Single-Molecule Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Single-Molecule SERS
2.1. Enhancement Factor
2.2. Experimental Evidence of Single-Molecule Event
2.3. Spectral Fluctuation and Data Analysis
3. Advanced Implementation of SM-SERS
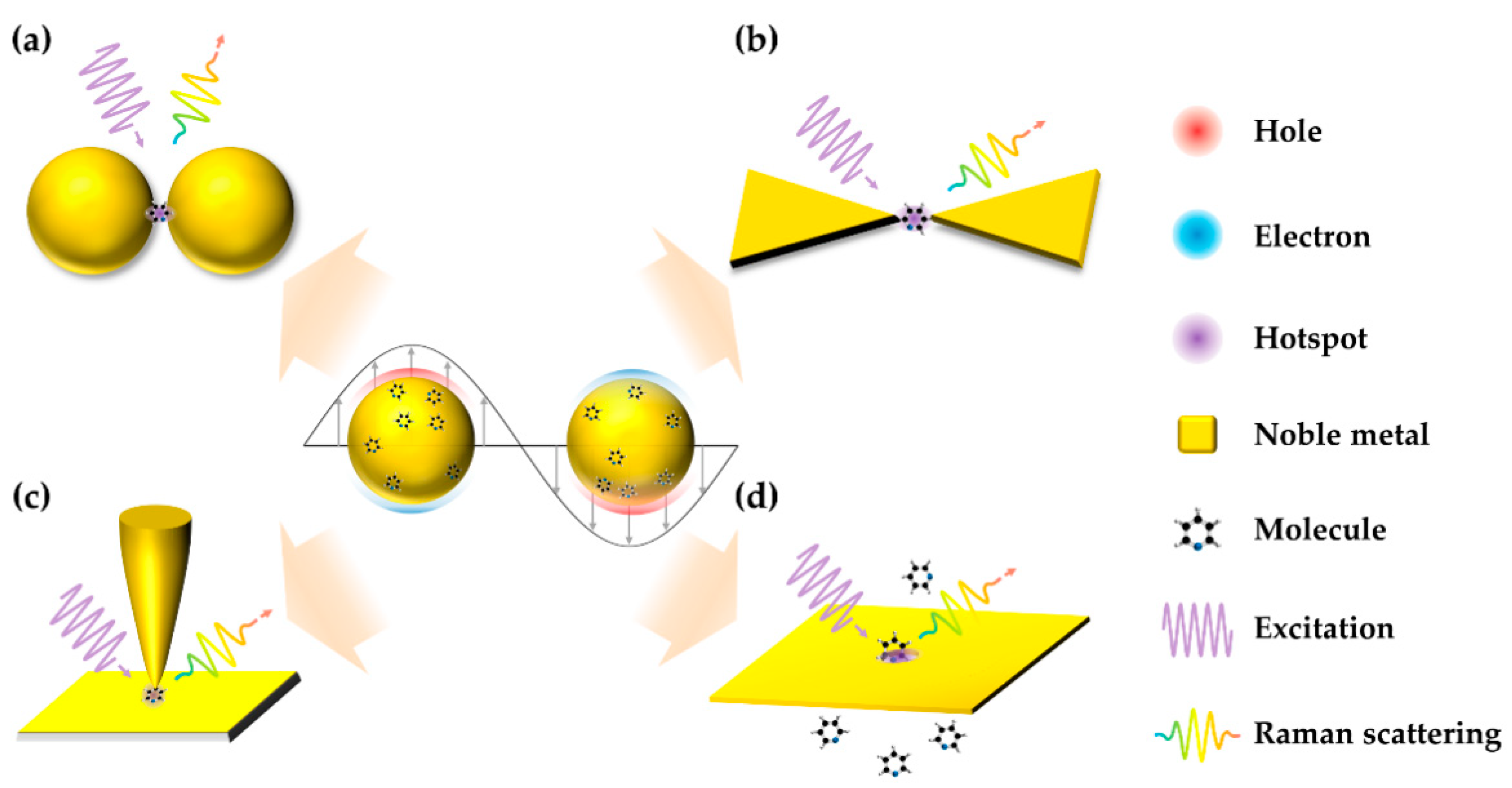
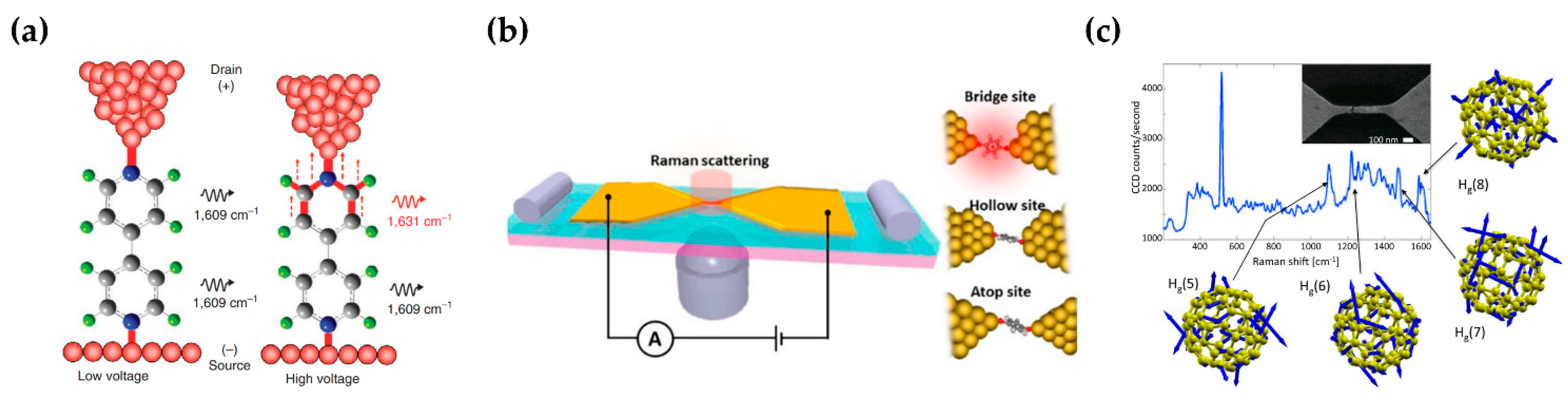
3.1. Substrates with Ultra-High EF and Reproducibility

3.2. Strategies for Improving the Probability of Molecules in Hotspots
3.3. Nonmetallic and Hybrid Substrate
4. Application of SM-SERS
4.1. In Situ Monitoring of Catalytic Reactions
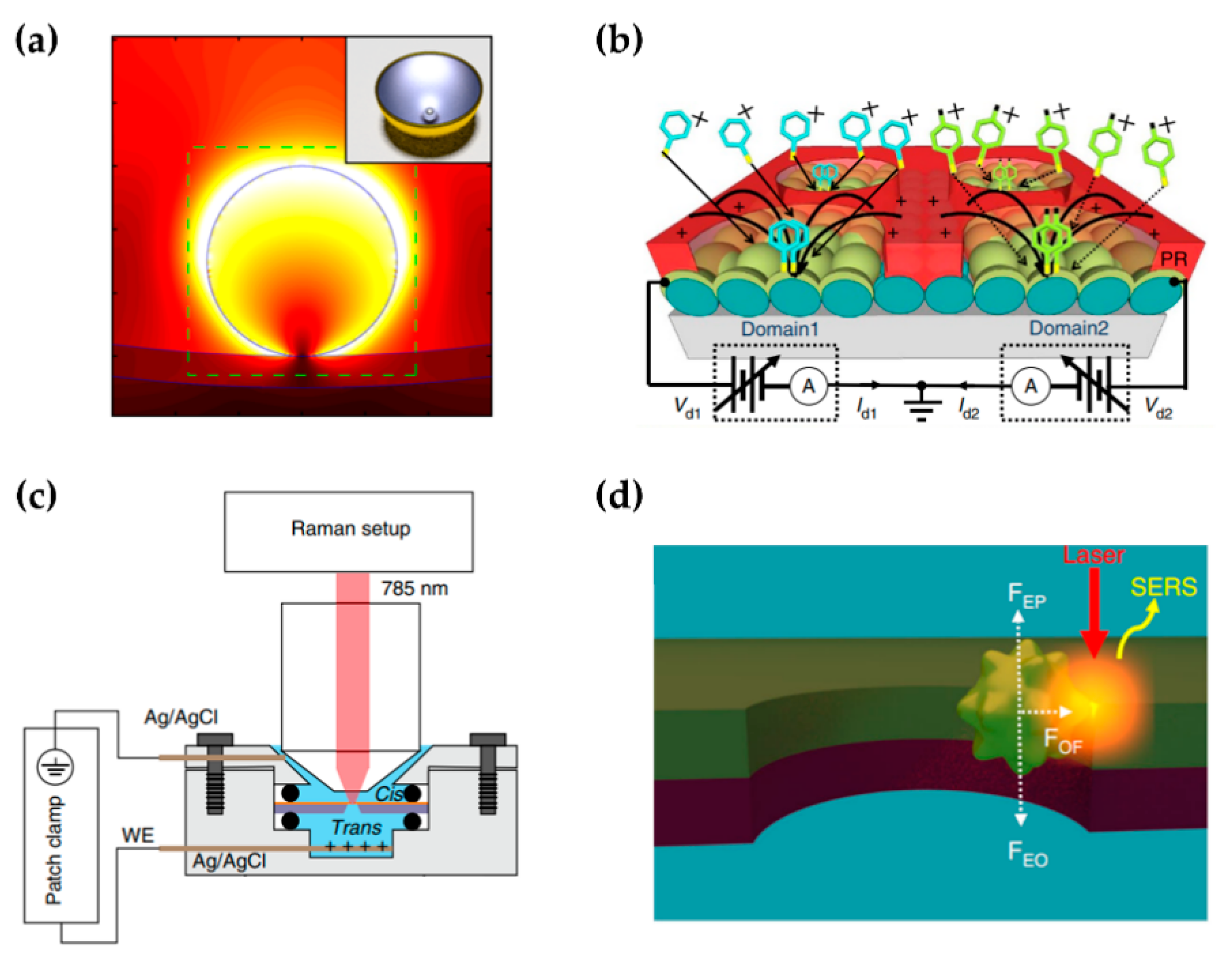
4.2. Characterization of Molecular Nanoelectronics
4.3. Single-Molecule Sensing
5. Several Challenges in SM-SERS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raman, C.V.; Krishnan, K.S. A new type of secondary radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Hanlon, E.B.; Manoharan, R.; Koo, T.W.; Shafer, K.E.; Motz, J.T.; Fitzmaurice, M.; Kramer, J.R.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Prospects for in vivo Raman spectroscopy. Phys. Med. Biol. 2000, 45, R1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman-spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Guerrini, L.; Graham, D. Molecularly-mediated assemblies of plasmonic nanoparticles for Surface-Enhanced Raman Spectroscopy applications. Chem. Soc. Rev. 2012, 41, 7085–7107. [Google Scholar] [CrossRef]
- Yamamoto, Y.S.; Itoh, T. Why and how do the shapes of surface-enhanced Raman scattering spectra change? Recent progress from mechanistic studies. J. Raman Spectrosc. 2016, 47, 78–88. [Google Scholar] [CrossRef]
- Ding, S.Y.; Yi, J.; Li, J.F.; Ren, B.; Wu, D.Y.; Panneerselvam, R.; Tian, Z.Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Kiguchi, M.; Aiba, A.; Fujii, S.; Kobayashi, S. Surface enhanced Raman scattering on molecule junction. Appl. Mater. Today 2019, 14, 76–83. [Google Scholar] [CrossRef]
- Spitzberg, J.D.; Zrehen, A.; van Kooten, X.F.; Meller, A. Plasmonic-nanopore biosensors for superior single-molecule detection. Adv. Mater. 2019, 31, 1900422. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, T.H.; Wu, Y.Z.; Li, W.J.; Zeng, Q.G.; Long, L.; Li, Z.Y. Roadmap for single-molecule surface-enhanced Raman spectroscopy. Adv. Photonics 2020, 2, 014002. [Google Scholar] [CrossRef] [Green Version]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.M.; Emery, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Claridge, S.A.; Schwartz, J.J.; Weiss, P.S. Electrons, photons, and force: Quantitative single-molecule measurements from physics to biology. ACS Nano 2011, 5, 693–729. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Nadappuram, B.P.; Cadinu, P.; Zhao, Z.Y.; Xue, L.; Yi, L.; Ren, R.; Wang, J.W.; Ivanov, A.P.; Edel, J.B. Combined quantum tunnelling and dielectrophoretic trapping for molecular analysis at ultra-low analyte concentrations. Nat. Commun. 2021, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Y.; Dong, Z.C.; Jiang, S.; Zhang, C.; Chen, L.G.; Zhang, L.; Liao, Y.; Aizpurua, J.; Luo, Y.; et al. Chemical mapping of a single molecule by plasmon-enhanced Raman scattering. Nature 2013, 498, 82–86. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Kerman, S.; Neutens, P.; Willems, K.; Cornelissen, S.; Lagae, L.; Stakenborg, T.; Van Dorpe, P. High spatial resolution nanoslit SERS for single-molecule nucleobase sensing. Nat. Commun. 2018, 9, 1733. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.I.; Dieringer, J.A.; Van Duyne, R.P. Creating, characterizing, and controlling chemistry with SERS hot spots. Phys. Chem. Chem. Phys. 2013, 15, 21–36. [Google Scholar] [CrossRef]
