Smartphone-Based Electrochemical Systems for Glucose Monitoring in Biofluids: A Review
Abstract
1. Introduction
| Biofluid | Physiological Range (mM) | Pathological Range (mM) |
|---|---|---|
| Blood | 3.9–6.1 [26] | 2–40 [27] |
| Sweat | 0.02–0.6 [28] | 0.01–1 [29] |
| Saliva | 0.23–0.38 [30] | 0.55–1.77 [31] |
| Tear | 0.05–0.5 [32] | 0.5–5 [30,33] |
| Interstitial Fluid | 3.9–6.6 [30] | 1.99–22.2 [34] |
| Urine | 2.78–5.55 [35] | >5.55 [35] |
| Ascitic Fluid | Similar to blood glucose [36] | <2.78 [37] |
2. Capabilities of Smartphones in Electrochemical Monitoring
| Technology Names | Transceiver Type | Operating Frequency | Data Rate | Working Ranges | Power Consumption | Power Source | Continuous Monitoring | Sensor Network | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Bluetooth | Active radio | 2.4~5 GHz | <24 Mbps | 10~100 m | Medium | Battery | Yes | Yes | [55,56,57,58,59] |
| NFC | Passive transponder | 13.56 MHz | <424 Kbps | <5 cm | Very Low | Battery-free | No | No | [60,61,62] |
| Zigbee | Active radio | 868 MHz, 915 MHz, 2.4 GHz | 250 Kbps | 10~100 m | Low | Battery | Yes | Yes | [63,64,65] |
| Infrared | Active radio | 330~350 THz | 0.1~1 Mbps | <10 m | Medium | Battery | Yes | No | [66,67] |
| WiFi | Active radio | 2.4~5 GHz | 54 Mbps | 10~100 m | High | Battery | Yes | Yes | [68,69] |
| Ultrawideband | Active radio | 3.1~10.6 GHz | <480 Mbps | <10 m | Medium | Battery | Yes | Yes | [70,71,72] |
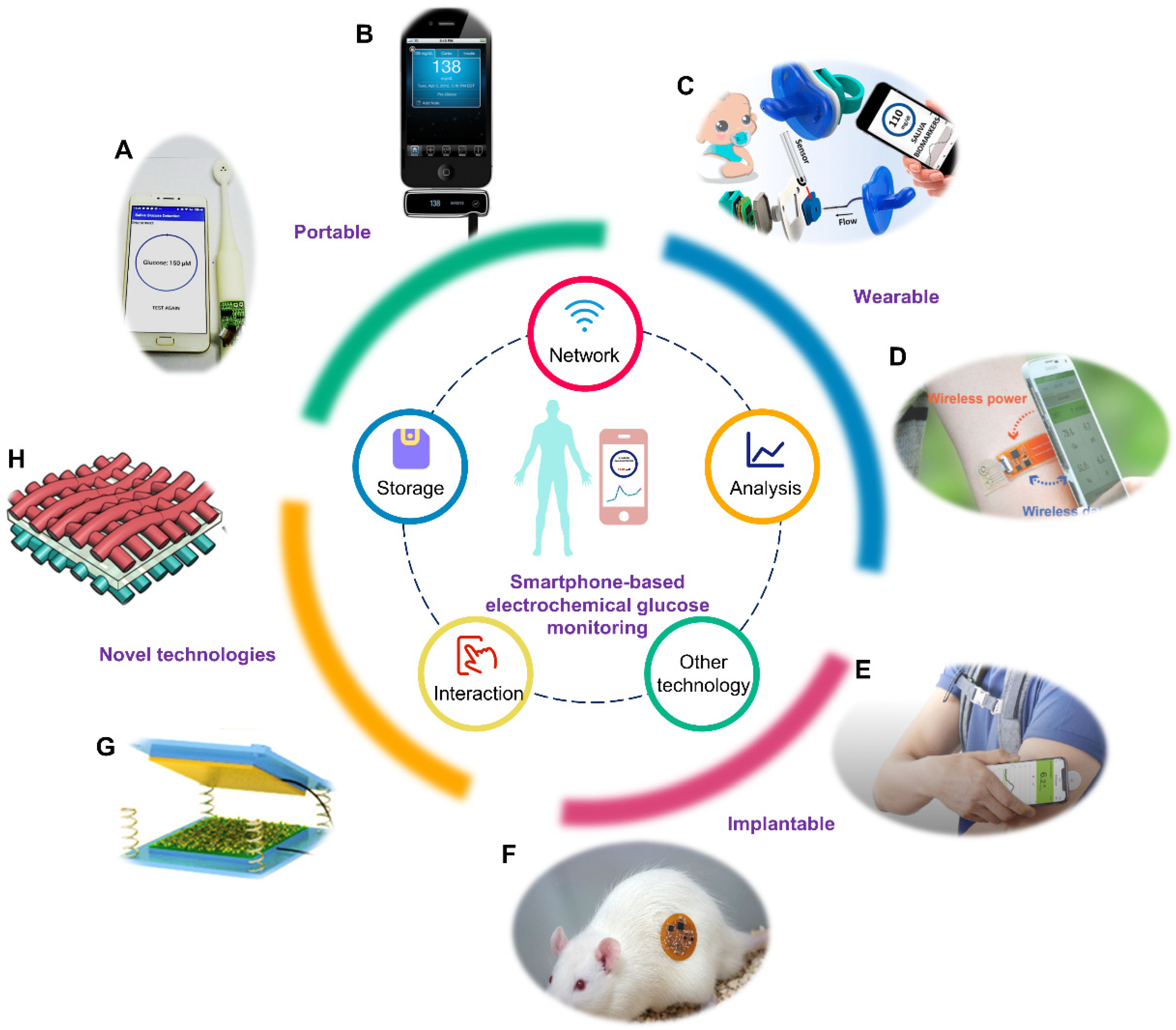
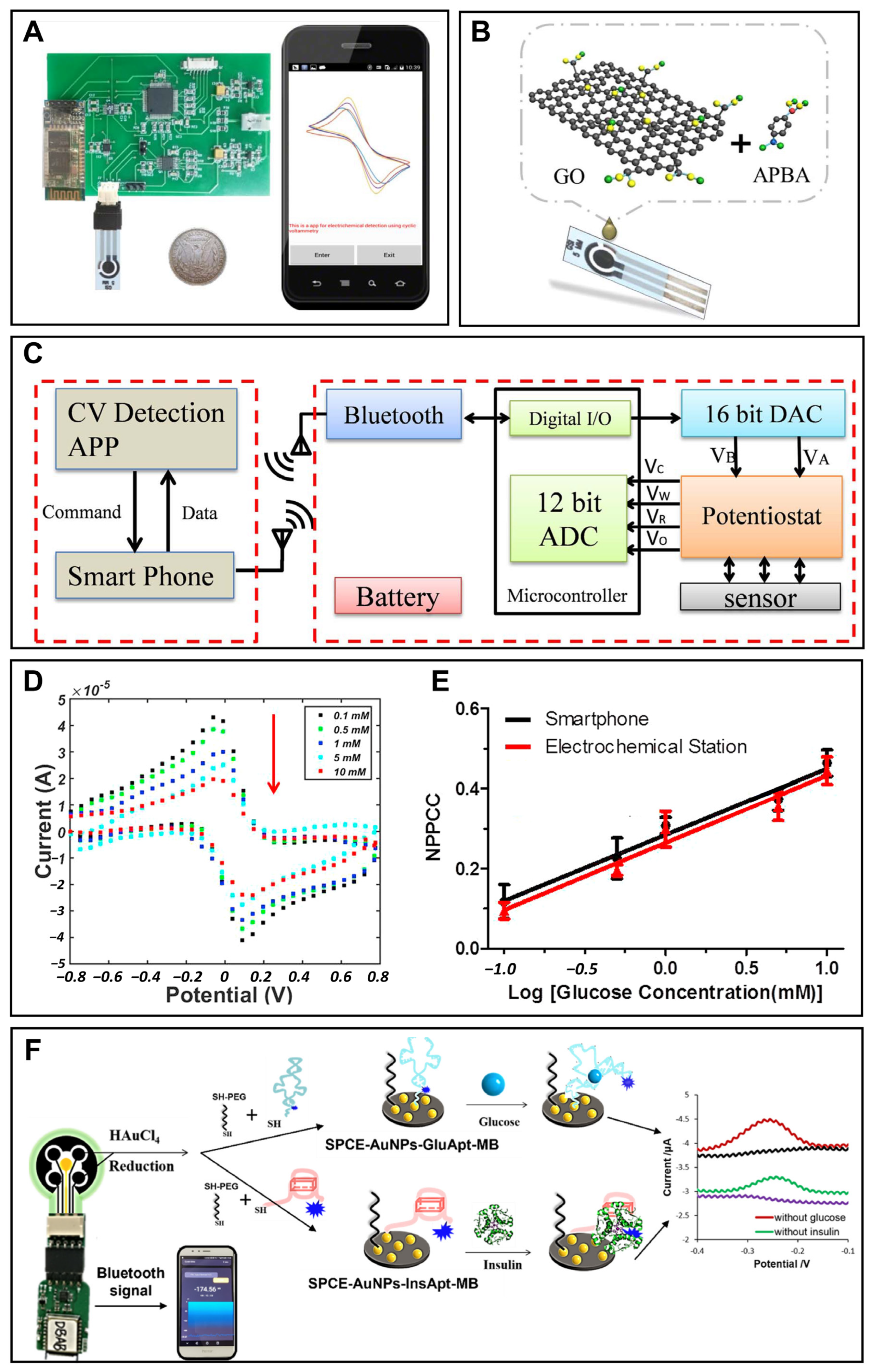
3. Smartphone-Based Portable Glucose Monitoring
3.1. Portable Blood Glucose Monitoring
3.2. Portable Urine Glucose Monitoring
3.3. Portable Saliva Glucose Monitoring
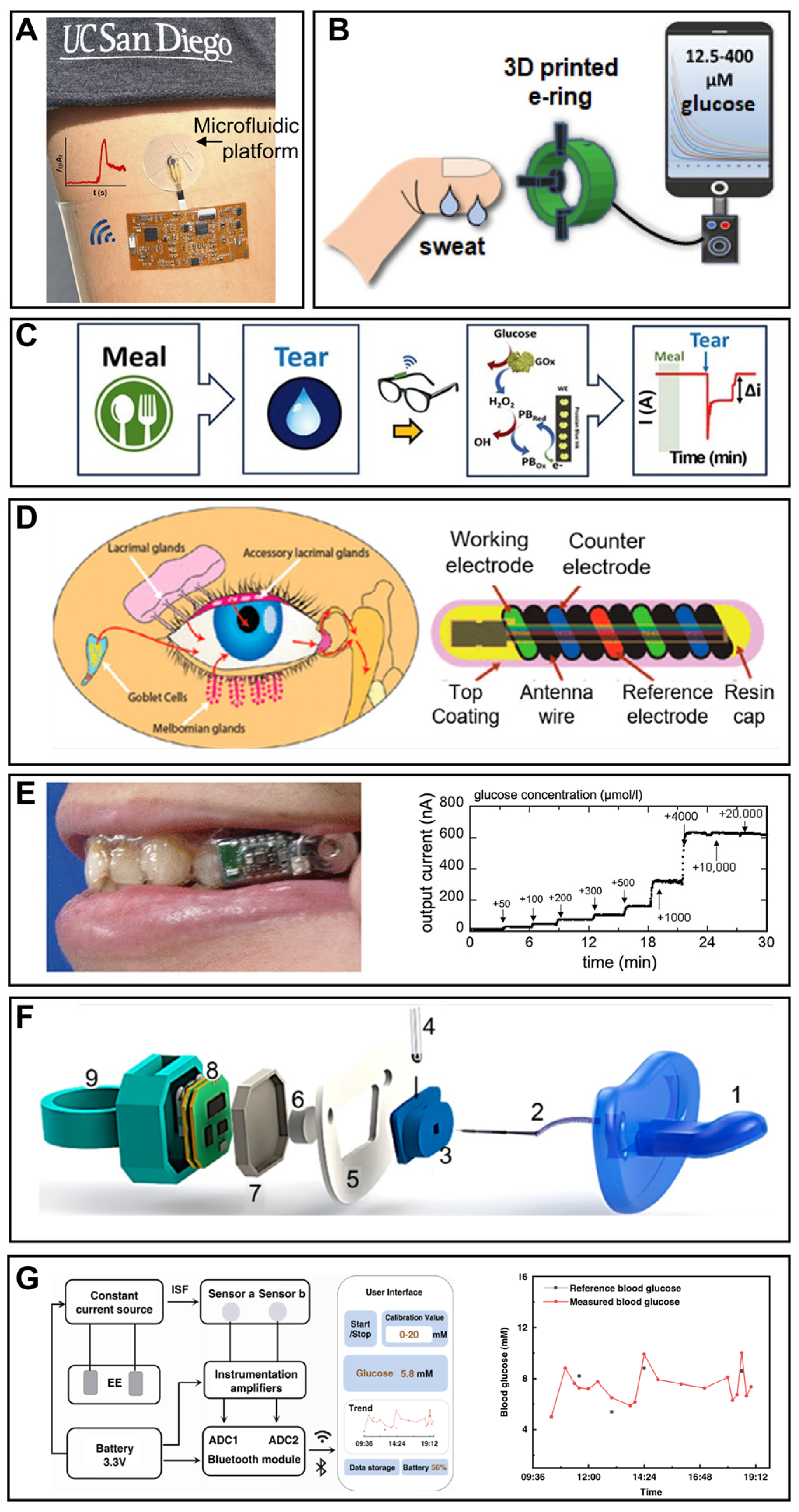
4. Smartphone-Based Wearable Glucose Monitoring
4.1. Wearable Sweat Glucose Monitoring
4.2. Wearable Tear Glucose Monitoring
4.3. Wearable Saliva Glucose Monitoring
4.4. Wearable Interstitial Glucose Monitoring
5. Smartphone-Based Implantable Glucose Monitoring
5.1. Implantable Blood Glucose Monitoring
5.2. Implantable Glucose Monitoring in Other Biofluids
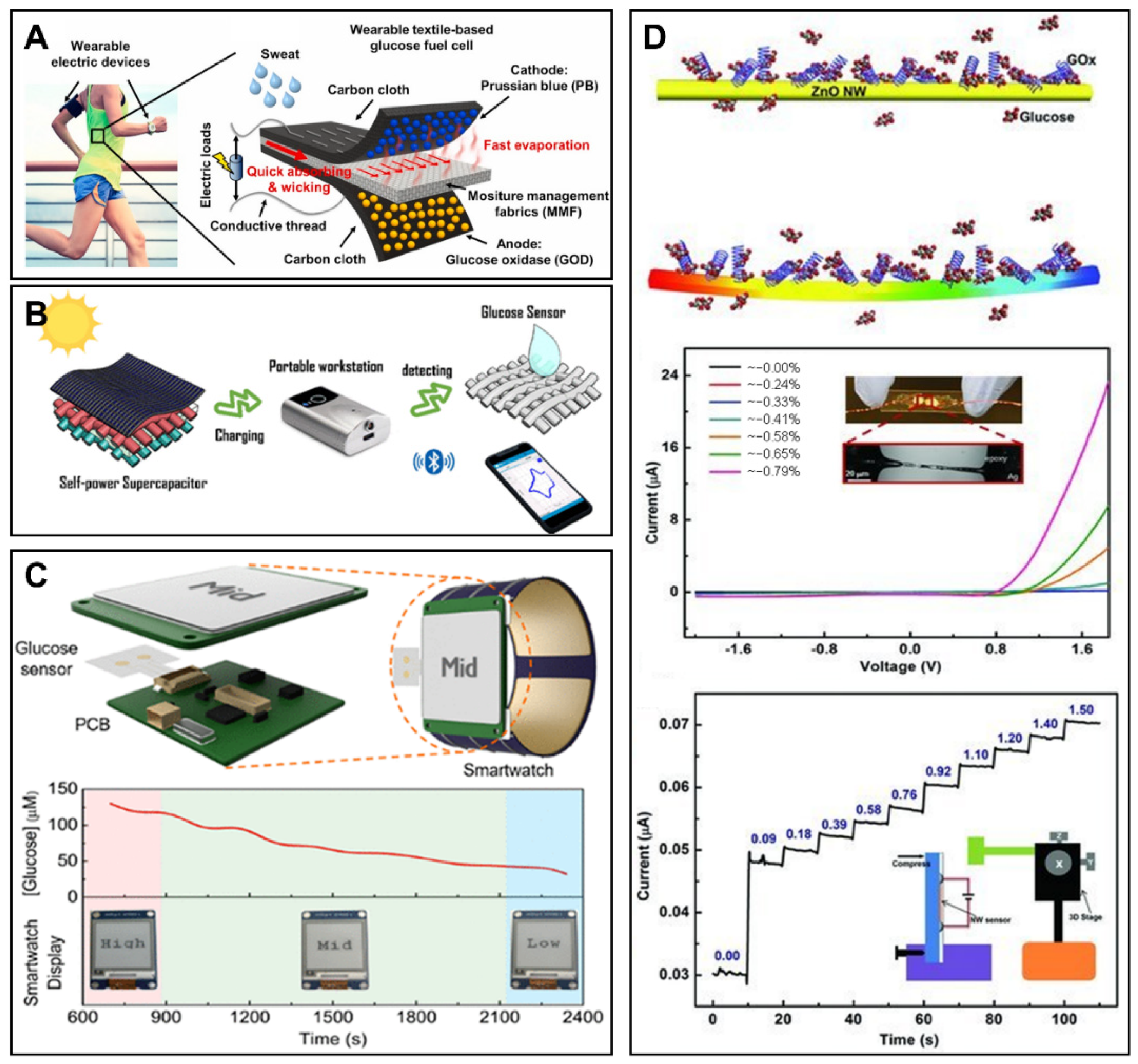
6. Novel Technologies for Glucose Monitoring
6.1. Biofuel Cells for Glucose Monitoring
6.2. Supercapacitors for Glucose Monitoring
6.3. Other Energy Supply Technologies for Glucose Sensors
7. Summary
8. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2017, 102, 4343–4410. [Google Scholar] [CrossRef]
- Ikegami, H.; Babaya, N.; Noso, S. Beta-Cell failure in diabetes: Common susceptibility and mechanisms shared between type 1 and type 2 diabetes. J. Diabetes Investig. 2021, 12, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Nanjo, K.; Tajima, N.; Kadowaki, T.; Kashiwagi, A.; Araki, E.; Ito, C.; Inagaki, N.; Iwamoto, Y.; Kasuga, M. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetol. Int. 2010, 1, 2–10. [Google Scholar] [CrossRef]
- Polak, M.; da Costa, A.T. Diagnostic Value of the Estimation of Glucose in Ascitic Fluid. Digestion 1973, 8, 347–352. [Google Scholar] [CrossRef]
- Heidari, K.; Amiri, M.; Kariman, H.; Bassiri, M.; Alimohammadi, H.; Hatamabadi, H.R. Differentiation of exudate from transudate ascites based on the dipstick values of protein, glucose, and pH. Am. J. Emerg. Med. 2013, 31, 779–782. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.A.; Pincus, M.R. Henry’s Clinical Diagnosis and Management by Laboratory Methods, 23rd ed.; Elsevier: New York, NY, USA, 2017; pp. 481–509. [Google Scholar]
- Dumitrescu, E.; Andreescu, S. Bioapplications of Electrochemical Sensors and Biosensors. In Enzymes as Sensors; Thompson, R.B., Fierke, C.A., Eds.; Elsevier: New York, NY, USA, 2017; Volume 589, pp. 301–350. [Google Scholar]
- Hussain, C.M. Handbook on Miniaturization in Analytical Chemistry: Application of Nanotechnology; Elsevier: New York, NY, USA, 2020. [Google Scholar]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Pohanka, M.; Skládal, P. Electrochemical biosensors—Principles and applications. J. Appl. Biomed. 2008, 6, 57–64. [Google Scholar] [CrossRef]
- Sehit, E.; Altintas, Z. Significance of nanomaterials in electrochemical glucose sensors: An updated review (2016–2020). Biosens. Bioelectron. 2020, 159, 112165. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Lee, A.-R. Recent developments in blood glucose sensors. J. Food Drug Anal. 2015, 23, 191–200. [Google Scholar] [CrossRef]
