocyte membranes. J. Phys. D Appl. Phys. 2003, 36, 369–374. [Google Scholar] [CrossRef]
- Cho, O.K.; Kim, Y.O.; Mitsumaki, H.; Kuwa, K. Noninvasive Measurement of Glucose by Metabolic Heat Conformation Method. Clin. Chem. 2004, 50, 1894–1898. [Google Scholar] [CrossRef]
- Tang, F.; Wang, X.; Wang, D.; Li, J. Non-Invasive Glucose Measurement by Use of Metabolic Heat Conformation Method. Sensors 2008, 8, 3335–3344. [Google Scholar] [CrossRef] [Green Version]
- Sim, J.Y.; Ahn, C.-G.; Jeong, E.-J.; Kim, B.K. In vivo Microscopic Photoacoustic Spectroscopy for Non-Invasive Glucose Monitoring Invulnerable to Skin Secretion Products. Sci. Rep. 2018, 8, 1059. [Google Scholar] [CrossRef] [Green Version]
- Pai, P.P.; Sanki, P.K.; De, A.; Banerjee, S. NIR photoacoustic spectroscopy for non-invasive glucose measurement. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 7978–7981. [Google Scholar] [CrossRef]
- Pleitez, M.A.; Lieblein, T.; Bauer, A.; Hertzberg, O.; von Lilienfeld-Toal, H.; Mäntele, W. In Vivo Noninvasive Monitoring of Glucose Concentration in Human Epidermis by Mid-Infrared Pulsed Photoacoustic Spectroscopy. Analyt. Chem. 2012, 85, 1013–1020. [Google Scholar] [CrossRef]
- Amir, O.; Weinstein, D.; Zilberman, S.; Less, M.; Perl-Treves, D.; Primack, H.; Weinstein, A.; Gabis, E.; Fikhte, B.; Karasik, A. Continuous Noninvasive Glucose Monitoring Technology Based on “Occlusion Spectroscopy”. J. Diabetes Sci. Technol. 2007, 1, 463–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, B.; Coté, G.L. Real-time, closed-loop dual-wavelength optical polarimetry for glucose monitoring. J. Biomed. Opt. 2010, 15, 017002. [Google Scholar] [CrossRef] [PubMed]
- Rawer, R.; Stork, W.; Kreiner, C.F. Non-invasive polarimetric measurement of glucose concentration in the anterior chamber of the eye. Graefe Arch. Clin. Exp. Ophthalmol. 2004, 242, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Larin, K.V.; Eledrisi, M.S.; Motamedi, M.; Esenaliev, R.O. Noninvasive blood glucose monitoring with optical coherence tomography: A pilot study in human subjects. Diabetes Care 2002, 25, 12, 2263–2267. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Backman, V. Imaging a full set of optical scattering properties of biological tissue by inverse spectroscopic optical coherence tomography. Opt. Lett. 2012, 37, 4443–4445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.; Ha, U.; Park, S.; Yoo, H.-J. An impedance and multi-wavelength near-infrared spectroscopy IC for non-invasive blood glucose estimation. In Proceedings of the 2014 Symposium on VLSI Circuits Digest of Technical Papers, Honolulu, HI, USA, 10–13 June 2014; pp. 1025–1037. [Google Scholar] [CrossRef]
- Ha, S.; Kim, C.; Chi, Y.M.; Akinin, A.; Maier, C.; Ueno, A.; Cauwenberghs, G. Integrated Circuits and Electrode Interfaces for Noninvasive Physiological Monitoring. IEEE Trans. Biomed. Eng. 2014, 61, 1522–1537. [Google Scholar] [CrossRef]