- Li, Z.Y. Mesoscopic and microscopic strategies for engineering plasmon-enhanced Raman scattering. Adv. Opt. Mater. 2018, 6, 1701097. [Google Scholar] [CrossRef]
- Pieczonka, N.P.W.; Aroca, R.F. Single molecule analysis by surfaced-enhanced Raman scattering. Chem. Soc. Rev. 2008, 37, 946–954. [Google Scholar] [CrossRef]
- Shim, S.; Stuart, C.M.; Mathies, R.A. Resonance Raman cross-sections and vibronic analysis of rhodamine 6G from broadband stimulated Raman spectroscopy. Chemphyschem 2008, 9, 697–699. [Google Scholar] [CrossRef]
- Blackie, E.J.; Le Ru, E.C.; Etchegoin, P.G. Single-molecule surface-enhanced Raman spectroscopy of nonresonant molecules. J. Am. Chem. Soc. 2009, 131, 14466–14472. [Google Scholar] [CrossRef]
- Morris, M.D. Resonance Raman spectroscopy-current applications and prospects. Anal. Chem. 1979, 51, 182. [Google Scholar] [CrossRef]
- Etchegoin, P.G.; Le Ru, E.C. A perspective on single molecule SERS: Current status and future challenges. Phys. Chem. Chem. Phys. 2008, 10, 6079–6089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, B.Q.; Li, Z.Y. Optical origin of subnanometer resolution in tip-enhanced Raman mapping. J. Phys. Chem. C 2015, 119, 11858–11871. [Google Scholar] [CrossRef]
- Fiederling, K.; Abasifard, M.; Richter, M.; Deckert, V.; Graefe, S.; Kupfer, S. The chemical effect goes resonant-a full quantum mechanical approach on TERS. Nanoscale 2020, 12, 6346–6359. [Google Scholar] [CrossRef] [PubMed]
- Fiederling, K.; Kupfer, S.; Grafe, S. Are charged tips driving TERS-resolution? A full quantum chemical approach. J. Chem. Phys. 2021, 154, 034106. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Jensen, L. A discrete interaction model/quantum mechanical method to describe the interaction of metal nanoparticles and molecular absorption. J. Chem. Phys. 2011, 135, 134103. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Etchegoin, P.G.; Meyer, M. Enhancement factor distribution around a single surface-enhanced Raman scattering hot spot and its relation to single molecule detection. J. Chem. Phys. 2006, 125, 204701. [Google Scholar] [CrossRef] [Green Version]
- Dieringer, J.A.; Lettan, R.B.; Scheidt, K.A.; Van Duyne, R.P. A frequency domain existence proof of single-molecule surface-enhanced Raman Spectroscopy. J. Am. Chem. Soc. 2007, 129, 16249–16256. [Google Scholar] [CrossRef]
- Zrimsek, A.B.; Chiang, N.H.; Mattei, M.; Zaleski, S.; McAnally, M.O.; Chapman, C.T.; Henry, A.I.; Schatz, G.C.; Van Duyne, R.P. Single-molecule chemistry with surface- and tip-enhanced Raman spectroscopy. Chem. Rev. 2017, 117, 7583–7613. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Meyer, M.; Etchegoin, P.G. Proof of single-molecule sensitivity in surface enhanced Raman scattering (SERS) by means of a two-analyte technique. J. Phys. Chem. B 2006, 110, 1944–1948. [Google Scholar] [CrossRef]