- Ferri, S.; Kojima, K.; Sode, K. Review of glucose oxidases and glucose dehydrogenases: A bird’s eye view of glucose sensing enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068. [Google Scholar] [CrossRef]
- Schleis, T.G. Interference of maltose, icodextrin, galactose, or xylose with some blood glucose monitoring systems. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2007, 27, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Lim, C.G.; Ellison, J.M.; Montandon, C.M. Review of Adverse Events Associated With False Glucose Readings Measured by GDH-PQQ–Based Glucose Test Strips in the Presence of Interfering Sugars. Diabetes Care 2010, 33, 728–729. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.-W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef]
- Cheng, C.; Li, X.; Xu, G.; Lu, Y.; Low, S.S.; Liu, G.; Zhu, L.; Li, C.; Liu, Q. Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat cortisol via near field communication. Biosens. Bioelectron. 2020, 172, 112782. [Google Scholar] [CrossRef]
- Xu, G.; Cheng, C.; Liu, Z.; Yuan, W.; Wu, X.; Lu, Y.; Low, S.S.; Liu, J.; Zhu, L.; Ji, D.; et al. Battery-Free and Wireless Epidermal Electrochemical System with All-Printed Stretchable Electrode Array for Multiplexed In Situ Sweat Analysis. Adv. Mater. Technol. 2019, 4, 1800658. [Google Scholar] [CrossRef]
- Heinemann, L. Finger pricking and pain: A never ending story. J. Diabetes Sci. Technol. 2008, 2, 919–921. [Google Scholar] [CrossRef]
- Jung, D.G.; Jung, D.; Kong, S.H. A lab-on-a-chip-based non-invasive optical sensor for measuring glucose in saliva. Sensors 2017, 17, 2607. [Google Scholar] [CrossRef]
- Shaker, G.; Smith, K.; Omer, A.E.; Liu, S.; Csech, C.; Wadhwa, U.; Safavi-Naeini, S.; Hughson, R. Non-Invasive Monitoring of Glucose Level Changes Utilizing a mm-Wave Radar System. Int. J. Mob. Hum. Comput. Interact. 2018, 10, 10–29. [Google Scholar] [CrossRef]
- Dimeski, G.; Jones, B.W.; Tilley, V.; Greenslade, M.N.; Russell, A.W. Glucose meters: Evaluation of the new formulation measuring strips from Roche (Accu-Chek) and Abbott (MediSense). Ann. Clin. Biochem. Int. J. Lab. Med. 2010, 47, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xiang, Z.; Fesenmaier, D.R. Smartphone Use in Everyday Life and Travel. J. Travel Res. 2016, 55, 52–63. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Moon, J.-M.; Wang, J. Touch-Based Fingertip Blood-Free Reliable Glucose Monitoring: Personalized Data Processing for Predicting Blood Glucose Concentrations. ACS Sens. 2021, 6, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation Between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef]
- Gupta, S.; Sandhu, S.V.; Bansal, H.; Sharma, D. Comparison of Salivary and Serum Glucose Levels in Diabetic Patients. J. Diabetes Sci. Technol. 2014, 9, 91–96. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. Ophthalmic Glucose Monitoring Using Disposable Contact Lenses—A Review. J. Fluoresc. 2004, 14, 617–633. [Google Scholar] [CrossRef]
- Koschinsky, T.; Heinemann, L. Sensors for glucose monitoring: Technical and clinical aspects. Diabetes Metab. Res. Rev. 2001, 17, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Urakami, T.; Morimoto, S.; Nitadori, Y.; Harada, K.; Owada, M.; Kitagawa, T. Urine Glucose Screening Program at Schools in Japan to Detect Children with Diabetes and Its Outcome-Incidence and Clinical Characteristics of Childhood Type 2 Diabetes in Japan. Pediatr. Res. 2007, 61, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Lu, X. Diagnostics, 9th ed.; People’s Medical Publishing House: Beijing, China, 2018. [Google Scholar]
- Burgess, E.A.; Sylvén, B. Changes in Glucose and Lactate Content of Ascites Fluid and Blood Plasma During Growth and Decay of the ELD Ascites Tumour. Br. J. Cancer 1962, 16, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, X.; Ju, Y.; Ma, B.; Zhao, C.; Liu, H. Electrocatalytic oxidation of glucose on bronze for monitoring of saliva glucose using a smart toothbrush. Sens. Actuators B Chem. 2019, 285, 56–61. [Google Scholar] [CrossRef]
