Real Time Monitoring of Wine Vinegar Supply Chain through MOX Sensors
Abstract
:1. Introduction
- Consequently, These Two Can Be Classified in Other Subclasses [11]
- Superficial or static fermentation (SFC): The obtained wine is maintained in an open-air container. Lactic acid bacteria (LAB) development will mainly be on the liquid surface, promoting the formation of a strong biofilm;
- Submerged (SMR) fermentation: The entire liquid is subjected to an addition of air, agitated/mixed with self-priming turbines to increase the surface of contact with air.
- carbon source/energy;
- inert support;
- carbon source/energy and inert support [12].
- analysis of alcohol content;
- pH analysis;
- optical control;
- acid-base titration.
2. Materials and Methods
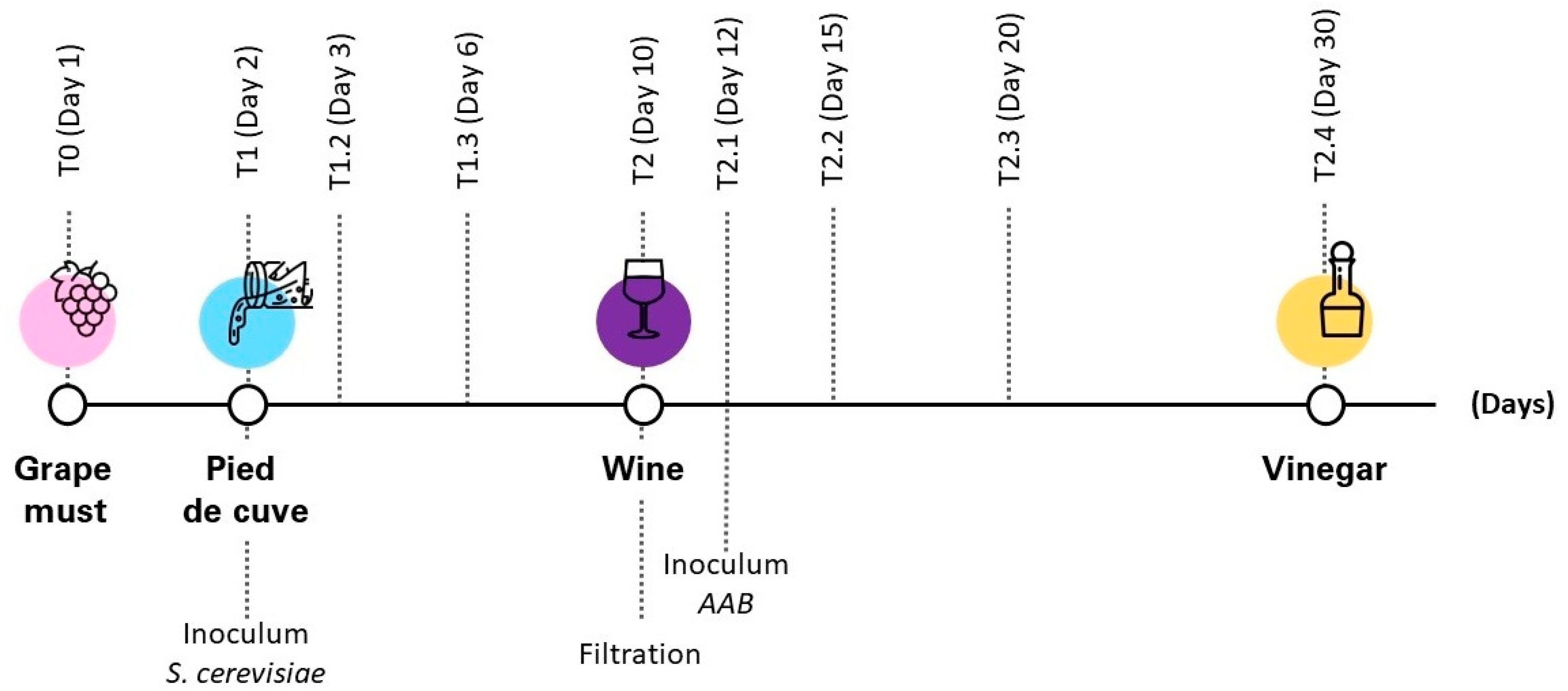
2.1. Sample Preparation
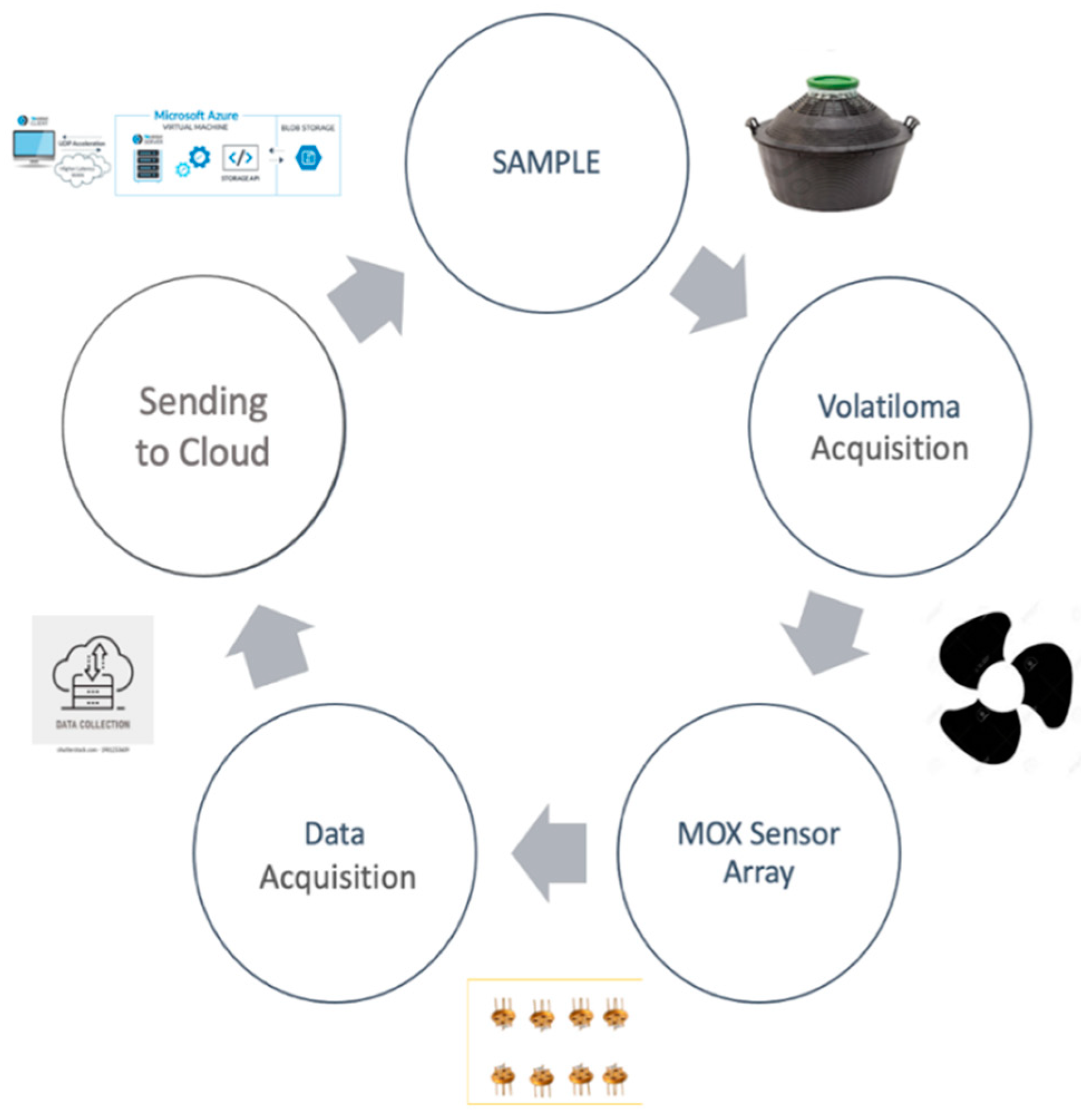
2.2. Small Sensor System (S3)
2.2.1. Analysis Conditions
- The steel chamber of the sensor contains the six MOX sensors. This allows the sensor to be separated from the environment, except for an inlet and an outlet path for the passage of volatile compounds. Other types of sensors are placed in order to control several parameters during the analysis. These are the temperature, humidity, and flow in the chamber;
- The dynamic fluid circuit consists of a pump (Knf, model: NMP05B), polyurethane tubes, an electro valve, and a metal cylinder, which contains activated carbon. Activated carbon plays the filtering role for any type of odor in the environment that may alter the final response. The solenoid valve is positioned at the inlet of the chamber to control the flow of the pump, with a maximum of 250 sccm;
- The electronic board processes the sensor responses by detecting the electrical resistance. It also controls the operating temperature of the sensors, which is an important parameter for the detection of volatile compounds. Finally, the system is able to send data to the Web App dedicated to the S3 device via an internet connection.