- Lahr, R.H.; Vikesland, P.J. Surface-enhanced Raman spectroscopy (SERS) cellular imaging of intracellulary biosynthesized gold nanoparticles. ACS Sustain. Chem. Eng. 2014, 2, 1599–1608. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, W.; Lu, A.X.; Wu, Y.; Liu, Y.; Yan, C.C.; Han, C.Q. Rapid classification of honey varieties by surface enhanced Raman scattering combining with deep learning. In Proceedings of the Cross Strait Quad-Regional Radio Science and Wireless Technology Conference (CSQRWC), Xuzhou, China, 21–24 July 2018. [Google Scholar]
- Zhang, R.; Xie, H.M.; Cai, S.N.; Hu, Y.; Liu, G.K.; Hong, W.J.; Tian, Z.Q. Transfer-learning-based Raman spectra identification. J. Raman Spectrosc. 2020, 51, 176–186. [Google Scholar] [CrossRef]
- Lindquist, N.C.; de Albuquerque, C.D.L.; Sobral-Filho, R.G.; Paci, I.; Brolo, A.G. High-speed imaging of surface-enhanced Raman scattering fluctuations from individual nanoparticles. Nat. Nanotechnol. 2019, 14, 981. [Google Scholar] [CrossRef] [PubMed]
- Kudelski, A.; Pettinger, B. Fluctuations of surface-enhanced Raman spectra of CO adsorbed on gold substrates. Chem. Phys. Lett. 2004, 383, 76–79. [Google Scholar] [CrossRef]
- Marshall, A.R.L.; Stokes, J.; Viscomi, F.N.; Proctor, J.E.; Gierschner, J.; Bouillard, J.S.G.; Adawi, A.M. Determining molecular orientation via single molecule SERS in a plasmonic nano-gap. Nanoscale 2017, 9, 17415–17421. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.L.; Willets, K.A. Correlated super-resolution optical and structural studies of surface-enhanced Raman scattering hot spots in silver colloid aggregates. J. Phys. Chem. Lett. 2011, 2, 1766–1770. [Google Scholar] [CrossRef]
- Maruyama, Y.; Ishikawa, M.; Futamata, M. Thermal activation of blinking in SERS signal. J. Phys. Chem. B 2004, 108, 673–678. [Google Scholar] [CrossRef]
- Galloway, C.M.; Le Ru, E.C.; Etchegoin, P.G. Single-molecule vibrational pumping in SERS. Phys. Chem. Chem. Phys. 2009, 11, 7372–7380. [Google Scholar] [CrossRef]
- Emory, S.R.; Jensen, R.A.; Wenda, T.; Han, M.Y.; Nie, S.M. Re-examining the origins of spectral blinking in single-molecule and single-nanoparticle SERS. Faraday Discuss. 2006, 132, 249–259. [Google Scholar] [CrossRef]
- Haran, G. Single molecule Raman spectroscopy and local work function fluctuations. Isr. J. Chem. 2004, 44, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.X.; Bjerneld, E.J.; Kall, M.; Borjesson, L. Spectroscopy of single hemoglobin molecules by surface enhanced Raman scattering. Phys. Rev. Lett. 1999, 83, 4357–4360. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Seong, N.H.; Dlott, D.D. Measurement of the distribution of site enhancements in surface-enhanced Raman scattering. Science 2008, 321, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Jeon, K.S.; Kim, H.M.; Nam, J.M.; Suh, Y.D. Nanogap-engineerable Raman-active nanodumbbells for single-molecule detection. Nat. Mater. 2010, 9, 60–67. [Google Scholar] [CrossRef]
- Li, L.; Hutter, T.; Steiner, U.; Mahajan, S. Single molecule SERS and detection of biomolecules with a single gold nanoparticle on a mirror junction. Analyst 2013, 138, 4574–4578. [Google Scholar] [CrossRef] [PubMed]
- Benz, F.; Tserkezis, C.; Herrmann, L.O.; de Nijs, B.; Sanders, A.; Sigle, D.O.; Pukenas, L.; Evans, S.D.; Aizpurua, J.; Baumberg, J.J. Nanooptics of molecular-shunted plasmonic nanojunctions. Nano Lett. 2015, 15, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Ma, L.; Li, J.; Zhang, Z. Nanoparticle-on-mirror cavity modes for huge and/or tunable plasmonic field enhancement. Nanotechnology 2017, 28, 105203. [Google Scholar] [CrossRef]