- Medgadget. Available online: https://www.medgadget.com/2012/05/ibgstar-glucometer-for-iphone-now-available-in-u-s.html (accessed on 28 June 2022).
- García-Carmona, L.; Martín, A.; Sempionatto, J.R.; Moreto, J.R.; González, M.C.; Wang, J.; Escarpa, A. Pacifier Biosensor: Toward Noninvasive Saliva Biomarker Monitoring. Anal. Chem. 2019, 91, 13883–13891. [Google Scholar] [CrossRef] [PubMed]
- Laboratories. Available online: https://freestylelibre.com.sg/ (accessed on 28 June 2022).
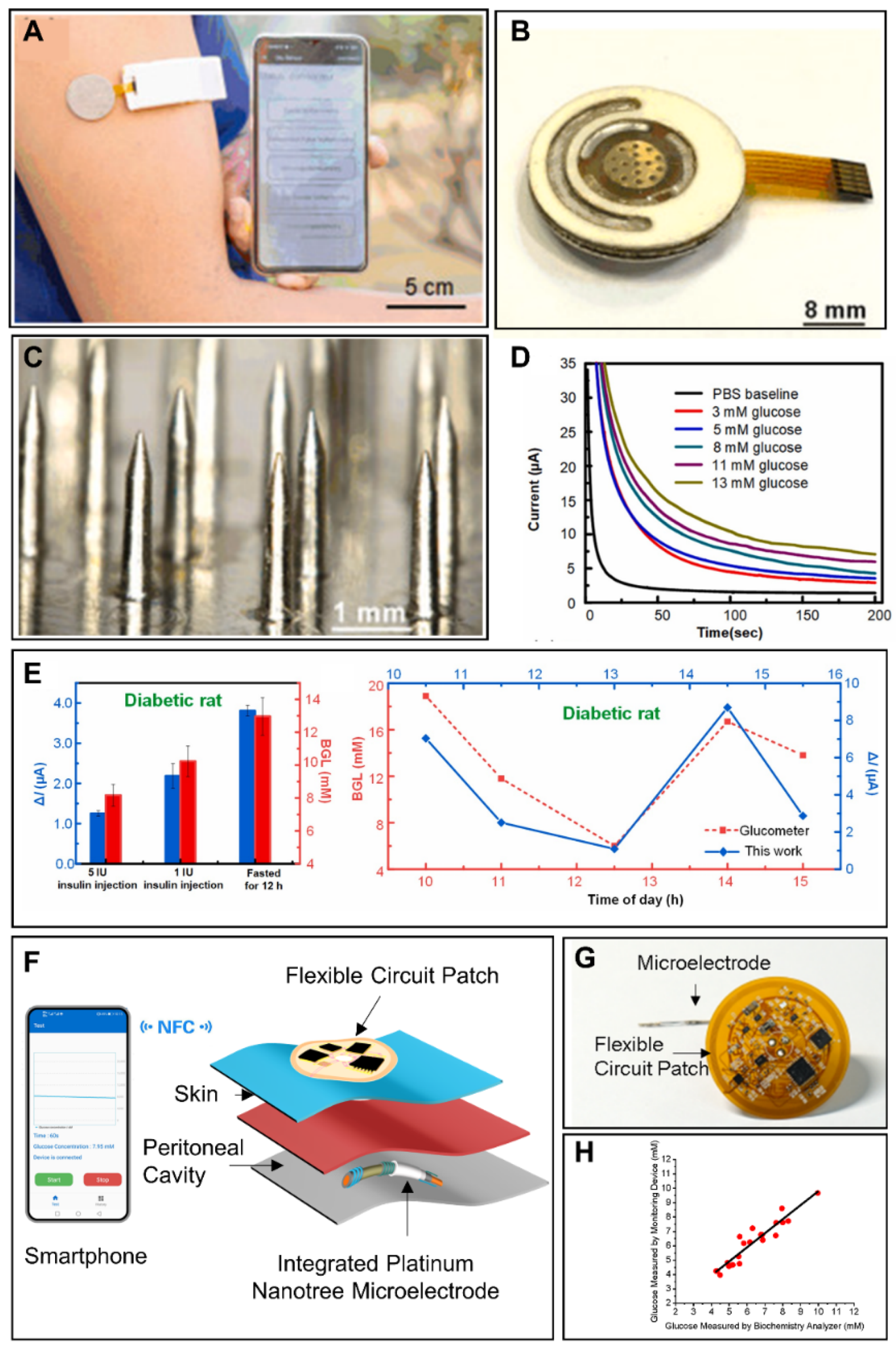
- Xu, J.; Cheng, C.; Li, X.; Lu, Y.; Hu, S.; Liu, G.; Zhu, L.; Wang, N.; Wang, L.; Cheng, P.; et al. Implantable platinum nanotree microelectrode with a battery-free electrochemical patch for peritoneal carcinomatosis monitoring. Biosens. Bioelectron. 2021, 185, 113265. [Google Scholar] [CrossRef]
- Lee, J.W.; Jung, S.; Jo, J.; Chae, H.Y.; Choi, K.; Kim, J.J.; Lee, J.H.; Yang, C.; Baik, J.M. High-Output Triboelectric Nanogenerator Based on Dual Inductive and Resonance Effects-Controlled Highly Transparent Polyimide for Self-Powered Sensor Network Systems. Adv. Energy Mater. 2019, 9, 1901987. [Google Scholar] [CrossRef]
- Sun, T.; Shen, L.; Jiang, Y.; Ma, J.; Lv, F.; Ma, H.; Chen, D.; Zhu, N. Wearable Textile Supercapacitors for Self-Powered Enzyme-Free Smartsensors. ACS Appl. Mater. Int. 2020, 12, 21779–21787. [Google Scholar] [CrossRef] [PubMed]
- Pahlavan, K.; Krishnamurthy, P. Principles of Wireless Networks; Prentice Hall PTR: Hoboken, NJ, USA, 2001; Volume 1. [Google Scholar]
- Kap, O.; Kılıç, V.; Hardy, J.G.; Horzum, N. Smartphone-based colorimetric detection systems for glucose monitoring in the diagnosis and management of diabetes. Analyst 2021, 146, 2784–2806. [Google Scholar] [CrossRef] [PubMed]
- Coskun, V.; Ozdenizci, B.; Ok, K. The Survey on Near Field Communication. Sensors 2015, 15, 13348–13405. [Google Scholar] [CrossRef]
- Kim, S.D.; Koo, Y.; Yun, Y. A Smartphone-Based Automatic Measurement Method for Colorimetric pH Detection Using a Color Adaptation Algorithm. Sensors 2017, 17, 1604. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.; Dinnes, J.; Chuchu, N.; Takwoingi, Y.; Bayliss, S.; Matin, R.N.; Jain, A.; Walter, F.M.; Williams, H.C.; Deeks, J.J. Algorithm based smartphone apps to assess risk of skin cancer in adults: Systematic review of diagnostic accuracy studies. BMJ 2020, 368, m127. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, S.; Liu, W.; Chen, J.; Yu, Q.; Zhang, Z.; Li, Y.; Liu, J.; Chen, X. Real time detection of 3-nitrotyrosine using smartphone-based electrochemiluminescence. Biosens. Bioelectron. 2021, 187, 113284. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Shah, V.; Akturk, H.K.; Beatson, C.; Snell-Bergeon, J.K. Role of Mobile Technology to Improve Diabetes Care in Adults with Type 1 Diabetes: The Remote-T1D Study iBGStar® in Type 1 Diabetes Management. Diabetes Ther. 2017, 8, 811–819. [Google Scholar] [CrossRef]
- Sami, M.A.; Tayyab, M.; Parikh, P.; Govindaraju, H.; Hassan, U. A modular microscopic smartphone attachment for imaging and quantification of multiple fluorescent probes using machine learning. Analyst 2021, 146, 2531–2541. [Google Scholar] [CrossRef]
- Hasan, M.K.; Haque, M.M.; Adib, R.; Tumpa, J.F.; Begum, A.; Love, R.R.; Kim, Y.L.; Sheikh, I.A. SmartHeLP: Smartphone-based hemoglobin level prediction using an artificial neural network. In Proceedings of the AMIA Annual Symposium Proceedings, San Francisco, CA, USA, 3–7 November 2018; p. 535. [Google Scholar]
- Ji, D.; Liu, Z.; Liu, L.; Low, S.S.; Lu, Y.; Yu, X.; Zhu, L.; Li, C.; Liu, Q. Smartphone-based integrated voltammetry system for simultaneous detection of ascorbic acid, dopamine, and uric acid with graphene and gold nanoparticles modified screen-printed electrodes. Biosens. Bioelectron. 2018, 119, 55–62. [Google Scholar] [CrossRef]
- Gargiulo, G.; Bifulco, P.; Cesarelli, M.; Ruffo, M.; Romano, M.; Calvo, R.A.; Jin, C.; van Schaik, A. An ultra-high input impedance ECG amplifier for long-term monitoring of athletes. Med. Devices 2010, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gil, B.; Anastasova, S.; Yang, G. A Smart Wireless Ear-Worn Device for Cardiovascular and Sweat Parameter Monitoring During Physical Exercise: Design and Performance Results. Sensors 2019, 19, 1616. [Google Scholar] [CrossRef]