2.2.2. S3 Data Analysis
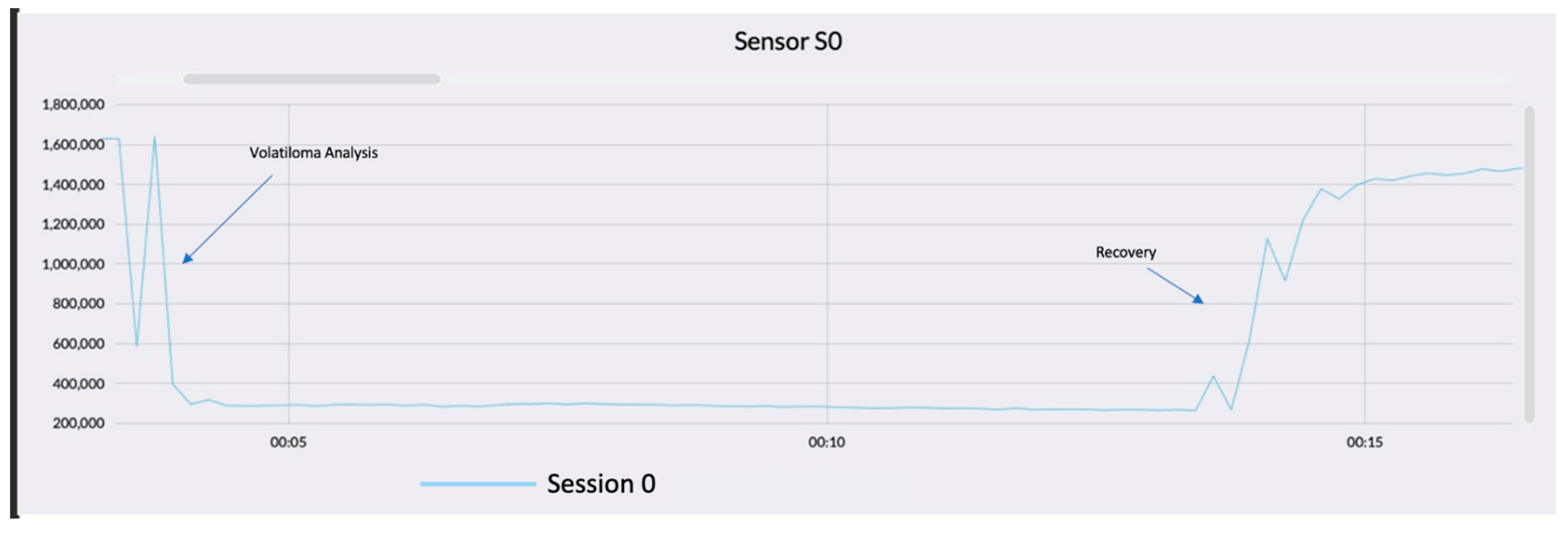
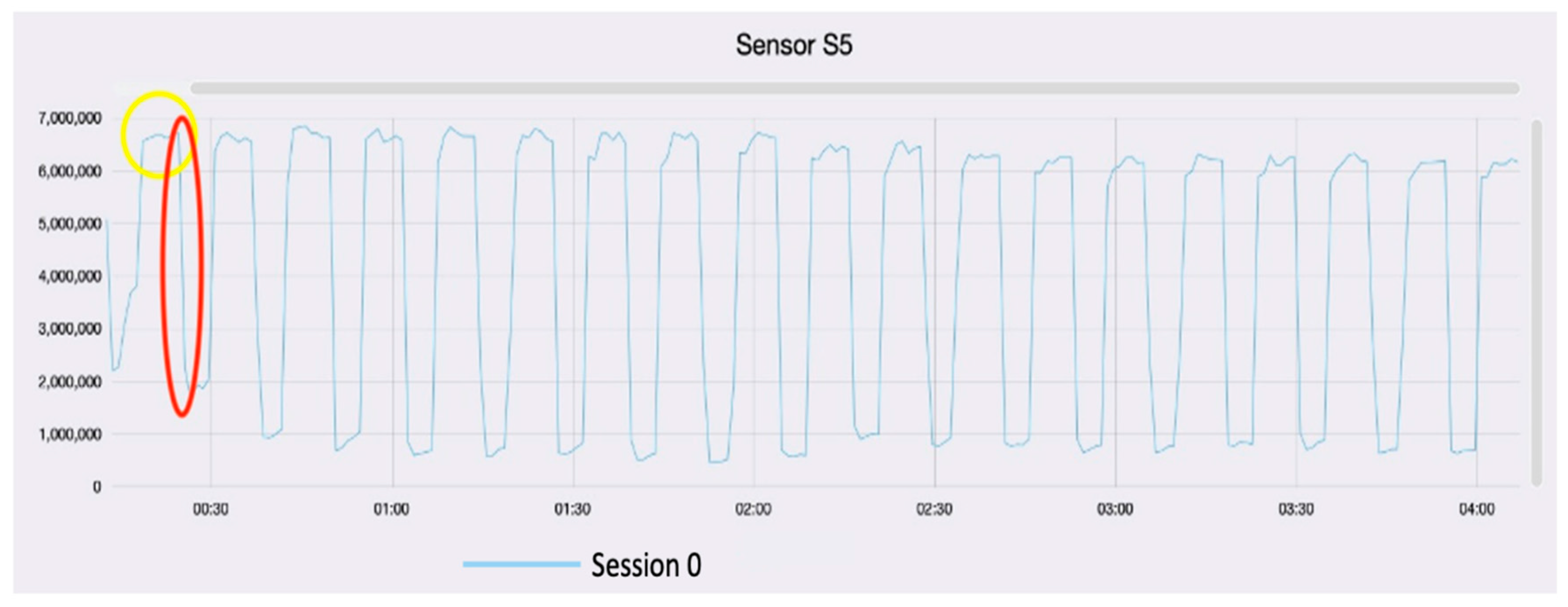
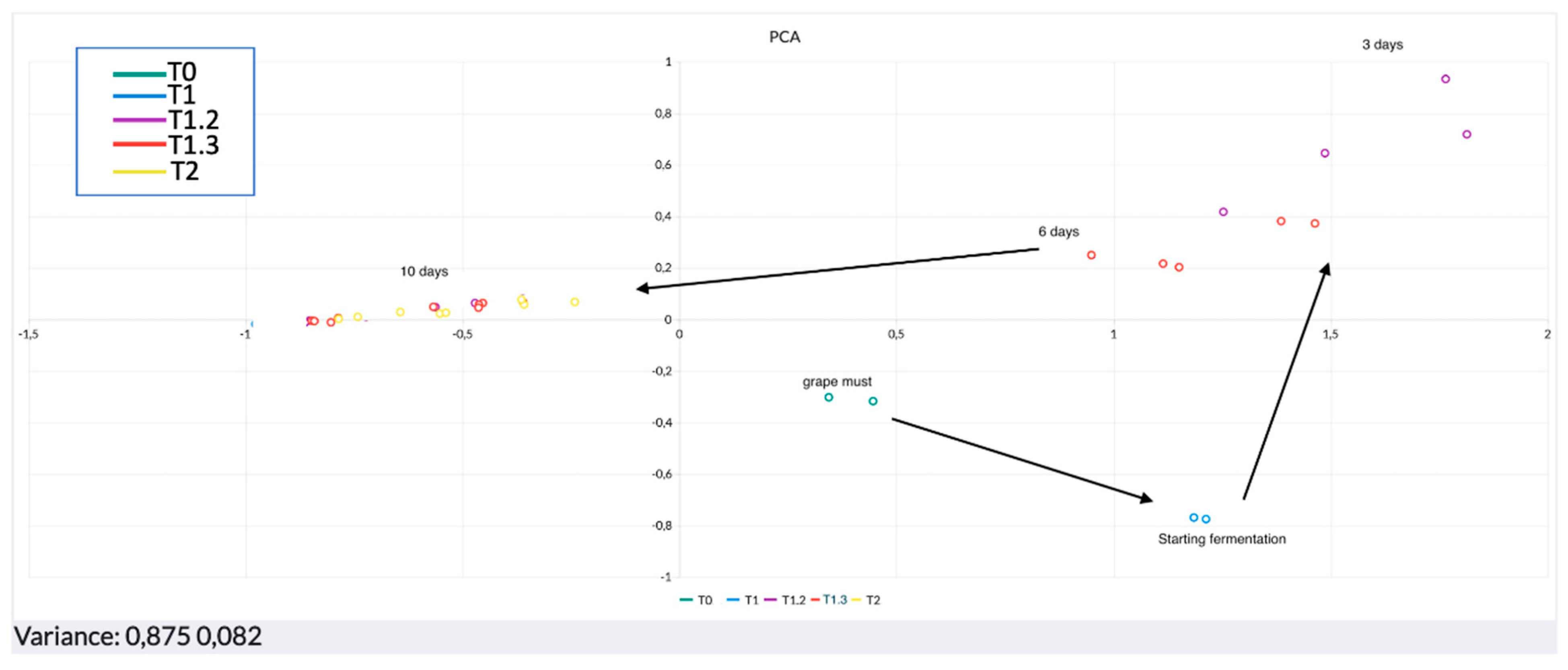
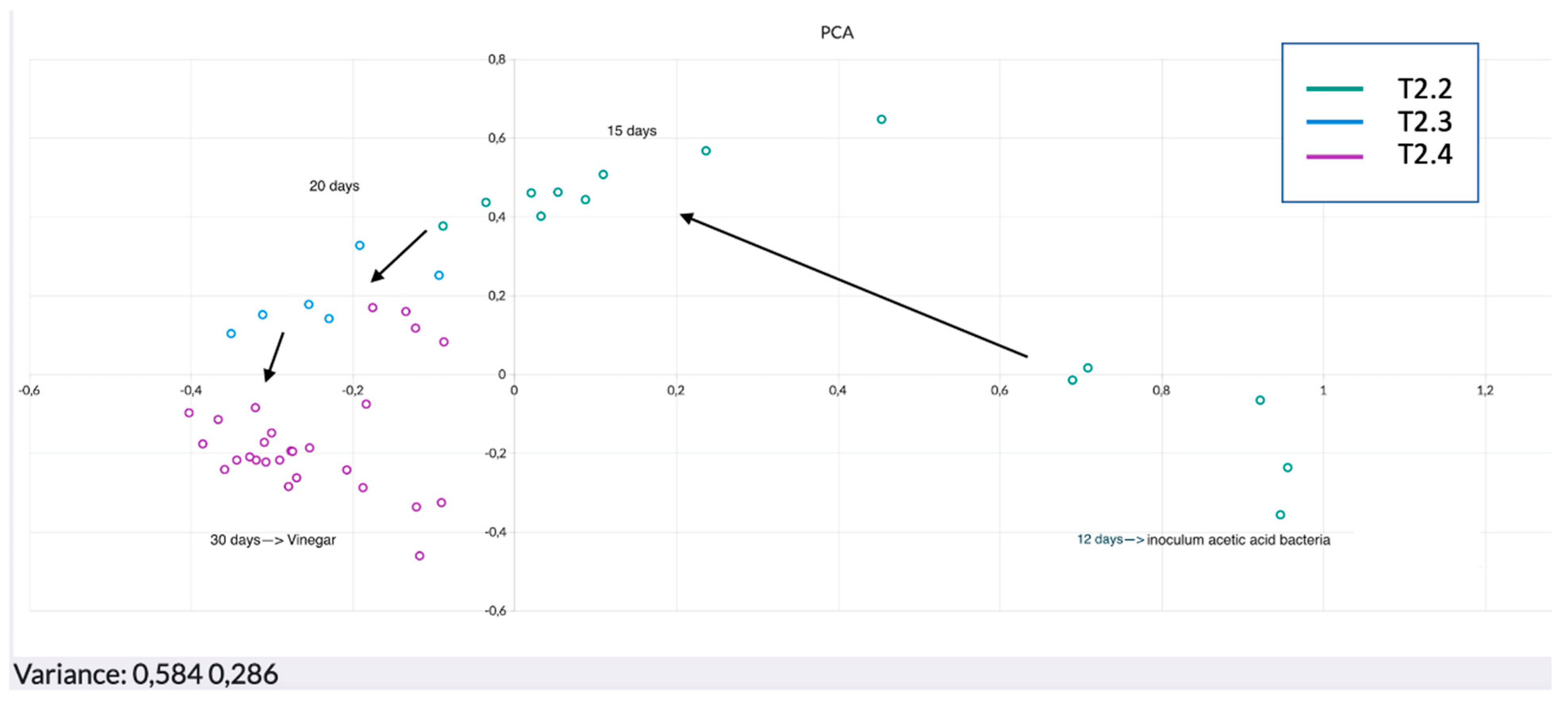
3. Results and Discussion
Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EU Food Legislation: EU MGP FAO/WHO 1990. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:1997:0176:FIN:EN:PDF (accessed on 4 July 2022).
- Gullo, M.; Caggia, C.; De Vero, L.; Giudici, P. Characterization of acetic acid bacteria in “traditional balsamic vinegar”. Int. J. Food Microbiol. 2006, 106, 209–212. [Google Scholar] [CrossRef]
- Hailu, S.; Admassu, S.; Jha, K. Vinegar production technology—An overview. Beverage Food World 2012, 2, 29–32. [Google Scholar]
- Adams, M.R. Vinegar. Microbiology of Fermented Foods; Springer: Boston, MA, USA, 1998; pp. 1–44. [Google Scholar]
- Cleenwerck, I.; Vandemeulebroecke, K.; Janssens, D.; Swings, J. Re-examination of the genus Acetobacter, with descriptions of Acetobacter cerevisiae sp. nov. and Acetobacter malorum sp. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 1551–1558. [Google Scholar] [PubMed]
- Fregapane, G.; Rubio-Fernández, H.; Salvador, M. Influence of fermentation temperature on semi-continuous acetification for wine vinegar production. Eur. Food Res. Technol. 2001, 213, 62–66. [Google Scholar] [CrossRef]
- Tripathi, N.M.; Sharma, D.; Ranga, P.; Aseri, G.K.; Singh, D. Microbial technologies for acetic acid production using fruit waste. In Microbial Resource Technologies for Sustainable Development; Elsevier: Amsterdam, The Netherlands, 2022; pp. 157–178. [Google Scholar]