- Carnegie, C.; Griffiths, J.; de Nijs, B.; Readman, C.; Chikkaraddy, R.; Deacon, W.M.; Zhang, Y.; Szabo, I.; Rosta, E.; Aizpurua, J.; et al. Room-temperature optical picocavities below 1 nm3 accessing single-atom geometries. J. Phys. Chem. Lett. 2018, 9, 7146–7151. [Google Scholar] [CrossRef]
- Gruenke, N.L.; Cardinal, M.F.; McAnally, M.O.; Frontiera, R.R.; Schatza, G.C.; Van Duyne, R.P. Ultrafast and nonlinear surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2016, 45, 2263–2290. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, Y.R.; Neumann, O.; Day, J.K.; Nordlander, P.; Halas, N.J. Coherent anti-Stokes Raman scattering with single-molecule sensitivity using a plasmonic Fano resonance. Nat. Commun. 2014, 5, 4424. [Google Scholar] [CrossRef]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Thacker, V.V.; Herrmann, L.O.; Sigle, D.O.; Zhang, T.; Liedl, T.; Baumberg, J.J.; Keyser, U.F. DNA origami based assembly of gold nanoparticle dimers for surface-enhanced Raman scattering. Nat. Commun. 2014, 5, 3448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, L.; Thacker, V.V.; Herrmann, L.O.; Hemmig, E.A.; Lombardi, A.; Keyser, U.F.; Baumberg, J.J. Gap-dependent coupling of Ag-Au nanoparticle heterodimers using DNA origami-based self-assembly. ACS Photonics 2016, 3, 1589–1595. [Google Scholar] [CrossRef] [Green Version]
- Tanwar, S.; Haldar, K.K.; Sen, T. DNA origami directed Au nanostar dimers for single-molecule surface-enhanced Raman scattering. J. Am. Chem. Soc. 2017, 139, 17639–17648. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.F.; Wen, T.; Wang, Z.G.; He, Y.B.; Shi, J.; Wang, T.; Liu, X.F.; Lu, G.W.; Ding, B.Q. DNA origami directed assembly of gold bowtie nanoantennas for single-molecule surface-enhanced Raman scattering. Angew. Chem. Int. Ed. 2018, 57, 2846–2850. [Google Scholar] [CrossRef]
- Chikkaraddy, R.; Turek, V.A.; Kongsuwan, N.; Benz, F.; Carnegie, C.; van de Goor, T.; de Nijs, B.; Demetriadou, A.; Hess, O.; Keyser, U.F.; et al. Mapping nanoscale hotspots with single-molecule emitters assembled into plasmonic nanocavities using DNA origami. Nano Lett. 2018, 18, 405–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapio, K.; Mostafa, A.; Kanehira, Y.; Suma, A.; Dutta, A.; Bald, I. A versatile DNA origami-based plasmonic nanoantenna for label-free single-molecule surface-enhanced Raman spectroscopy. ACS Nano 2021, 15, 7065–7077. [Google Scholar] [CrossRef]
- Stockle, R.M.; Suh, Y.D.; Deckert, V.; Zenobi, R. Nanoscale chemical analysis by tip-enhanced Raman spectroscopy. Chem. Phys. Lett. 2000, 318, 131–136. [Google Scholar] [CrossRef]
- Vasconcelos, T.L.; Archanjo, B.S.; Fragneaud, B.; Oliveira, B.S.; Riikonen, J.; Li, C.F.; Ribeiro, D.S.; Rabelo, C.; Rodrigues, W.N.; Jorio, A.; et al. Tuning localized surface plasmon resonance in scanning near-field optical microscopy probes. ACS Nano 2015, 9, 6297–6304. [Google Scholar] [CrossRef]
- Jaculbia, R.B.; Imada, H.; Miwa, K.; Iwasa, T.; Takenaka, M.; Yang, B.; Kazuma, E.; Hayazawa, N.; Taketsugu, T.; Kim, Y. Single-molecule resonance Raman effect in a plasmonic nanocavity. Nat. Nanotechnol. 2020, 15, 105. [Google Scholar] [CrossRef]
