ZnO Nanorods Coated Single-Mode–Multimode–Single-Mode Optical Fiber Sensor for VOC Biomarker Detection
Abstract
:1. Introduction
2. Materials and Methodss
2.1. Materials and Reagents
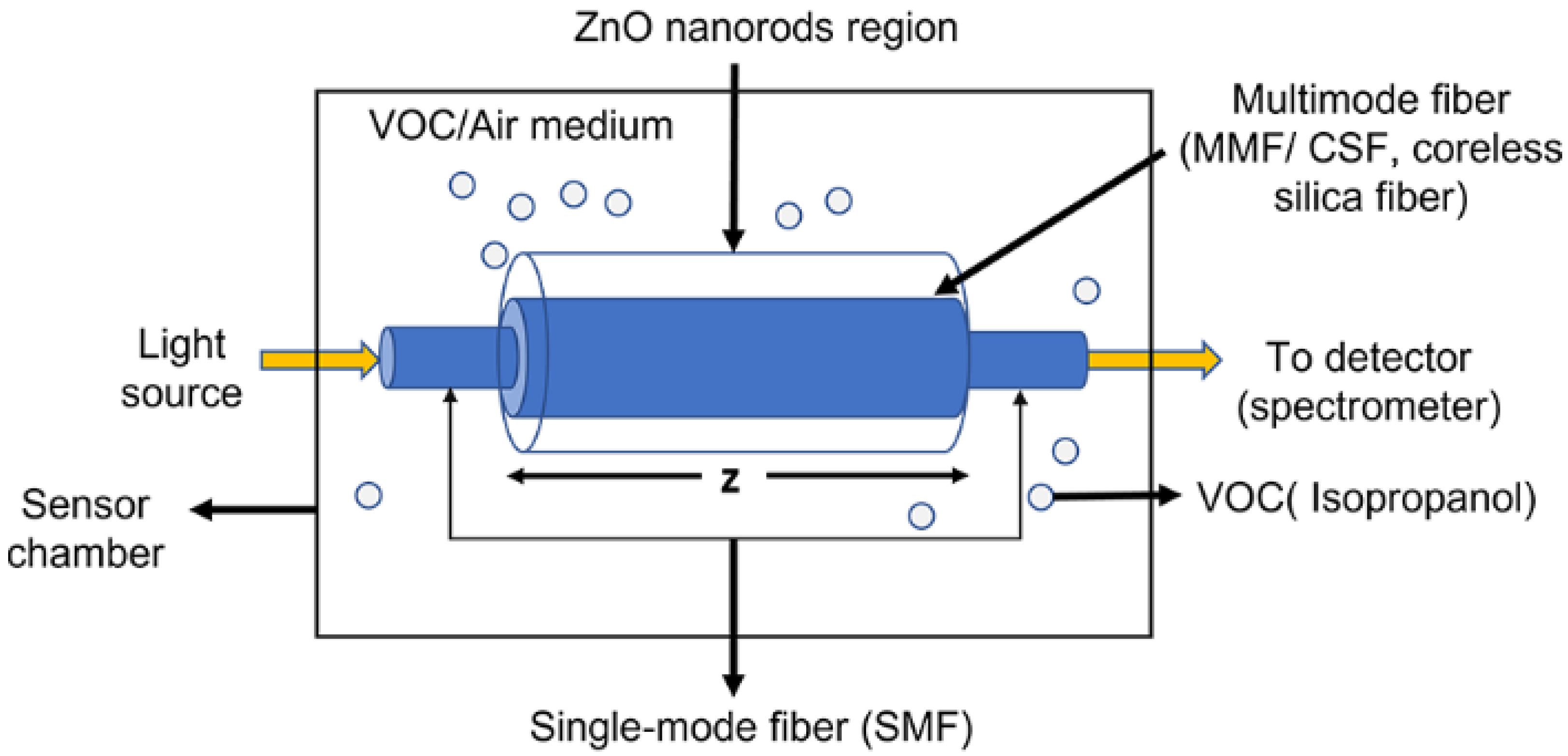