- Solieri, L.; Giudici, P. Vinegars of the World; Springer: Milano, Italy, 2009; pp. 1–16. [Google Scholar]
- Singh, B.; Gupta, M.; Verma, A. Mechanical behaviour of particulate hybrid composite laminates as potential building materials. Constr. Build. Mater. 1995, 9, 39–44. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Gullo, M.; Verzelloni, E.; Canonico, M. Aerobic submerged fermentation by acetic acid bacteria for vinegar production: Process and biotechnological aspects. Process Biochem. 2014, 49, 1571–1579. [Google Scholar] [CrossRef]
- Farris, G.A.; Gobbetti, M.; Neviani, E.; Vincenzini, M. Microbiologia dei Prodotti Alimentari Microrganismi, Controllo delle Fermentazioni, Indicatori di Qualità, Igiene degli Alimenti Fermentati e Non; Zanichelli, CEA: Modena, Italy, 2012. [Google Scholar]
- Elmi, I.; Zampolli, S.; Cozzani, E.; Mancarella, F.; Cardinali, G.C. Development of ultra-low-power consumption MOX sensors with ppb-level VOC detection capabilities for emerging applications. Sens. Actuators B Chem. 2008, 135, 342–351. [Google Scholar] [CrossRef]
- Abbatangelo, M.; Núñez-Carmona, E.; Duina, G.; Sberveglieri, V. Multidisciplinary Approach to Characterizing the Fingerprint of Italian EVOO. Molecules 2019, 24, 1457. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Wang, Y.; Zhang, R.; Li, J.; Li, X.; Zang, Z. Conductometric room temperature ammonia sensors based on titanium dioxide nanoparticles decorated thin black phosphorus nanosheets. Sens. Actuators B Chem. 2021, 349, 130770. [Google Scholar] [CrossRef]
- Bamforth, W.C. Vinegar. Food, Fermentation and Micro-Organisms; Blachwell Science: Kundli, India, 2005; pp. 154–159. [Google Scholar]