- Huang, Z.; Hao, Y.; Li, Y.; Hu, H.; Wang, C.; Nomoto, A.; Pan, T.; Gu, Y.; Chen, Y.; Zhang, T.; et al. Three-dimensional integrated stretchable electronics. Nat. Electron. 2018, 1, 473–480. [Google Scholar] [CrossRef]
- Jang, J.; Hyun, B.G.; Ji, S.; Cho, E.; An, B.W.; Cheong, W.H.; Park, J.-U. Rapid production of large-area, transparent and stretchable electrodes using metal nanofibers as wirelessly operated wearable heaters. NPG Asia Mater. 2017, 9, e432. [Google Scholar] [CrossRef]
- Khan, Y.; Garg, M.; Gui, Q.; Schadt, M.; Gaikwad, A.; Han, D.; Yamamoto, N.A.D.; Hart, P.; Welte, R.; Wilson, W.; et al. Flexible Hybrid Electronics: Direct Interfacing of Soft and Hard Electronics for Wearable Health Monitoring. Adv. Funct. Mater. 2016, 26, 8764–8775. [Google Scholar] [CrossRef]
- Jeong, H.; Wang, L.; Ha, T.; Mitbander, R.; Yang, X.; Dai, Z.; Qiao, S.; Shen, L.; Sun, N.; Lu, N. Modular and Reconfigurable Wireless E-Tattoos for Personalized Sensing. Adv. Mater. Technol. 2019, 4, 1900117. [Google Scholar] [CrossRef]
- Kim, J.; Banks, A.; Xie, Z.; Heo, S.Y.; Gutruf, P.; Lee, J.W.; Xu, S.; Jang, K.-I.; Liu, F.; Brown, G.; et al. Miniaturized Flexible Electronic Systems with Wireless Power and Near-Field Communication Capabilities. Adv. Funct. Mater. 2015, 25, 4761–4767. [Google Scholar] [CrossRef]
- Krishnan, S.R.; Su, C.J.; Xie, Z.; Patel, M.; Madhvapathy, S.R.; Xu, Y.; Freudman, J.; Ng, B.; Heo, S.Y.; Wang, H. Wireless, battery-free epidermal electronics for continuous, quantitative, multimodal thermal characterization of skin. Small 2018, 14, 1803192. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jung, S.M.; Lee, C.K.; Jeong, K.S.; Cho, G.; Yoo, S.K. Wearable ECG monitoring system using conductive fabrics and active electrodes. In Proceedings of the International Conference on Human-Computer Interaction, San Diego, CA, USA, 19–24 July 2009; pp. 778–783. [Google Scholar]
- Llorente-Alonso, A.; Peña-Poza, J.; de Arcas, G.; Garcia-Heras, M.; López, J.; Villegas, M.-A. Interface electronic system for measuring air acidity with optical sensors. Sens. Actuators A Phys. 2013, 194, 67–74. [Google Scholar] [CrossRef]
- Lorwongtragool, P.; Sowade, E.; Watthanawisuth, N.; Baumann, R.R.; Kerdcharoen, T. A Novel Wearable Electronic Nose for Healthcare Based on Flexible Printed Chemical Sensor Array. Sensors 2014, 14, 19700–19712. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, P.; Wang, B.; Wang, J.; Wang, Q.; Chen, Y.; Pfefer, T.J. Evaluation of Mobile Phone Performance for Near-Infrared Fluorescence Imaging. IEEE Trans. Biomed. Eng. 2016, 64, 1650–1653. [Google Scholar] [CrossRef]
- Pügner, T.; Knobbe, J.; Grüger, H. Near-Infrared Grating Spectrometer for Mobile Phone Applications. Appl. Spectrosc. 2016, 70, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Farshad, A.; Li, J.; Marina, M.K.; Garcia, F.J. A microscopic look at WiFi fingerprinting for indoor mobile phone localization in diverse environments. In Proceedings of the International Conference on Indoor Positioning and Indoor Navigation, Montbeliard, France, 28–31 October 2013; pp. 1–10. [Google Scholar]
- Zou, H.; Chen, Z.; Jiang, H.; Xie, L.; Spanos, C. Accurate indoor localization and tracking using mobile phone inertial sensors, WiFi and iBeacon. In Proceedings of the 2017 IEEE International Symposium on Inertial Sensors and Systems (INERTIAL), Kauai, HI, USA, 27–30 March 2017; pp. 1–4. [Google Scholar]
- Gomez-Villanueva, R.; Linares-y-Miranda, R.; Tirado-Mendez, J.A.; Jardon-Aguilar, H. Ultra-wideband planar inverted-F antenna (PIFA) for mobile phone frequencies and ultra-wideband applications. Prog. Electromagn. Res. C 2013, 43, 109–120. [Google Scholar] [CrossRef][Green Version]
- Wu, C.-H.; Wong, K.-L. Ultrawideband PIFA With a Capacitive Feed for Penta-Band Folder-Type Mobile Phone Antenna. IEEE Trans. Antennas Propag. 2009, 57, 2461–2464. [Google Scholar] [CrossRef]
- Zhang, Z.; Langer, J.-C.; Li, K.; Iskander, M. Design of Ultrawideband Mobile Phone Stubby Antenna (824 MHz-6 GHz). IEEE Trans. Antennas Propag. 2008, 56, 2107–2111. [Google Scholar] [CrossRef]
