Pilot Study for Correlation of Heart Rate Variability and Dopamine Transporter Brain Imaging in Patients with Parkinsonian Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Selection
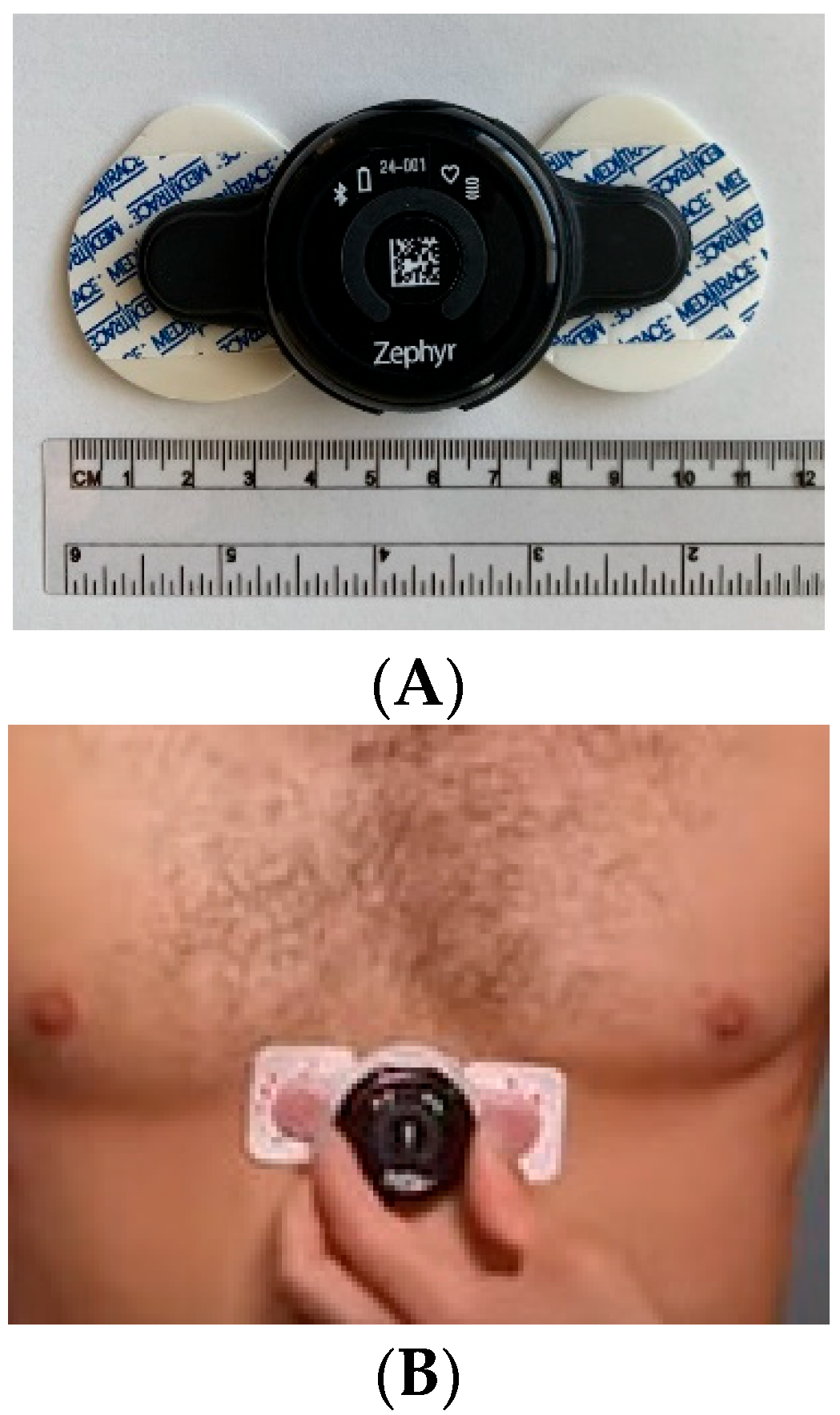
2.2. HRV Data Acquisition
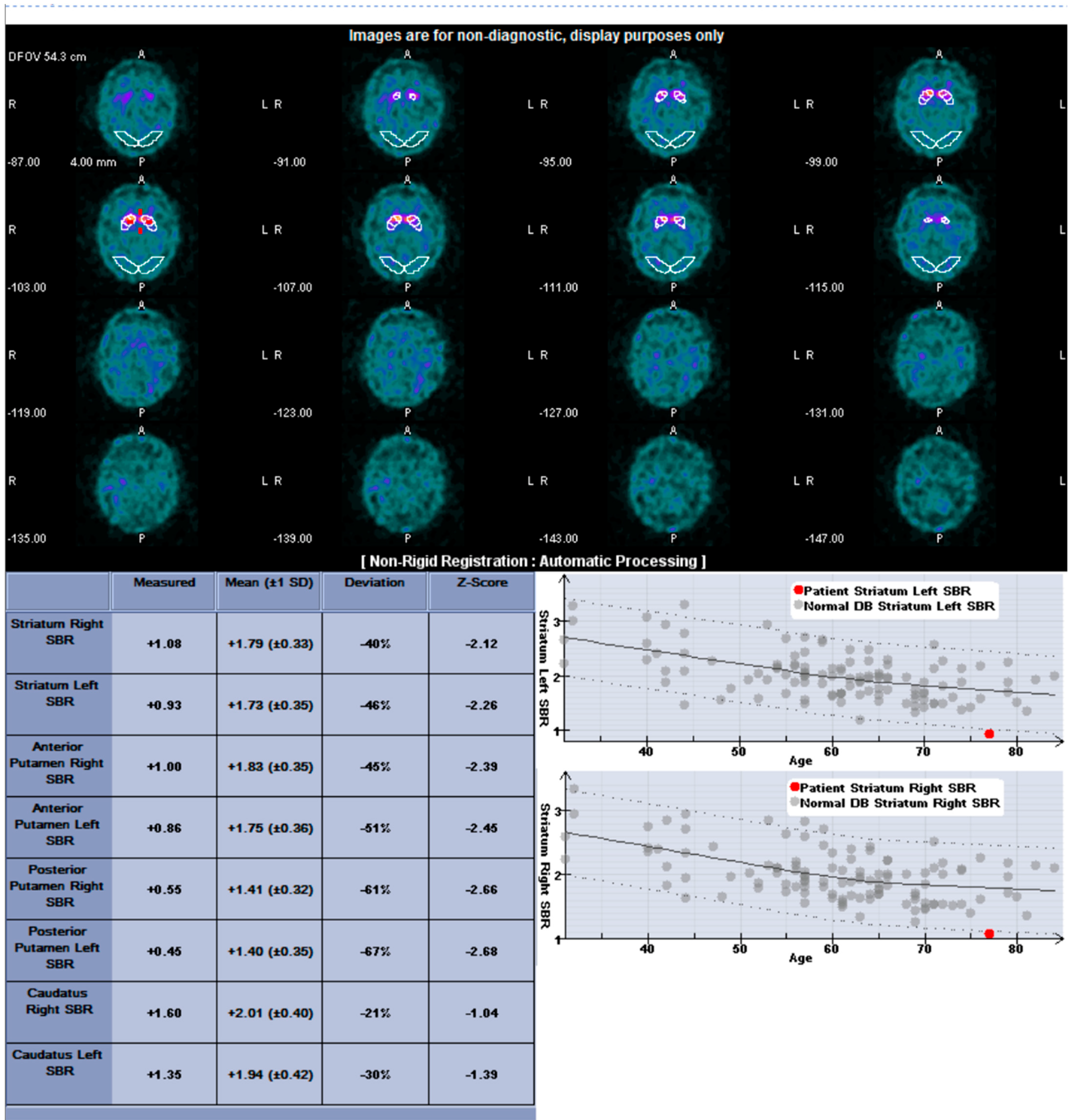
2.3. I-123 Ioflupane SPECT Protocol and Quantification
2.4. Statistical Methods
3. Results
3.1. Patients
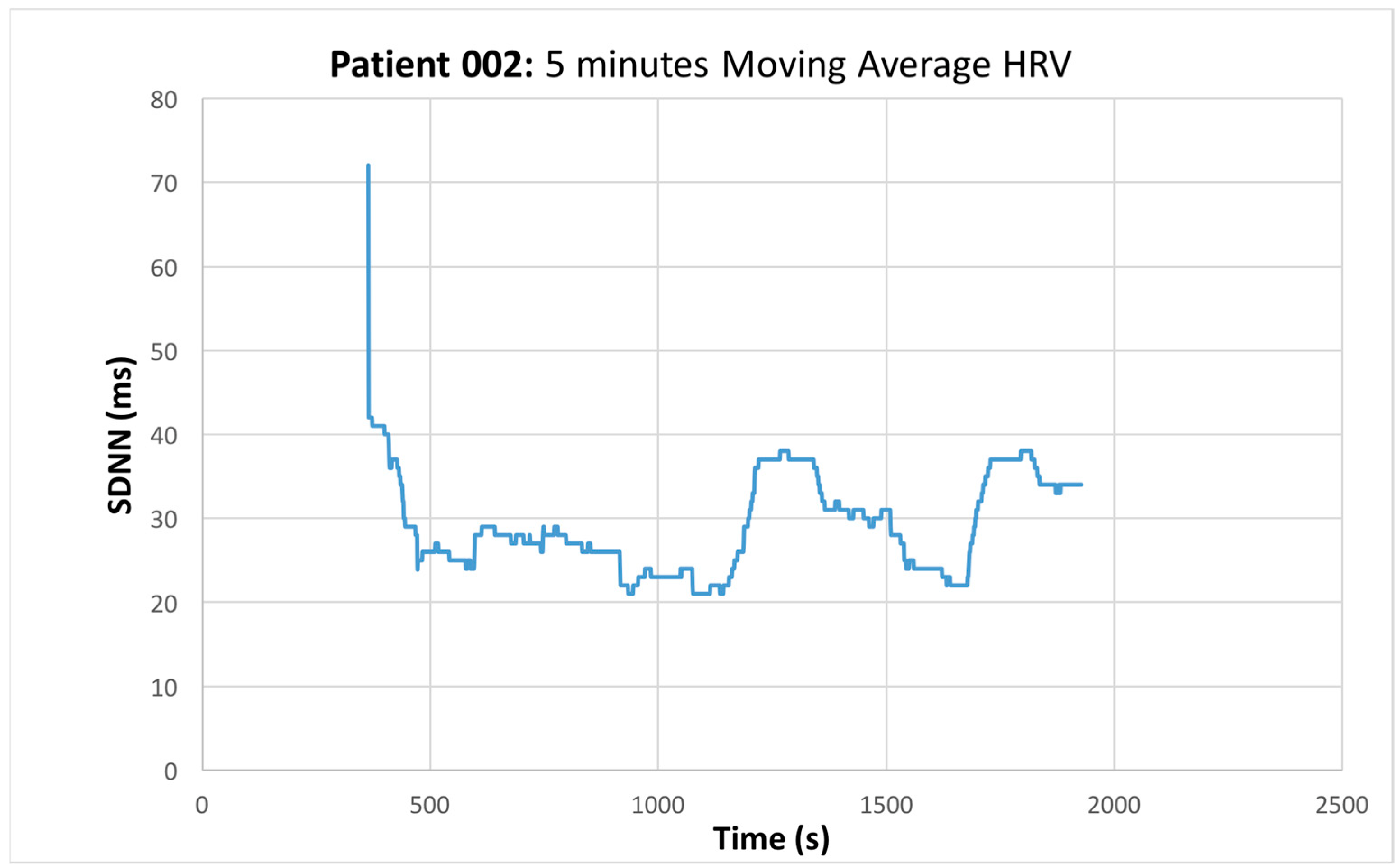
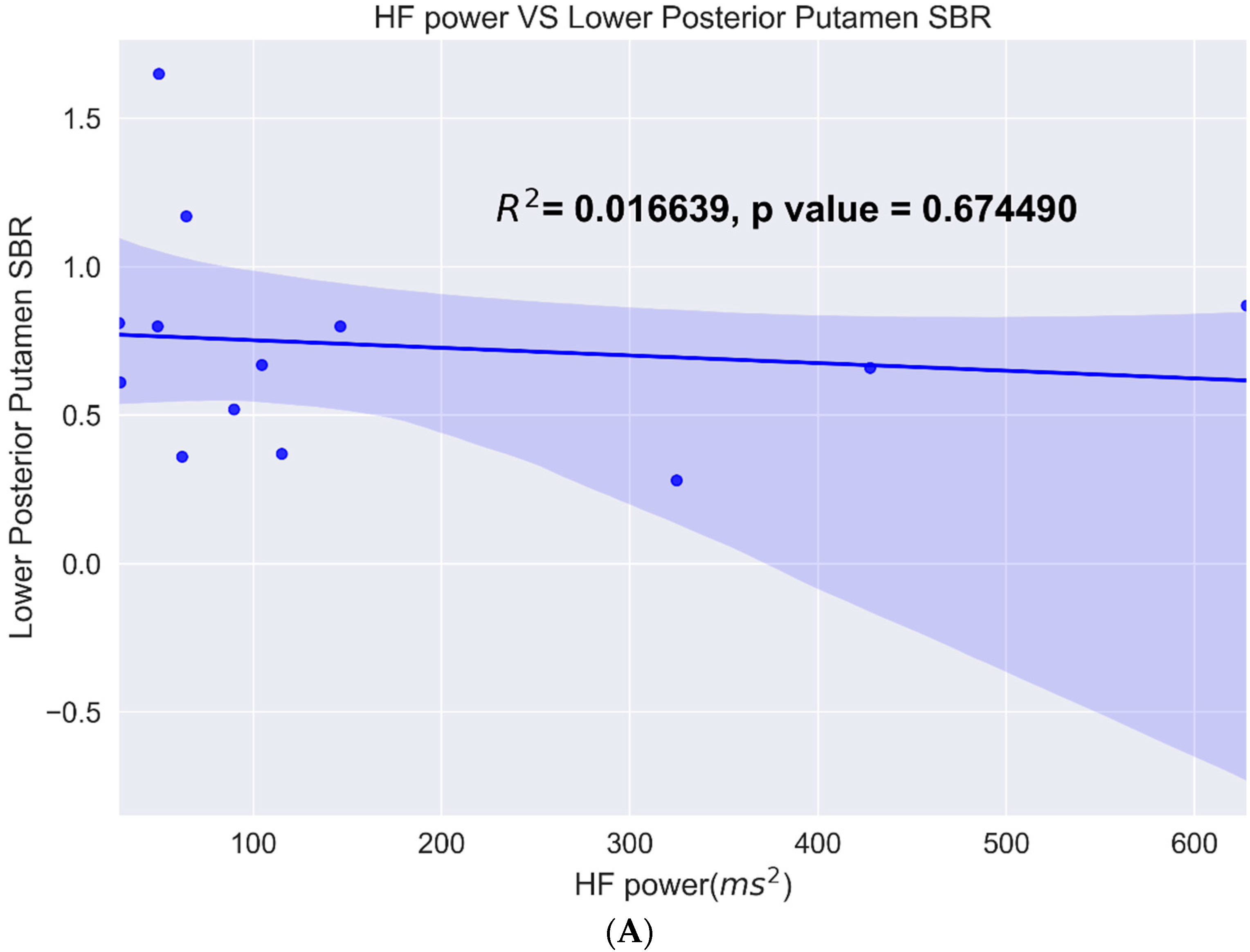
3.2. Heart Rate Variability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Williams, D.R.; Litvan, I. Parkinsonian syndromes. Continuum 2013, 19, 1189–1212. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Davie, C.A. A review of Parkinson’s disease. Br. Med. Bulletin 2008, 86, 109–127. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Benito, M.; Granado, N.; García-Sanz, P.; Michel, A.; Dumoulin, M.; Moratalla, R. Modeling Parkinson’s Disease with the Alpha-Synuclein Protein. Front. Pharmacol. 2020, 11, 356. [Google Scholar] [CrossRef]
- Harnod, D.; Wen, S.H.; Chen, S.Y.; Harnod, T. The association of heart rate variability with parkinsonian motor symptom duration. Yonsei Med. J. 2014, 55, 1297–1302. [Google Scholar] [CrossRef] [Green Version]
- De Pablo-Fernandez, E.; Tur, C.; Revesz, T.; Lees, A.J.; Holton, J.L.; Warner, T.T. Association of Autonomic Dysfunction with Disease Progression and Survival in Parkinson Disease. JAMA Neurol. 2017, 74, 970–976. [Google Scholar] [CrossRef]