- Camara, G.A.; Iwasita, T. Parallel pathways of ethanol oxidation: The effect of ethanol concentration. J. Electroanal. Chem. 2005, 578, 315–321. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeasts in fruit and fruit products. In Yeasts in Food: Beneficial and Detrimental Aspects; Boekhout, T., Robert, V., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2003; pp. 267–288. [Google Scholar]
- Ubeda Iranzo, J.F.; Gonzalez Magana, F.; Gonzalez Vinas, M.A. Evaluation of the formation of volatiles and sensory characteristics in the industrial production of white wines using different commercial strains of the genus Saccharomyces. Food Control 2000, 11, 143–147. [Google Scholar] [CrossRef]
- Li, E.; Liu, C.; Liu, Y. Evaluation of yeast diversity during wine fermentations with direct inoculation and pied de cuve method at an industrial scale. J. Microbiol. Biotechnol. 2012, 22, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, A.; Calderon, I.L.; Paneque, P. Yeast assessment during alcoholic fermentation inoculated with a natural ‘pied de cuve’ or a commercial yeast strain. World J. Microbiol. Biotechnol. 2011, 27, 1569–1577. [Google Scholar] [CrossRef]
- Giudici, P.; De Vero, L.; Landi, S.; Gullo, M.; Solieri, L. Starter Cultures for the Production of Traditional Balsamic Vinegar: Practical and Applicative Aspects; Beverage Industries No. 196; Beverage Industries: Reggio Emilia, Italy, 2005; pp. 104–108. [Google Scholar]
- Baek, C.H.; Jeong, D.-H.; Baek, S.Y.; Choi, J.-H.; Park, H.-Y.; Choi, H.S.; Yeo, S.-H. Quality characteristics of farm-made brown rice vinegar via traditional static fermentation. Korean J. Food Preserv. 2013, 20, 564–572. [Google Scholar] [CrossRef]