- Hatada, M.; Wilson, E.; Khanwalker, M.; Probst, D.; Okuda-Shimazaki, J.; Sode, K. Current and future prospective of biosensing molecules for point-of-care sensors for diabetes biomarker. Sens. Actuators B Chem. 2022, 351, 130914. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Marmisollé, W.A.; Knoll, W.; Azzaroni, O. Highly sensitive urine glucose detection with graphene field-effect transistors functionalized with electropolymerized nanofilms. Sens. Diagn. 2022, 1, 139–148. [Google Scholar] [CrossRef]
- Jędrzak, A.; Kuznowicz, M.; Rębiś, T.; Jesionowski, T. Portable glucose biosensor based on polynorepinephrine@magnetite nanomaterial integrated with a smartphone analyzer for point-of-care application. Bioelectrochemistry 2022, 145, 108071. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.Y.; Seo, M.; Kim, Y.; Hong, H.; Chung, T.D. Paper-based electrochromic glucose sensor with polyaniline on indium tin oxide nanoparticle layer as the optical readout. Biosens. Bioelectron. 2022, 203, 114002. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Liu, L.; Li, S.; Chen, C.; Lu, Y.; Wu, J.; Liu, Q. Smartphone-based cyclic voltammetry system with graphene modified screen printed electrodes for glucose detection. Biosens. Bioelectron. 2017, 98, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Kawahara, S.; Fuchigami, Y.; Shimokawa, S.; Nakamura, Y.; Fukayama, K.; Kamahori, M.; Uno, S. Portable Electrochemical Sensing System Attached to Smartphones and Its Incorporation with Paper-based Electrochemical Glucose Sensor. Int. J. Electr. Comput. Eng. IJECE 2017, 7, 1423. [Google Scholar] [CrossRef][Green Version]
- Guo, J.; Huang, X.; Ma, X. Clinical identification of diabetic ketosis/diabetic ketoacidosis acid by electrochemical dual channel test strip with medical smartphone. Sens. Actuators B Chem. 2018, 275, 446–450. [Google Scholar] [CrossRef]
- Felix, S.; Grace, A.N.; Jayavel, R. Sensitive electrochemical detection of glucose based on Au-CuO nanocomposites. J. Phys. Chem. Solids 2018, 122, 255–260. [Google Scholar] [CrossRef]
- Janmee, N.; Preechakasedkit, P.; Rodthongkum, N.; Chailapakul, O.; Potiyaraj, P.; Ruecha, N. A non-enzymatic disposable electrochemical sensor based on surface-modified screen-printed electrode CuO-IL/rGO nanocomposite for a single-step determination of glucose in human urine and electrolyte drinks. Anal Methods 2021, 13, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shen, Z.; Deng, L.; Liu, G. Smartphone assisted portable biochip for non-invasive simultaneous monitoring of glucose and insulin towards precise diagnosis of prediabetes/diabetes. Biosens. Bioelectron. 2022, 209, 114251. [Google Scholar] [CrossRef] [PubMed]
- Marsenic, O. Glucose Control by the Kidney: An Emerging Target in Diabetes. Am. J. Kidney Dis. 2009, 53, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Zeni, L.; Norden, A.G.W.; Cancarini, G.; Unwin, R.J. A more tubulocentric view of diabetic kidney disease. J. Nephrol. 2017, 30, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Jha, S.K. Smartphone based non-invasive salivary glucose biosensor. Anal. Chim. Acta 2017, 996, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Abbas, G.; Li, N.; Afzal, A.; Haider, Z.; Ahmed, S.; Xu, X.; Pan, C.; Peng, Z. Significance of Flexible Substrates for Wearable and Implantable Devices: Recent Advances and Perspectives. Adv. Mater. Technol. 2021, 7, 2100773. [Google Scholar] [CrossRef]
- Li, M.S.; Wong, H.L.; Ip, Y.L.; Peng, Z.; Yiu, R.; Yuan, H.; Wong, J.K.W.; Chan, Y.K. Current and Future Perspectives on Microfluidic Tear Analytic Devices. ACS Sens. 2022, 7, 1300–1314. [Google Scholar] [CrossRef]
- Bariya, M.; Davis, N.; Gillan, L.; Jansson, E.; Kokkonen, A.; McCaffrey, C.; Hiltunen, J.; Javey, A. Resettable Microfluidics for Broad-Range and Prolonged Sweat Rate Sensing. ACS Sens. 2022, 7, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Su, R.; Teng, L.; Tian, Q.; Han, F.; Li, H.; Cao, Z.; Xie, R.; Li, G.; Liu, X.; et al. Recent advances in flexible and wearable sensors for monitoring chemical molecules. Nanoscale 2022, 14, 1653–1669. [Google Scholar] [CrossRef]
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal Microfluidic Electrochemical Detection System: Enhanced Sweat Sampling and Metabolite Detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef]