- Gourzi, M.; Rouane, A.; Guelaz, R.; Alavi, M.S.; McHugh, M.B.; Nadi, M.; Roth, P. Non-invasive glycaemia blood measurements by electromagnetic sensor: Study in static and dynamic blood circulation. J. Med. Eng. Technol. 2005, 29, 22–26. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Khalil, O.S. Noninvasive Photonic-Crystal Material for Sensing Glucose in Tears. Clin. Chem. 2004, 50, 2236–2237. [Google Scholar] [CrossRef]
- Liao, Y.; Yao, H.; Lingley, A.; Parviz, B.; Otis, B.P. A 3µW CMOS Glucose Sensor for Wireless Contact-Lens Tear Glucose Monitoring. IEEE J. Solid-State Circuits 2012, 47, 335–344. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.-S.; Kim, K.; Ji, S.; Kim, Y.-T.; Park, J.; Na, K.; Bae, K.-H.; Kim, H.K.; et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Arata, S.; Xu, G.; Murakami, S.; Bui, C.D.; Doike, T.; Matsunaga, M.; Kobavashi, A.; Niitsu, K. A 385μm × 385μm 0.165 V 0.27 nW Fully-integrated supply-modulated OOK CMOS TX in 65 nm CMOS for glasses-free, self-powered, and fuel-cell-embedded continuous glucose monitoring contact lens. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Soni, A.; Jha, S.K. A paper strip based non-invasive glucose biosensor for salivary analysis. Biosens. Bioelectron. 2015, 67, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Kuroki, Y.; Nitta, H.; Chouhan, P.; Toma, K.; Sawada, S.-I.; Takeuchi, S.; Sekita, T.; Akiyoshi, K.; Minakuchi, S.; et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron. 2016, 84, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, J.F. Some useful properties of Teager’s energy operators. In Proceedings of the 1993 IEEE International Conference on Acoustics, Speech, and Signal Processing, Minneapolis, MN, USA, 27–30 April 1993; pp. 149–152. [Google Scholar] [CrossRef]
- Kvedalen, E. Signal Processing Using the Teager Energy Operator and Other Nonlinear Operators. Master’s Thesis, University of Oslo, Oslo, Norway, 2003. [Google Scholar]
- Maragos, P.; Kaiser, J.; Quatieri, T. Energy separation in signal modulations with application to speech analysis. IEEE Trans. Signal Process. 1993, 41, 3024–3051. [Google Scholar] [CrossRef]
- Doane, D.P.; Seward, L.E. Measuring skewness, a forgotten statistic. J. Stat. Educ. 2011, 19, 1–18. [Google Scholar] [CrossRef]
- Li, W.; Bourlard, H. Sub-band based log-energy and its dynamic range stretching for robust in-car speech recognition. In Proceedings of the 13th Annual Conference of the International Speech Communication Association (InterSpeech), Portland, OR, USA, 9–13 September 2012; pp. 314–317. [Google Scholar] [CrossRef]
- Lee, J.; Chon, K.H. An Autoregressive Model-Based Particle Filtering Algorithms for Extraction of Respiratory Rates as High as 90 Breaths Per Minute from Pulse Oximeter. IEEE Trans. Biomed. Eng. 2010, 57, 2158–2167. [Google Scholar] [CrossRef]
- Toh, A.M.; Togneri, R.B. Spectral entropy as speech features for speech recognition. Proc. PEECS 2005, 1, 92. [Google Scholar]
- Yingthawornsuk, T. Spectral entropy in speech for classification of depressed speakers. In Proceedings of the 2016 12th International Conference on Signal-Image Technology & Internet-Based Systems (SITIS), Naples, Italy, 28 November–1 December 2016; pp. 679–682. [Google Scholar]