- Núñez-Carmona, E.; Abbatangelo, M.; Sberveglieri, V. Internet of Food (IoF), Tailor-Made Metal Oxide Gas Sensors to Support Tea Supply Chain. Sensors 2021, 21, 4266. [Google Scholar] [CrossRef]
- Navarini, L.; Rivetti, D. Water quality for Espresso coffee. Food Chem. 2010, 122, 424–428. [Google Scholar] [CrossRef]
- Nunez-Carmona, E.; Abbatangelo, M.; Zappa, D.; Comini, E.; Sberveglieri, G.; Sberveglieri, V. Nanostructured MOS Sensor for the Detection, Follow up, and Threshold Pursuing of Campylobacter Jejuni Development in Milk Samples. Sensors 2020, 20, 2009. [Google Scholar] [CrossRef]
- Greco, G.; Carmona, E.N.; Sberveglieri, G.; Genzardi, D.; Sberveglieri, V. A New Kind of Chemical Nanosensors for Discrimination of Espresso Coffee. Chemosensors 2022, 10, 186. [Google Scholar] [CrossRef]
- Núñez-Carmona, E.; Abbatangelo, M.; Zottele, I.; Piccoli, P.; Tamanini, A.; Comini, E.; Sberveglieri, V. Nanomaterial gas sensors for online monitoring system of fruit jams. Foods 2019, 8, 632. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Tongson, E.; Fuentes, S. Integrating a Low-Cost Electronic Nose and Machine Learning Modelling to 292 Assess Coffee Aroma Profile and Intensity. Sensors 2021, 21, 2016. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.S.; Lee, J.H.; Han, K.M.; Kim, J.W.; Cho, S.; Kim, J. Discrimination of commercial cheeses from fatty acid profiles and phytosterol contents obtained by GC and PCA. Food Chem. 2014, 143, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Betto, G.; Sberveglieri, V.; Carmona, E.N.; Comini, E.; Giudici, P. A new approach to evaluate vinegars quality: Application of Small Sensor System (S3) device coupled with enfleurage. Procedia Eng. 2016, 168, 456–459. [Google Scholar] [CrossRef]