- Zhu, X.; Ju, Y.; Chen, J.; Liu, D.; Liu, H. Nonenzymatic Wearable Sensor for Electrochemical Analysis of Perspiration Glucose. ACS Sens. 2018, 3, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Katseli, V.; Economou, A.; Kokkinos, C. Smartphone-Addressable 3D-Printed Electrochemical Ring for Nonenzymatic Self-Monitoring of Glucose in Human Sweat. Anal. Chem. 2021, 93, 3331–3336. [Google Scholar] [CrossRef]
- Han, J.H.; Cho, Y.C.; Koh, W.-G.; Bin Choy, Y. Preocular sensor system for concurrent monitoring of glucose levels and dry eye syndrome using tear fluids. PLoS ONE 2020, 15, e0239317. [Google Scholar] [CrossRef] [PubMed]
- Kownacka, A.E.; Vegelyte, D.; Joosse, M.; Anton, N.; Toebes, B.J.; Lauko, J.; Buzzacchera, I.; Lipinska, K.; Wilson, D.A.; Geelhoed-Duijvestijn, N.; et al. Clinical Evidence for Use of a Noninvasive Biosensor for Tear Glucose as an Alternative to Painful Finger-Prick for Diabetes Management Utilizing a Biopolymer Coating. Biomacromolecules 2018, 19, 4504–4511. [Google Scholar] [CrossRef] [PubMed]
- Keum, D.H.; Kim, S.-K.; Koo, J.; Lee, G.-H.; Jeon, C.; Mok, J.W.; Mun, B.H.; Lee, K.J.; Kamrani, E.; Joo, C.-K.; et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 2020, 6, eaba3252. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Kuroki, Y.; Nitta, H.; Chouhan, P.; Toma, K.; Sawada, S.-I.; Takeuchi, S.; Sekita, T.; Akiyoshi, K.; Minakuchi, S.; et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron. 2016, 84, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Tomoto, K.; Nitta, H.; Toma, K.; Takeuchi, S.; Sekita, T.; Minakuchi, S.; Mitsubayashi, K. A Wearable Cellulose Acetate-Coated Mouthguard Biosensor for In Vivo Salivary Glucose Measurement. Anal. Chem. 2020, 92, 12201–12207. [Google Scholar] [CrossRef]
- Kim, J.; Sempionatto, J.R.; Imani, S.; Hartel, M.C.; Barfidokht, A.; Tang, G.; Campbell, A.S.; Mercier, P.P.; Wang, J. Simultaneous Monitoring of Sweat and Interstitial Fluid Using a Single Wearable Biosensor Platform. Adv. Sci. 2018, 5, 1800880. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Li, H.; Zhang, N.; Jiang, X.; Yu, X.; Yang, Q.; Jin, Z.; Meng, H.; Chang, L. Highly integrated watch for noninvasive continual glucose monitoring. Microsyst. Nanoeng. 2022, 8, 25. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Brazaca, L.C.; García-Carmona, L.; Bolat, G.; Campbell, A.S.; Martin, A.; Tang, G.; Shah, R.; Mishra, R.K.; Kim, J.; et al. Eyeglasses-based tear biosensing system: Non-invasive detection of alcohol, vitamins and glucose. Biosens. Bioelectron. 2019, 137, 161–170. [Google Scholar] [CrossRef]
- Kim, S.; Lee, G.; Jeon, C.; Han, H.H.; Kim, S.; Mok, J.W.; Joo, C.; Shin, S.; Sim, J.; Myung, D.; et al. Bimetallic Nanocatalysts Immobilized in Nanoporous Hydrogels for Long-Term Robust Continuous Glucose Monitoring of Smart Contact Lens. Adv. Mater. 2022, 34, 2110536. [Google Scholar] [CrossRef]
- Malon, R.S.P.; Sadir, S.; Balakrishnan, M.; Córcoles, E.P. Saliva-Based Biosensors: Noninvasive Monitoring Tool for Clinical Diagnostics. Biomed Res. Int. 2014, 2014, 962903. [Google Scholar] [CrossRef] [PubMed]
- García-Guzmán, J.J.; Pérez-Ràfols, C.; Cuartero, M.; Crespo, G.A. Microneedle based electrochemical (Bio)Sensing: Towards decentralized and continuous health status monitoring. TrAC Trends Anal. Chem. 2021, 135, 116148. [Google Scholar] [CrossRef]
- Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Wu, X.; Liu, P.; Li, Y.; Saiyin, H.; et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.; Bode, B.W.; Christiansen, M.P.; Klaff, L.J.; Alva, S. The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol. Ther. 2015, 17, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Gong, X.; Yang, J.; Zheng, G.; Zheng, Y.; Li, Y.; Xu, Y.; Nie, G.; Xie, X.; Chen, M.; et al. A touch-actuated glucose sensor fully integrated with microneedle array and reverse iontophoresis for diabetes monitoring. Biosens. Bioelectron. 2022, 203, 114026. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huang, W.; Chen, Z.; Yi, C.; Jiang, L. Simultaneous detection of glucose, uric acid and cholesterol using flexible microneedle electrode array-based biosensor and multi-channel portable electrochemical analyzer. Sens. Actuators B Chem. 2019, 287, 102–110. [Google Scholar] [CrossRef]