- Verma, P. Tip-enhanced Raman spectroscopy: Technique and recent advances. Chem. Rev. 2017, 117, 6447–6466. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Zhang, R.; Hu, C.R.; Liao, M.H.; Luo, Y.; Yang, J.L.; Dong, Z.C.; Hou, J.G. Distinguishing adjacent molecules on a surface using plasmon-enhanced Raman scattering. Nat. Nanotechnol. 2015, 10, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Muller, E.A.; Kravtsov, V.; Sass, P.M.; Dreyer, J.; Atkin, J.M.; Raschke, M.B. Variable-temperature tip-enhanced Raman spectroscopy of single-molecule fluctuations and dynamics. Nano Lett. 2016, 16, 479–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Ding, S.Y.; Chen, Z.B.; Wang, X.; Tian, J.H.; Anema, J.R.; Zhou, X.S.; Wu, D.Y.; Mao, B.W.; Xu, X.; et al. Revealing the molecular structure of single-molecule junctions in different conductance states by fishing-mode tip-enhanced Raman spectroscopy. Nat. Commun. 2011, 2, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.; Gordon, R. Single molecule directivity enhanced Raman scattering using nanoantennas. Nano Lett. 2012, 12, 2625–2630. [Google Scholar] [CrossRef]
- Wang, D.X.; Zhu, W.Q.; Best, M.D.; Camden, J.P.; Crozier, K.B. Directional Raman scattering from single molecules in the feed gaps of optical antennas. Nano Lett. 2013, 13, 2194–2198. [Google Scholar] [CrossRef]
- Li, J.F.; Mu, J.J.; Wang, B.L.; Ding, W.; Liu, J.; Guo, H.L.; Li, W.X.; Gu, C.Z.; Li, Z.Y. Direct laser writing of symmetry-broken spiral tapers for polarization-insensitive three-dimensional plasmonic focusing. Laser Photonics Rev. 2014, 8, 602–609. [Google Scholar] [CrossRef]
- Mu, J.J.; Liu, Z.G.; Li, J.F.; Hao, T.T.; Wang, Y.J.; Sun, S.S.; Li, Z.Y.; Li, J.J.; Li, W.X.; Gu, C.Z. Direct laser writing of pyramidal plasmonic structures with apertures and asymmetric gratings towards efficient subwavelength light focusing. Opt. Express 2015, 23, 22564–22571. [Google Scholar] [CrossRef]
- Weekes, S.M.; Ogrin, F.Y.; Murray, W.A.; Keatley, P.S. Macroscopic arrays of magnetic nanostructures from self-assembled nanosphere templates. Langmuir 2007, 23, 1057–1060. [Google Scholar] [CrossRef]
- Tao, A.; Kim, F.; Hess, C.; Goldberger, J.; He, R.R.; Sun, Y.G.; Xia, Y.N.; Yang, P.D. Langmuir-Blodgett silver nanowire monolayers for molecular sensing using surface-enhanced Raman spectroscopy. Nano Lett. 2003, 3, 1229–1233. [Google Scholar] [CrossRef]
- Zhang, L.; Lang, X.Y.; Hirata, A.; Chen, M.W. Wrinkled nanoporous gold films with ultrahigh surface-enhanced Raman scattering enhancement. ACS Nano 2011, 5, 4407–4413. [Google Scholar] [CrossRef]
- Mao, P.; Liu, C.X.; Favraud, G.; Chen, Q.; Han, M.; Fratalocchi, A.; Zhang, S. Broadband single molecule SERS detection designed by warped optical spaces. Nat. Commun. 2018, 9, 5428. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.C.; Fang, J.; Park, S.C.; Johnson, F.W.; Jacobs, H.O. Effective localized collection and identification of airborne species through electrodynamic precipitation and SERS-based detection. Nat. Commun. 2013, 4, 1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.A.; Mousavi, M.Z.; Zhao, Y.Q.; Hubarevich, A.; Omeis, F.; Giovannini, G.; Schutte, M.; Garoli, D.; De Angelis, F. SERS discrimination of single DNA bases in single oligonucleotides by electro-plasmonic trapping. Nat. Commun. 2019, 10, 5321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garoli, D.; Yamazaki, H.; Maccaferri, N.; Wanunu, M. Plasmonic nanopores for single-molecule detection and manipulation: Toward sequencing applications. Nano Lett. 2019, 19, 7553–7562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Y.J.; Gordon, R. Optical trapping of a single protein. Nano Lett. 2012, 12, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi, B.; Ahmadi, V.; Faramarzi, V. Nano-plasmonic-based structures for DNA sequencing. Opt. Lett. 2016, 41, 4229–4232. [Google Scholar] [CrossRef]