- Vanderlei, L.C.; Pastre, C.M.; Hoshi, R.A.; Carvalho, T.D.; Godoy, M.F. Basic notions of heart rate variability and its clinical applicability. Rev. Bras Cir. Cardiovasc. 2009, 24, 205–217. [Google Scholar] [CrossRef] [Green Version]
- Benichou, T.; Pereira, B.; Mermillod, M.; Tauveron, I.; Pfabigan, D.; Maqdasy, S.; Dutheil, F. Heart rate variability in type 2 diabetes mellitus: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0195166. [Google Scholar] [CrossRef] [Green Version]
- Mani, A.R.; Montagnese, S.; Jackson, C.D.; Jenkins, C.W.; Head, I.M.; Stephens, R.C.; Moore, K.P.; Morgan, M.Y. Decreased heart rate variability in patients with cirrhosis relates to the presence and degree of hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G330–G338. [Google Scholar] [CrossRef]
- Alonso, A.; Huang, X.; Mosley, T.H.; Heiss, G.; Chen, H. Heart rate variability and the risk of Parkinson disease: The Atherosclerosis Risk in Communities study. Ann. Neurol. 2015, 77, 877–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbons, C.H.; Simon, D.K.; Huang, M.; Tilley, B.; Aminoff, M.J.; Bainbridge, J.L.; Brodsky, M.; Freeman, R.; Goudreau, J.; Hamill, R.W.; et al. Autonomic and electrocardiographic findings in Parkinson’s disease. Auton Neurosci. 2017, 205, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zandstra, T.E.; Notenboom, R.G.E.; Wink, J.; Kiès, P.; Vliegen, H.W.; Egorova, A.D.; Schalij, M.J.; De Ruiter, M.C.; Jongbloed, M.R.M. Asymmetry and Heterogeneity: Part and Parcel in Cardiac Autonomic Innervation and Function. Front. Physiol. 2021, 12, 665298. [Google Scholar] [CrossRef]