2.2. Sensor Design and Concept
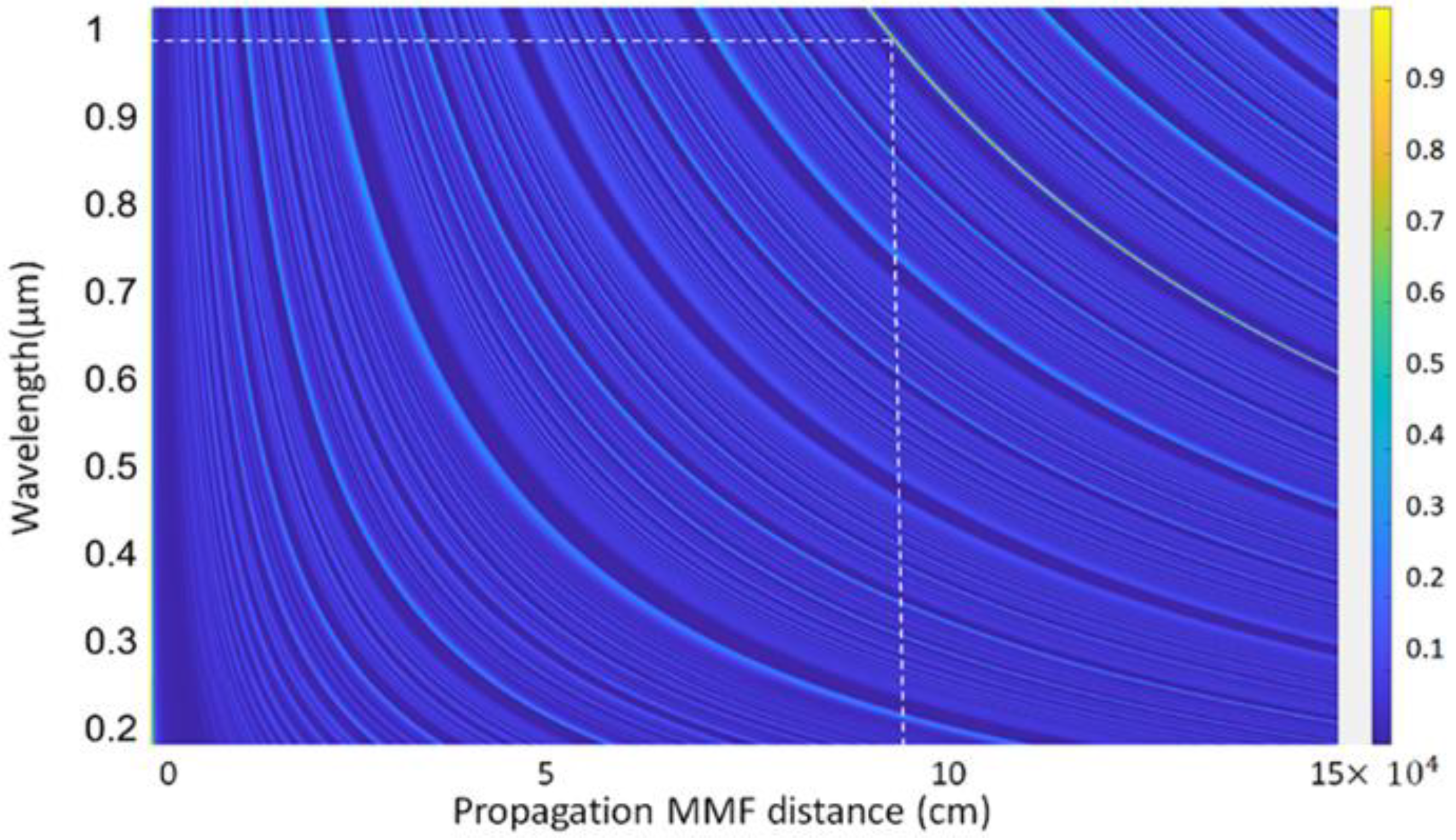
2.3. Numerical Modelling