- Ubeda, C.; Callejón, R.M.; Hidalgo, C.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Determination of major volatile compounds during the production of fruit vinegars by static headspace gas chromatography–mass spectrometry method. Food Res. Int. 2011, 44, 259–268. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.L.; Silva, P.; Câmara, J.S. Establishment of the Volatile Signature of Wine-Based Aromatic Vinegars Subjected to Maceration. Molecules 2018, 23, 499. [Google Scholar] [CrossRef]
- Chinnici, F.; Durán Guerrero, E.; Sonni, F.; Natali, N.; Natera Marín, R.; Riponi, C. Gas Chromatography−Mass Spectrometry (GC−MS) Characterization of Volatile Compounds in Quality Vinegars with Protected European Geographical Indication. J. Agric. Food Chem. 2009, 57, 4784–4792. [Google Scholar] [CrossRef]
- Budak, N.; Aykin, E.; Seydim, A.; Greene, A.K.; Guzel-Seydim, Z.B. Functional properties of vinegar. J. Food Sci. 2014, 79, R757–R764. [Google Scholar] [CrossRef]
- Xu, W.; Huang, Z.; Zhang, X.; Li, Q.; Lu, Z.; Shi, J.; Xu, Z.; Ma, Y. Monitoring the microbial community during solid-state acetic acid fermentation of Zhenjiang aromatic vinegar. Food Microbiol. 2011, 28, 1175–1181. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, A.; Zhao, J.; Ouyang, Q.; Sun, Z.; Huang, L. Monitoring vinegar acetic fermentation using a colorimetric sensor array. Sens. Actuators B Chem. 2013, 183, 608–616. [Google Scholar] [CrossRef]