- Yang, S.C.; Wu, C.C.; Hsieh, Y.J. Left stellate ganglion block, a rescue treatment for ventricular arrhythmia refractory to radiofrequency catheter ablation. Medicine 2019, 98, e17790. [Google Scholar] [CrossRef] [PubMed]
- Loyalka, P.; Hariharan, R.; Gholkar, G.; Gregoric, I.D.; Tamerisa, R.; Nathan, S.; Kar, B. Left stellate ganglion block for continuous ventricular arrhythmias during percutaneous left ventricular assist device support. Tex. Heart Inst. J. 2011, 38, 409–411. [Google Scholar]
- Kuo, P.H.; Lei, H.H.; Avery, R.; Krupinski, E.A.; Bauer, A.; Sherman, S.; McMillan, N.; Seibyl, J.; Zubal, G.I. Evaluation of an Objective Striatal Analysis Program for Determining Laterality in Uptake of 123I-Ioflupane SPECT Images: Comparison to Clinical Symptoms and to Visual Reads. J. Nucl. Med. Technol. 2014, 42, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Calle, S.; Dawood, L.; Tripathee, N.R.; Cai, C.; Kaur, H.; Wan, D.Q.; Ibekwe, H.; Gayed, I.W. Identification of patterns of abnormalities seen on DaTscan™ SPECT imaging in patients with non-Parkinson’s movement disorders. Rep. Med. Imaging 2019, 12, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Defazio, G.; Ercoli, T.; Erro, R.; Pellicciari, R.; Mascia, M.M.; Fabbrini, G.; Albanese, A.; Lalli, S.; Eleopra, R.; Barone, P.; et al. Idiopathic Non-task-Specific Upper Limb Dystonia, a Neglected Form of Dystonia. Mov. Disord. 2020, 35, 2038–2045. [Google Scholar] [CrossRef]