- Schmidtke, D.W.; Heller, A. Accuracy of the One-Point in Vivo Calibration of “Wired” Glucose Oxidase Electrodes Implanted in Jugular Veins of Rats in Periods of Rapid Rise and Decline of the Glucose Concentration. Anal. Chem. 1998, 70, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, D.W.; Freeland, A.C.; Heller, A.; Bonnecaze, R.T. Measurement and modeling of the transient difference between blood and subcutaneous glucose concentrations in the rat after injection of insulin. Proc. Natl. Acad. Sci. USA 1998, 95, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhao, C.; Zhu, X.; Yuan, S.; Dong, X.; Zuo, Y.; Liu, H. Nonenzymatic Electrochemical Sensor for Wearable Interstitial Fluid Glucose Monitoring. Electroanalysis 2021, 34, 415–422. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Jamaledin, R.; Yiu, C.K.; Zare, E.N.; Niu, L.; Vecchione, R.; Chen, G.; Gu, Z.; Tay, F.R.; Makvandi, P. Advances in Antimicrobial Microneedle Patches for Combating Infections. Adv. Mater. 2020, 32, e2002129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, X.; Zhou, L.; Su, B. An Overview of Wearable and Implantable Electrochemical Glucose Sensors. Electroanalysis 2021, 34, 237–245. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Ramakrishna, S.; Aberle, A.G. Recent progress in flexible–wearable solar cells for self-powered electronic devices. Energy Environ. Sci. 2020, 13, 685–743. [Google Scholar] [CrossRef]
- Zheng, Q.; Tang, Q.; Wang, Z.L.; Li, Z. Self-powered cardiovascular electronic devices and systems. Nat. Rev. Cardiol. 2021, 18, 7–21. [Google Scholar] [CrossRef]
- Huang, C.; Chen, G.; Nashalian, A.; Chen, J. Advances in self-powered chemical sensing via a triboelectric nanogenerator. Nanoscale 2021, 13, 2065–2081. [Google Scholar] [CrossRef]
- Ferrari, I.V.; Pasquini, L.; Narducci, R.; Sgreccia, E.; Di Vona, M.L.; Knauth, P. A Short Overview of Biological Fuel Cells. Membranes 2022, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Jeerapan, I.; Sempionatto, J.R.; Pavinatto, A.; You, J.-M.; Wang, J. Stretchable biofuel cells as wearable textile-based self-powered sensors. J. Mater. Chem. A 2016, 4, 18342–18353. [Google Scholar] [CrossRef]
- Wang, C.; Shim, E.; Chang, H.-K.; Lee, N.; Kim, H.R.; Park, J. Sustainable and high-power wearable glucose biofuel cell using long-term and high-speed flow in sportswear fabrics. Biosens. Bioelectron. 2020, 169, 112652. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Song, Y.; Han, M.; Zhang, H. Portable and wearable self-powered systems based on emerging energy harvesting technology. Microsyst. Nanoeng. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nassar, J.; Xu, C.; Min, J.; Yang, Y.; Dai, A.; Doshi, R.; Huang, A.; Song, Y.; Gehlhar, R.; et al. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 2020, 5, eaaz7946. [Google Scholar] [CrossRef]
- Kil, H.-J.; Kim, S.-R.; Park, J.-W. A Self-Charging Supercapacitor for a Patch-Type Glucose Sensor. ACS Appl. Mater. Int. 2022, 14, 3838–3848. [Google Scholar] [CrossRef]
- Kim, J.; Khan, S.; Wu, P.; Park, S.; Park, H.; Yu, C.; Kim, W. Self-charging wearables for continuous health monitoring. Nano Energy 2021, 79, 105419. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, Y.; Wu, J.; Nyein, H.Y.Y.; Bariya, M.; Tai, L.-C.; Chao, M.; Ji, W.; Zhang, G.; Fan, Z.; et al. A Fully Integrated and Self-Powered Smartwatch for Continuous Sweat Glucose Monitoring. ACS Sens. 2019, 4, 1925–1933. [Google Scholar] [CrossRef]
- Yu, R.; Pan, C.; Chen, J.; Zhu, G.; Wang, Z.L. Enhanced Performance of a ZnO Nanowire-Based Self-Powered Glucose Sensor by Piezotronic Effect. Adv. Funct. Mater. 2013, 23, 5868–5874. [Google Scholar] [CrossRef]
- Balkourani, G.; Damartzis, T.; Brouzgou, A.; Tsiakaras, P. Cost Effective Synthesis of Graphene Nanomaterials for Non-Enzymatic Electrochemical Sensors for Glucose: A Comprehensive Review. Sensors 2022, 22, 355. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Wang, T.; Li, L.; Gong, J.; Zhang, L.; Chen, W. Copper oxide nanoleaves covered with loose nickel oxide nanoparticles for sensitive and selective non-enzymatic nitrite sensors. Mater. Res. Bull. 2022, 149, 111712. [Google Scholar] [CrossRef]