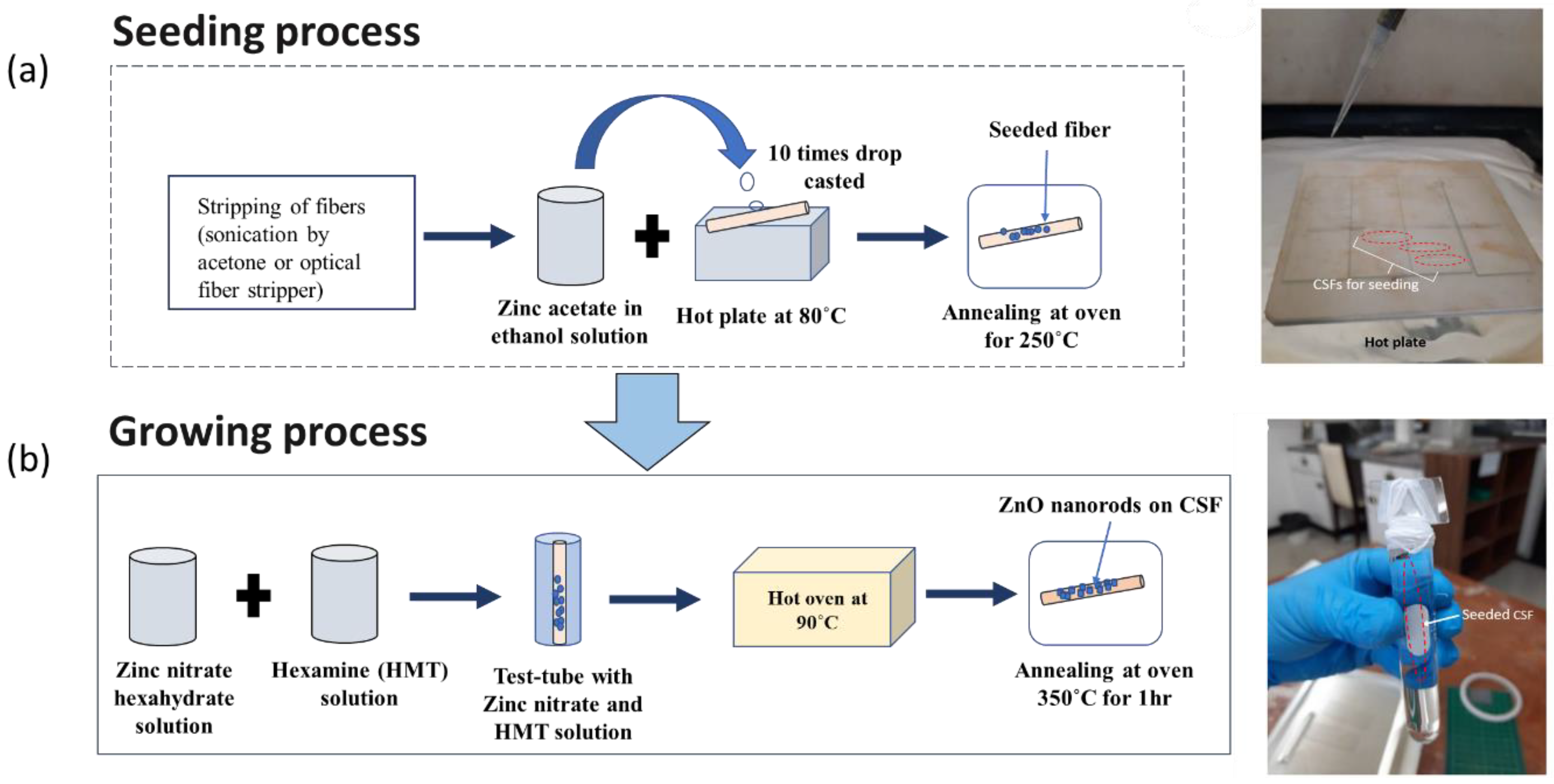
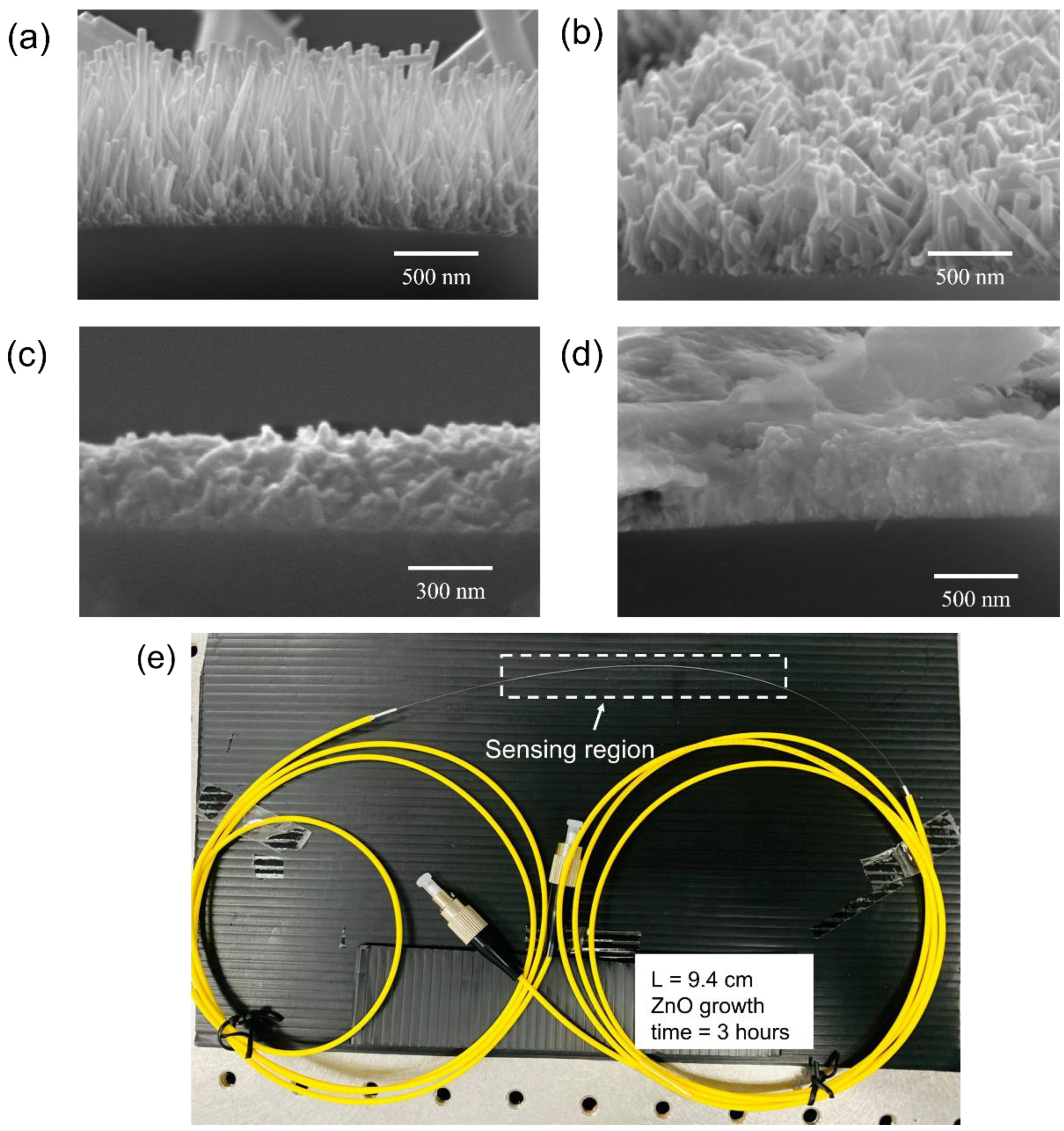