- Hallett, M.; Aybek, S.; Dworetzky, B.A.; McWhirter, L.; Staab, J.P.; Stone, J. Functional neurological disorder: New subtypes and shared mechanisms. Lancet Neurol. 2022, 21, 537–550. [Google Scholar] [CrossRef]
- Razjouyan, J.; Grewal, G.S.; Rishel, C.; Parthasarathy, S.; Mohler, J.; Najafi, B. Activity Monitoring and Heart Rate Variability as Indicators of Fall Risk: Proof-of-Concept for Application of Wearable Sensors in the Acute Care Setting. J. Gerontol. Nurs. 2017, 43, 53–62. [Google Scholar] [CrossRef]
- Razjouyan, J.; Grewal, G.S.; Talal, T.K.; Armstrong, D.G.; Mills, J.L.; Najafi, B. Does Physiological Stress Slow Down Wound Healing in Patients With Diabetes? J. Diabetes Sci. Technol. 2017, 11, 685–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makowski, D.; Pham, T.; Lau, Z.J.; Brammer, J.C.; Lespinasse, F.; Pham, H.; Schölzel, C.; Chen, S.H. NeuroKit2: A Python toolbox for neurophysiological signal processing. Behav. Res. Methods 2021, 53, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Maaten, L.V.D.; Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Iranzo, A.; Stefani, A.; Niñerola-Baizan, A.; Stokner, H.; Serradell, M.; Vilas, D.; Holzknecht, E.; Gaig, C.; Pavia, J.; Lomeña, F.; et al. Left-hemispheric predominance of nigrostriatal deficit in isolated REM sleep behavior disorder. Neurology 2020, 94, e1605–e1613. [Google Scholar] [CrossRef]