- Hong, S.Y.; Jee, S.M.; Ko, Y.; Cho, J.; Lee, K.H.; Yeom, B.; Kim, H.; Son, J.G. Intrinsically Stretchable and Printable Lithium-Ion Battery for Free-Form Configuration. ACS Nano 2022, 16, 2271–2281. [Google Scholar] [CrossRef]
- Costa, G.; Lopes, P.A.; Sanati, A.L.; Silva, A.F.; Freitas, M.C.; de Almeida, A.T.; Tavakoli, M. 3D Printed Stretchable Liquid Gallium Battery. Adv. Funct. Mater. 2022, 32, 2113232. [Google Scholar] [CrossRef]
- Sosale, B.; Sosale, A.R.; Murthy, H.; Sengupta, S.; Naveenam, M. Medios—An offline, smartphone-based artificial intelligence algorithm for the diagnosis of diabetic retinopathy. Indian J. Ophthalmol. 2020, 68, 391–395. [Google Scholar] [CrossRef]
- Chen, H.-C.; Tzeng, S.-S.; Hsiao, Y.-C.; Chen, R.-F.; Hung, E.-C.; Lee, O.K. Smartphone-Based Artificial Intelligence–Assisted Prediction for Eyelid Measurements: Algorithm Development and Observational Validation Study. JMIR Mhealth Uhealth 2021, 9, e32444. [Google Scholar] [CrossRef]
- Zajdel, T.J.; Shim, G.; Wang, L.; Rossello-Martinez, A.; Cohen, D.J. SCHEEPDOG: Programming Electric Cues to Dynamically Herd Large-Scale Cell Migration. Cell Syst. 2020, 10, 506–514. [Google Scholar] [CrossRef]
- Zhou, X.; Qu, M.; Tebon, P.; Jiang, X.; Wang, C.; Xue, Y.; Zhu, J.; Zhang, S.; Oklu, R.; Sengupta, S.; et al. Screening Cancer Immunotherapy: When Engineering Approaches Meet Artificial Intelligence. Adv. Sci. 2020, 7, 2001447. [Google Scholar] [CrossRef] [PubMed]
| Biofluid | Limit of Detection (μM) | Working Range (mM) | Ref. |
|---|---|---|---|
| Blood | 26 | 0.1–10 | [77] |
| Blood | 0 | 0–10 | [78] |
| Blood | - | 6.9–23.1 | [79] |
| Blood | - | 1.1–33.3 | [51] |
| Urine | 1.4 | 0.005–0.65 | [80] |
| Urine | 0.14 | 0.03–7 | [81] |
| Saliva | 6.64 | 0–0.32 | [38] |
| Saliva | 80 | 0.1–50 | [82] |
| Biofluid | Limit of Detection (μM) | Working Range (mM) | Ref. |
|---|---|---|---|
| Sweat | 50 | 2–10 | [90] |
| Sweat | - | 0.1–0.5 | [20] |
| Sweat | 15 | 0.03–1.1 | [91] |
| Sweat | 1.2 | 0.0125–0.4 | [92] |
| Tear | 0 | 0–1 | [93] |
| Tear | 0 | 0–20 | [94] |
| Tear | 0 | 0–2.78 | [95] |
| Saliva | - | 0.005–1 | [96] |
| Saliva | - | 0.00175–10 | [97] |
| Interstitial Fluid | 0 | 0–0.16 | [98] |
| Interstitial Fluid | - | 0.02–0.2 | [99] |
| Biofluid | Limit of Detection (μM) | Working Range (mM) | Ref. |
|---|---|---|---|
| Blood | 50 | 2.5–7 | [104] |
| Interstitial Fluid | - | 2.22–27.78 | [105] |
| Interstitial Fluid | 920 | 3–13 | [106] |
| Interstitial Fluid | 260 | 2–12 | [107] |
| Ascitic Fluid | 53.9 | 1–20 | [42] |
| System | Main Technologies | Convenience | Continuous Detection | Glucose Monitoring in Biofluids | ||
|---|---|---|---|---|---|---|
| Biofluid | Sampling Method | Correlation with Blood Glucose Level | ||||
| Portable system | Smartphone-related technologies, Miniaturized electrochemical circuit | Medium | No | Blood | Invasive | Equal |
| Urine, Saliva | Non-invasive | Debatable | ||||
| Wearable system | Smartphone-related technologies, Miniaturized electrochemical circuit, Flexible electronics | High | Yes | Sweat, Tear, Saliva | Non-invasive | Debatable |
| Interstitial fluid | Non-invasive, Irritation from RI | Correlated | ||||
| Implantable system | Smartphone-related technologies, Miniaturized electrochemical circuit, Flexible electronics, Biocompatible and antifouling microelectrode | High | Yes | Interstitial fluid | Minimally invasive | Correlated |
| Blood | Minimally invasive | Equal | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Yan, Z.; Liu, Q. Smartphone-Based Electrochemical Systems for Glucose Monitoring in Biofluids: A Review. Sensors 2022, 22, 5670. https://doi.org/10.3390/s22155670
Xu J, Yan Z, Liu Q. Smartphone-Based Electrochemical Systems for Glucose Monitoring in Biofluids: A Review. Sensors. 2022; 22(15):5670. https://doi.org/10.3390/s22155670
Chicago/Turabian StyleXu, Jie, Zupeng Yan, and Qingjun Liu. 2022. "Smartphone-Based Electrochemical Systems for Glucose Monitoring in Biofluids: A Review" Sensors 22, no. 15: 5670. https://doi.org/10.3390/s22155670
APA StyleXu, J., Yan, Z., & Liu, Q. (2022). Smartphone-Based Electrochemical Systems for Glucose Monitoring in Biofluids: A Review. Sensors, 22(15), 5670. https://doi.org/10.3390/s22155670