- Durán, E.; Palma, M.; Natera, R.; Castro, R.; Barroso, C.G. New FT-IR method to control the evolution of the volatile constituents of vinegar during the acetic fermentation process. Food Chem. 2010, 121, 575–579. [Google Scholar] [CrossRef]
- Sun, Z.B.; Zhao, J.W.; Zou, X.B.; Chen, Q.S.; Li, G.D. Changes of characteristic aroma component in Zhenjiang fragrance vinegar during processing and analysis of their formation mechanism. J. Chin. Inst. Food Sci. Technol. 2010, 10, 120–127. [Google Scholar]
- Arshak, K.; Adley, C.; Moore, E.; Cunniffe, C.; Campion, M.; Harris, J. Characterisation of polymer nanocomposite sensors for quantification of bacterial cultures. Sens. Actuators B Chem. 2007, 126, 226–231. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genzardi, D.; Greco, G.; Núñez-Carmona, E.; Sberveglieri, V. Real Time Monitoring of Wine Vinegar Supply Chain through MOX Sensors. Sensors 2022, 22, 6247. https://doi.org/10.3390/s22166247
Genzardi D, Greco G, Núñez-Carmona E, Sberveglieri V. Real Time Monitoring of Wine Vinegar Supply Chain through MOX Sensors. Sensors. 2022; 22(16):6247. https://doi.org/10.3390/s22166247
Chicago/Turabian StyleGenzardi, Dario, Giuseppe Greco, Estefanía Núñez-Carmona, and Veronica Sberveglieri. 2022. "Real Time Monitoring of Wine Vinegar Supply Chain through MOX Sensors" Sensors 22, no. 16: 6247. https://doi.org/10.3390/s22166247
APA StyleGenzardi, D., Greco, G., Núñez-Carmona, E., & Sberveglieri, V. (2022). Real Time Monitoring of Wine Vinegar Supply Chain through MOX Sensors. Sensors, 22(16), 6247. https://doi.org/10.3390/s22166247