- Jang, W.; Lee, J.Y.; Kim, J.Y.; Lee, S.J.; Kim, T.Y.; Choi, Y.Y.; Kim, H.T.; Kim, C.K. Intrasubject relationship between striatal 18F-FP-CIT uptake and cardiac 123I-MIBG uptake differs by motor subtype in early Parkinson disease. Medicine 2021, 100, e26995. [Google Scholar] [CrossRef]




| Patients | ||||||
|---|---|---|---|---|---|---|
| Sex (M/F) | Age (years) | Weight (kg) | Height (m) | BMI (kg/m2) | Co-Morbidities | |
| 1 | F | 75 | 116.12 | 1.74 | 38.36 | PD, Headaches, Cancer, COPD, HTN |
| 2 | M | 84 | 77.11 | 1.83 | 23.06 | Diabetes, Cancer |
| 3 | F | 68 | 81.65 | 1.7 | 28.19 | Asthma, Vertigo, |
| * 5 | M | 74 | 77.56 | 1.73 | 26 | PD, Neuropathy, HTN, Pain |
| 7 | M | 73 | 95.25 | 1.8 | 29.29 | MSA, GERD, Hypercholesterolemia |
| 8 | M | 53 | 81.65 | 1.83 | 24.41 | ADHD, Anxiety, Seizures |
| * 9 | M | 70 | 69.4 | 1.78 | 21.95 | Hypercholesterolemia, Diabetes, HTN, Dementia |
| * 11 | M | 71 | 86.18 | 1.78 | 27.26 | PD, GERD, HTN, COPD, BPH |
| 12 | F | 62 | 63.5 | 1.6 | 24.8 | Pain, ADHD, Anxiety, Mental Health, Depression, GERD, Cancer, Pancreatitis |
| 13 | M | 62 | 71.67 | 1.75 | 23.33 | PD |
| 16 | M | 78 | 83.91 | 1.78 | 26.54 | PS, HTN, Mood Disorder, Dementia, Sleep Apnea, Neuropathy |
| 17 | F | 76 | 63.5 | 1.63 | 24.03 | PS, Cancer, HTN, Depression, Colitis, Raynaud’s Syndrome, Hypercholesterolemia, Pain |