2.4. Sensor Fabrication
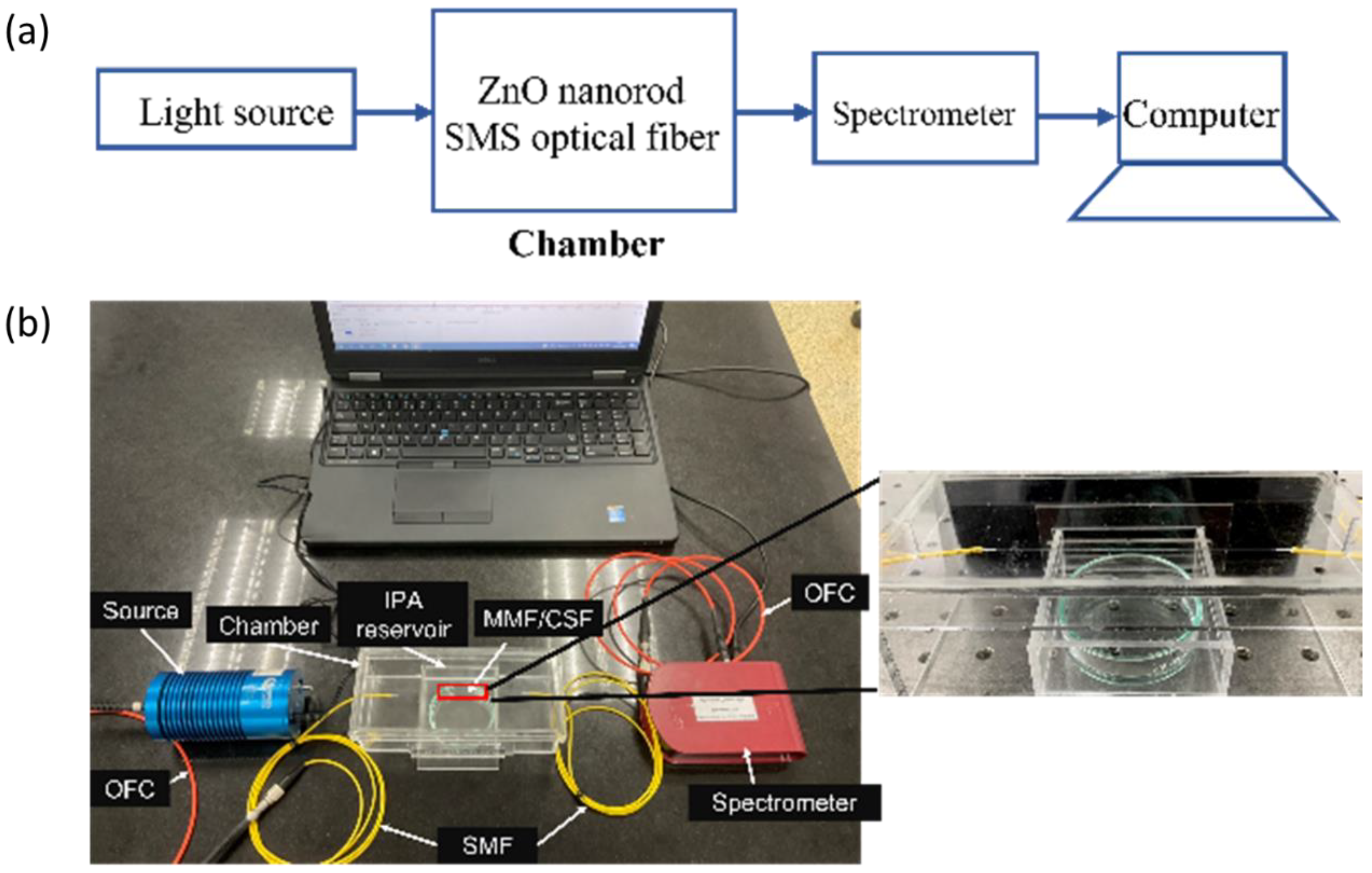
2.5. Experimental Setup
2.6. Isopropanol (IPA) Vapor Preparation