- Hemert, J.P.V. Automatic segmentation of speech. IEEE Trans. Signal Process. 1991, 39, 1008–1012. [Google Scholar] [CrossRef]
- Djawad, Y.A.; Mu’Nisa, A.; Rusung, P.; Kurniawan, A.; Idris, I.S.; Taiyeb, M. Essential Feature Extraction of Photoplethysmography Signal of Men and Women in Their 20 s. Eng. J. 2017, 21, 259–272. [Google Scholar] [CrossRef]
- Wu, Q. On a Feature Extraction and Classification Study for PPG Signal Analysis. J. Comput. Commun. 2021, 9, 153–160. [Google Scholar] [CrossRef]
- Clarke, W.L. The Original Clarke Grid Error Analysis. Diabetes Technol. Ther. 2005, 7, 776–779. [Google Scholar] [CrossRef]







| SVR-Fine Gaussian | SVR Quadratic | Linear Regression | En. Boosted Trees | |||||
|---|---|---|---|---|---|---|---|---|
| Combination of Features | mARD | RMSE | mARD | RMSE | mARD | RMSE | mARD | RMSE |
| ARPPG, KTEσ, KTEµ, KTEiqr, KTEskew, ARKTE, LogEσ, LogEµ, LogEiqr, ARLogE, SEσ, SEµ, SEiqr, SEskew (14 Features) | 8.36 | 11.29 | 18.27 | 25.21 | 22.57 | 33.85 | 18.27 | 25.21 |
| KTEσ, KTEµ, KTEiqr, KTEskew, LogEσ, LogEµ, LogEiqr, SEσ, SEµ, SEiqr, SEskew (11 Features) | 10.16 | 12.31 | 15.01 | 46.00 | 14.66 | 26.00 | 15.24 | 21.64 |
| KTEσ, KTEµ, KTEiqr, KTEskew, LogEσ, LogEiqr, SEσ, SEµ, SEiqr, SEskew (10 Features) | 13.66 | 21.93 | 22.09 | 44.41 | 16.19 | 29.94 | 16.18 | 23.00 |
| KTEσ, KTEµ, KTEiqr, KTEskew, LogEσ, LogEiqr, SEσ, SEµ, SEiqr, SEskew (8 Features) | 12.17 | 21.6 | 22.05 | 50 | 19.19 | 25.86 | 16.05 | 23.21 |
| KTEσ, KTEµ, LogEσ, LogEμ, SEσ, SEµ (6 Features) | 7.62 | 11.20 | 21.10 | 42.90 | 13.22 | 23.35 | 9.67 | 13.00 |
| Machine Learning Algorithm | mARD | RMSE |
|---|---|---|
| Linear Regression | 8.25 | 12.35 |
| Fine Gaussian | 7.36 | 11.20 |
| Non-Linear Medium Gaussian | 6.52 | 10.15 |
| Ensemble Boosted Trees | 5.83 | 8.65 |
| Author (Reference) | Number of Features | Machine Learning Technique | R2 |
|---|---|---|---|
| Monte-Moreno E. [38] | 33 | Random Forest | 0.88 |
| Habbu S. et al. [42] | 28 | Neural Networks | 0.91 |
| Yadav J. et al. [43] | 17 | Neural Networks | 0.96 |
| Hina A. et al. [45] | 6 | Fine Gaussian SVR | 0.937 |
| Hina A. et al. [47] | 6 | Ensemble Boosted Trees | 0.956 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hina, A.; Saadeh, W. Noninvasive Blood Glucose Monitoring Systems Using Near-Infrared Technology—A Review. Sensors 2022, 22, 4855. https://doi.org/10.3390/s22134855
Hina A, Saadeh W. Noninvasive Blood Glucose Monitoring Systems Using Near-Infrared Technology—A Review. Sensors. 2022; 22(13):4855. https://doi.org/10.3390/s22134855
Chicago/Turabian StyleHina, Aminah, and Wala Saadeh. 2022. "Noninvasive Blood Glucose Monitoring Systems Using Near-Infrared Technology—A Review" Sensors 22, no. 13: 4855. https://doi.org/10.3390/s22134855
APA StyleHina, A., & Saadeh, W. (2022). Noninvasive Blood Glucose Monitoring Systems Using Near-Infrared Technology—A Review. Sensors, 22(13), 4855. https://doi.org/10.3390/s22134855