| 18 | F | 81 | 53.52 | 1.55 | 22.3 | Depression, Lupus, Stroke, HTN, Hypercholesterolemia |
| 19 | M | 69 | 104.33 | 1.83 | 31.19 | |
| 20 | M | 80 | 73.48 | 1.68 | 26.15 | Hypercholesterolemia, Diabetes, HTN |
| 21 | F | 53 | 108.86 | 1.65 | 39.94 | Angina, HTN |
| 22 | F | 75 | 61.23 | 1.68 | 21.79 | GERD, Pain, Fibromyalgia, Hypercholesterolemia |
| 23 | M | 75 | 86.18 | 1.65 | 31.62 | |
| 24 | M | 82 | 99.79 | 1.75 | 32.49 | HTN, Osteoarthritis, BPH, GERD, Gout |
| 25 | M | 73 | 83.01 | 1.8 | 25.52 | GERD, Heart Disease, Hypercholesterolemia, Asthma |
| * 26 | F | 84 | 64.41 | 1.6 | 25.15 | Pain, Arthritis, HTN |
| 27 | F | 73 | 56.7 | 1.5 | 25.2 | PS, Supranuclear Palsy, Depression, Arthritis |
| 29 | M | 65 | 72.57 | 1.68 | 25.8 | HTN, Stroke, Neuropathy, Lymphoma |
| 30 | M | 83 | 60.33 | 1.73 | 20.2 | HTN, Hyperlipidemia, Seizures |
| 31 | M | 74 | 77.11 | 1.63 | 29.2 | Depression, Tremors, HTN |
| 32 | F | 71 | 81.65 | 1.57 | 32.9 | Hypothyrodism, Migraines, Sleep Apnea, GERD, Depression, Hypercholesterolemia |
| 33 | F | 71 | 58.51 | 1.68 | 20.8 | PS, Cancer, Hypercholesterolemia, HTN, Dementia, Depression, Anxiety, Hypothyroidism |
| 34 | M | 73 | 86.18 | 1.75 | 28.1 | HTN, Depression, GERD |
| 35 | M | 72 | 73.48 | 1.68 | 26.1 | HTN, GERD, BPH, Depression, Hypercholesterolemia |
| * 36 | M | 41 | 102.97 | 1.85 | 29.9 | PS, HTN |
| 37 | F | 71 | 52.16 | 1.65 | 19.1 | Dementia, Tremors, Hypercholesterolemia, Neuropathy, Diarrhea |