- Alessandri, I.; Lombardi, J.R. Enhanced Raman scattering with dielectrics. Chem. Rev. 2016, 116, 14921–14981. [Google Scholar] [CrossRef]
- Lin, W. A durable plastic substrate for surface-enhanced Raman spectroscopy. Appl. Phys. Mater. Sci. Processing 2011, 102, 121–125. [Google Scholar] [CrossRef]
- Woods, D.A.; Bain, C.D. Total internal reflection Raman spectroscopy. Analyst 2012, 137, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Connell, G.A.N.; Nemanich, R.J.; Tsai, C.C. Interference enhanced Raman-scattering from very thin absorbing films. Appl. Phys. Lett. 1980, 36, 31–33. [Google Scholar] [CrossRef]
- Alessandri, I. Enhancing Raman scattering without plasmons: Unprecedented sensitivity achieved by TiO2 shell-based resonators. J. Am. Chem. Soc. 2013, 135, 5541–5544. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, P.A.; Baranov, D.G.; Milichko, V.A.; Makarov, S.V.; Mukhin, I.S.; Samusev, A.K.; Krasnok, A.E.; Belov, P.A.; Kivshar, Y.S. Resonant Raman scattering from silicon nanoparticles enhanced by magnetic response. Nanoscale 2016, 8, 9721–9726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.H.; Cong, S.; Gong, W.B.; Xuan, J.N.; Li, G.H.; Lu, W.B.; Geng, F.X.; Zhao, Z.G. Semiconductor SERS enhancement enabled by oxygen incorporation. Nat. Commun. 2017, 8, 1993. [Google Scholar] [CrossRef] [Green Version]
- Cong, S.; Yuan, Y.Y.; Chen, Z.G.; Hou, J.Y.; Yang, M.; Su, Y.L.; Zhang, Y.Y.; Li, L.; Li, Q.W.; Geng, F.X.; et al. Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015, 6, 7800. [Google Scholar] [CrossRef]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Label-free SERS quantum semiconductor probe for molecular-level and in vitro cellular detection: A noble-metal-free methodology. ACS Appl. Mater. Interfaces 2018, 10, 34886–34904. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Su, S.; Xu, T.T.; Zhong, Y.L.; Zapien, J.A.; Li, J.; Fan, C.H.; Lee, S.T. Silicon nanowires-based highly-efficient SERS-active platform for ultrasensitive DNA detection. Nano Today 2011, 6, 122–130. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Joseph, V.; Engelbrekt, C.; Zhang, J.D.; Gernert, U.; Ulstrup, J.; Kneipp, J. Characterizing the kinetics of nanoparticle-catalyzed reactions by surface-enhanced Raman scattering. Angew. Chem. Int. Ed. 2012, 51, 7592–7596. [Google Scholar] [CrossRef]
- Kang, L.L.; Xu, P.; Zhang, B.; Tsai, H.H.; Han, X.J.; Wang, H.L. Laser wavelength- and power-dependent plasmon-driven chemical reactions monitored using single particle surface enhanced Raman spectroscopy. Chem. Commun. 2013, 49, 3389–3391. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, J.J.; Ji, J.; Liu, B.H. Synthesis of micro-sized shell-isolated 3D plasmonic superstructures for in situ single-particle SERS monitoring. Nanoscale 2016, 8, 7871–7875. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Kneipp, J. Mapping the inhomogeneity in plasmonic catalysis on supported gold nanoparticles using surface-enhanced Raman scattering microspectroscopy. Anal. Chem. 2018, 90, 9199–9205. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Schlucker, S. Hot electron-induced reduction of small molecules on photorecycling metal surfaces. Nat. Commun. 2015, 6, 7570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Deckert-Gaudig, T.; Singh, P.; Deckert, V. Single molecule level plasmonic catalysis-a dilution study of p-nitrothiophenol on gold dimers. Chem. Commun. 2015, 51, 3069–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Liu, Y.J.; Wang, Y.N.; Zhao, J.J.; Liu, B.H. Direct SERS tracking of a chemical reaction at a single 13 nm gold nanoparticle. Chem. Sci. 2019, 10, 1741–1745. [Google Scholar] [CrossRef] [Green Version]
- De Nijs, B.; Benz, F.; Barrow, S.J.; Sigle, D.O.; Chikkaraddy, R.; Palma, A.; Carnegie, C.; Kamp, M.; Sundararaman, R.; Narang, P.; et al. Plasmonic tunnel junctions for single-molecule redox chemistry. Nat. Commun. 2017, 8, 994. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.Q.; Ren, B.; Li, J.F.; Yang, Z.L. Expanding generality of surface-enhanced Raman spectroscopy with borrowing SERS activity strategy. Chem. Commun. 2007, 34, 3514–3534. [Google Scholar] [CrossRef] [Green Version]
- Joachim, C.; Gimzewski, J.K.; Aviram, A. Electronics using hybrid-molecular and mono-molecular devices. Nature 2000, 408, 541–548. [Google Scholar] [CrossRef]
- Kiguchi, M.; Tal, O.; Wohlthat, S.; Pauly, F.; Krieger, M.; Djukic, D.; Cuevas, J.C.; van Ruitenbeek, J.M. Highly conductive molecular junctions based on direct binding of benzene to platinum electrodes. Phys. Rev. Lett. 2008, 101, 046801. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, S.; Murai, D.; Marques-Gonzalez, S.; Nakamura, H.; Komoto, Y.; Fujii, S.; Nishino, T.; Ikeda, K.; Tsukagoshi, K.; Kiguchi, M. Site-selection in single-molecule junction for highly reproducible molecular electronics. J. Am. Chem. Soc. 2016, 138, 1294–1300. [Google Scholar] [CrossRef] [Green Version]
- Han, R.; Song, W.; Wang, X.; Mao, Z.; Han, X.X.; Zhao, B. Investigation of charge transfer at the TiO2-MBA-Au interface based on surface-enhanced Raman scattering: SPR contribution. Phys. Chem. Chem. Phys. 2018, 20, 5666–5673. [Google Scholar] [CrossRef]
- Li, Y.; Doak, P.; Kronik, L.; Neaton, J.B.; Natelson, D. Voltage tuning of vibrational mode energies in single-molecule junctions. Proc. Natl. Acad. Sci. USA 2014, 111, 1282–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhou, J.; Maccaferri, N.; Krahne, R.; Wang, K.; Garoli, D. Enhanced optical spectroscopy for multiplexed DNA and protein-sequencing with plasmonic nanopores: Challenges and prospects. Anal. Chem. 2022, 94, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zeng, J.B.; Zhao, F.S.; Lin, S.H.; Raja, B.; Strych, U.; Willson, R.C.; Shih, W.C. Label-free, in situ SERS monitoring of individual DNA hybridization in microfluidics. Nanoscale 2014, 6, 8521–8526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, P.L.; Shen, Q.; Ahmed, S.A.; Pan, X.T.; Liu, H.L.; Ding, X.L.; Li, J.; Wang, K.; Xia, X.H. Probing multidimensional structural information of single molecules transporting through a sub-10 nm conical plasmonic nanopore by SERS. Anal. Chem. 2021, 93, 11679–11685. [Google Scholar] [CrossRef]