| 38 | M | 73 | 127.01 | 1.88 | 35.9 | HTN, BPH, Hypercholesterolemia, Sleep Apnea, CABG |
| 39 | M | 77 | 84.82 | 1.75 | 27.6 | Tremor, Achalasia, Cancer, CABG, Hypothyroidism, Hypercholesterolemia, Osteoporosis |
| 40 | F | 66 | 95.25 | 1.52 | 41 | MI, Diabetes, Osteoarthritis, CKD, Hypothyroidism, Depression, Hypercholesterolemia, GERD, BPH |
| 41 | M | 64 | 108.86 | 1.78 | 34.4 | PS, HTN, Hypercholesterolemia, Hypothyroidism |
| * 42 | M | 76 | 85.28 | 1.7 | 29.4 | HTN, Neuropathy, Pain, Gait Instability |
| 43 | M | 69 | 81.65 | 1.73 | 27.4 | PD, Stroke, HTN, Hypercholesterolemia, BPH, Depression, Arthritis, Diabetes |
| 44 | M | 84 | 81.65 | 1.78 | 25.8 | BPH, Pain, Dementia, Neuropathy, Glaucoma |
| 45 | M | 75 | 87.54 | 1.85 | 25.5 | Diabetes, Glaucoma, GERD |
| 46 | M | 77 | 62.5 | HTN | ||
| 47 | F | 60 | 67.59 | 1.63 | 25.6 | HTN, Dystonia, Arthritis |
| 48 | M | 78 | 76.2 | 1.7 | 26.3 | HTN, Stroke, Abdominal Aneurysm |
| 49 | M | 68 | 72.57472 | 1.73 | 24.3 | |
| 50 | M | 74 | 123.83062 | 1.75 | 40.3 | |
| 51 | M | 75 | 89.36 | 1.73 | 29.7 | HTN, Cancer, Hypothyroidism |
| Medications | Number of Patients | Number of Analyzed Patients | |
|---|---|---|---|
| Antihypertensives | β-blocker | 14 | 1 |
| ACE-I/ARB | 19 | 6 | |
| Other | 12 | 2 | |
| PD/Parkinsonian/Dementia | Levadopa/Carbidopa | 12 | 4 |
| Cholinergic | 4 | 2 | |
| Other | 6 | 1 | |
| Additional Medications | Gabapentin/Anti-seizure | 12 | 4 |
| Opiate/Opioid | 5 | 1 | |