- Olschewski, K.; Kammer, E.; Stockel, S.; Bocklitz, T.; Deckert-Gaudig, T.; Zell, R.; Cialla-May, D.; Weber, K.; Deckert, V.; Popp, J. A manual and an automatic TERS based virus discrimination. Nanoscale 2015, 7, 4545–4552. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Han, Z.H.; Kizer, M.; Linhardt, R.J.; Wang, X.; Sinyukov, A.M.; Wang, J.Z.; Deckert, V.; Sokolov, A.V.; Hu, J.; et al. Tip-enhanced Raman imaging of single-stranded DNA with single base resolution. J. Am. Chem. Soc. 2019, 141, 753–757. [Google Scholar] [CrossRef]
- He, Z.; Qiu, W.W.; Kizer, M.E.; Wang, J.Z.; Chen, W.C.; Sokolov, A.V.; Wang, X.; Hu, J.; Scully, M.O. Resolving the sequence of RNA strands by tip-enhanced Raman spectroscopy. ACS Photonics 2021, 8, 424–430. [Google Scholar] [CrossRef]
- Hubarevich, A.; Huang, J.A.; Giovannini, G.; Schirato, A.; Zhao, Y.Q.; Maccaferri, N.; De Angelis, F.; Alabastri, A.; Garoli, D. lambda-DNA through porous materials-surface-enhanced Raman scattering in a simple plasmonic nanopore. J. Phys. Chem. C 2020, 124, 22663–22670. [Google Scholar] [CrossRef]
- Belkin, M.; Chao, S.H.; Jonsson, M.P.; Dekker, C.; Aksimentiev, A. Plasmonic nanopores for trapping, controlling displacement, and sequencing of DNA. ACS Nano 2015, 9, 10598–10611. [Google Scholar] [CrossRef]
- Dekker, C. Solid-state nanopores. Nat. Nanotechnol. 2007, 2, 209–215. [Google Scholar] [CrossRef]
- Cao, J.; Liu, H.L.; Yang, J.M.; Li, Z.Q.; Yang, D.R.; Ji, L.N.; Wang, K.; Xia, X.H. SERS detection of nucleobases in single silver plasmonic nanopores. ACS Sens. 2020, 5, 2198–2204. [Google Scholar] [CrossRef]
- Yang, J.M.; Jin, L.; Pan, Z.Q.; Zhou, Y.; Liu, H.L.; Ji, L.N.; Xia, X.H.; Wang, K. Surface-enhanced Raman scattering probing the translocation of DNA and Amino acid through plasmonic nanopores. Anal. Chem. 2019, 91, 6275–6280. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Chen, C.J.; Wang, X.; Hu, P.; Liu, G.K.; Ren, B. Single-molecule level rare events revealed by dynamic surface-enhanced Raman spectroscopy. Anal. Chem. 2020, 92, 15806–15810. [Google Scholar] [CrossRef] [PubMed]
- Oyamada, N.; Minamimoto, H.; Murakoshi, K. Room-Temperature Molecular Manipulation via Plasmonic Trapping at Electrified Interfaces. J. Am. Chem. Soc. 2022, 144, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Chen, J.F.; Yu, H.K.; Li, Z.Y. Strong optics force of a molecule enabled by the plasmonic nanogap hot spot in a tip-enhanced Raman spectroscopy system. Photonics Res. 2020, 8, 1573–1579. [Google Scholar] [CrossRef]
- Keller, E.L.; Brandt, N.C.; Cassabaum, A.A.; Frontiera, R.R. Ultrafast surface-enhanced Raman spectroscopy. Analyst 2015, 140, 4922–4931. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Kuang, C.; Liu, X.; Tang, L. Single-Molecule Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 4889. https://doi.org/10.3390/s22134889
Qiu Y, Kuang C, Liu X, Tang L. Single-Molecule Surface-Enhanced Raman Spectroscopy. Sensors. 2022; 22(13):4889. https://doi.org/10.3390/s22134889
Chicago/Turabian StyleQiu, Yuxuan, Cuifang Kuang, Xu Liu, and Longhua Tang. 2022. "Single-Molecule Surface-Enhanced Raman Spectroscopy" Sensors 22, no. 13: 4889. https://doi.org/10.3390/s22134889
APA StyleQiu, Y., Kuang, C., Liu, X., & Tang, L. (2022). Single-Molecule Surface-Enhanced Raman Spectroscopy. Sensors, 22(13), 4889. https://doi.org/10.3390/s22134889