| Psychiatric Medications | 20 | 2 | |
| β-agonist | 2 | 2 | |
| Steroid | 3 | ||
| NSAIDs | 17 | 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warhadpande, D.S.; Huo, J.; Libling, W.A.; Stuehm, C.; Najafi, B.; Sherman, S.; Lei, H.; Roveda, J.M.; Kuo, P.H. Pilot Study for Correlation of Heart Rate Variability and Dopamine Transporter Brain Imaging in Patients with Parkinsonian Syndrome. Sensors 2022, 22, 5055. https://doi.org/10.3390/s22135055
Warhadpande DS, Huo J, Libling WA, Stuehm C, Najafi B, Sherman S, Lei H, Roveda JM, Kuo PH. Pilot Study for Correlation of Heart Rate Variability and Dopamine Transporter Brain Imaging in Patients with Parkinsonian Syndrome. Sensors. 2022; 22(13):5055. https://doi.org/10.3390/s22135055
Chicago/Turabian StyleWarhadpande, Devdutta S., Jiayan Huo, William A. Libling, Carol Stuehm, Bijan Najafi, Scott Sherman, Hong Lei, Janet Meiling Roveda, and Phillip H. Kuo. 2022. "Pilot Study for Correlation of Heart Rate Variability and Dopamine Transporter Brain Imaging in Patients with Parkinsonian Syndrome" Sensors 22, no. 13: 5055. https://doi.org/10.3390/s22135055
APA StyleWarhadpande, D. S., Huo, J., Libling, W. A., Stuehm, C., Najafi, B., Sherman, S., Lei, H., Roveda, J. M., & Kuo, P. H. (2022). Pilot Study for Correlation of Heart Rate Variability and Dopamine Transporter Brain Imaging in Patients with Parkinsonian Syndrome. Sensors, 22(13), 5055. https://doi.org/10.3390/s22135055