3. Results and Discussion
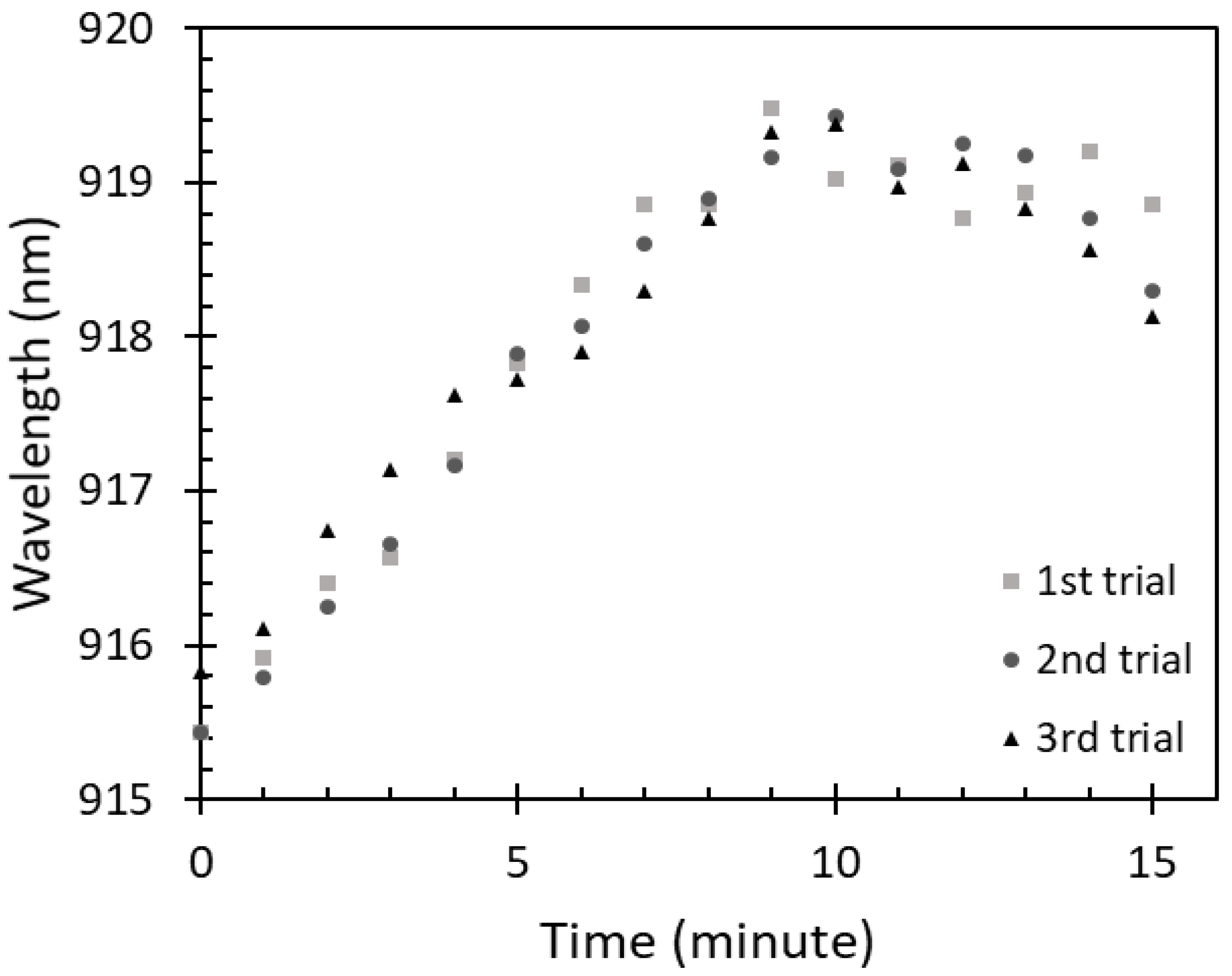
3.1. Saturation Time
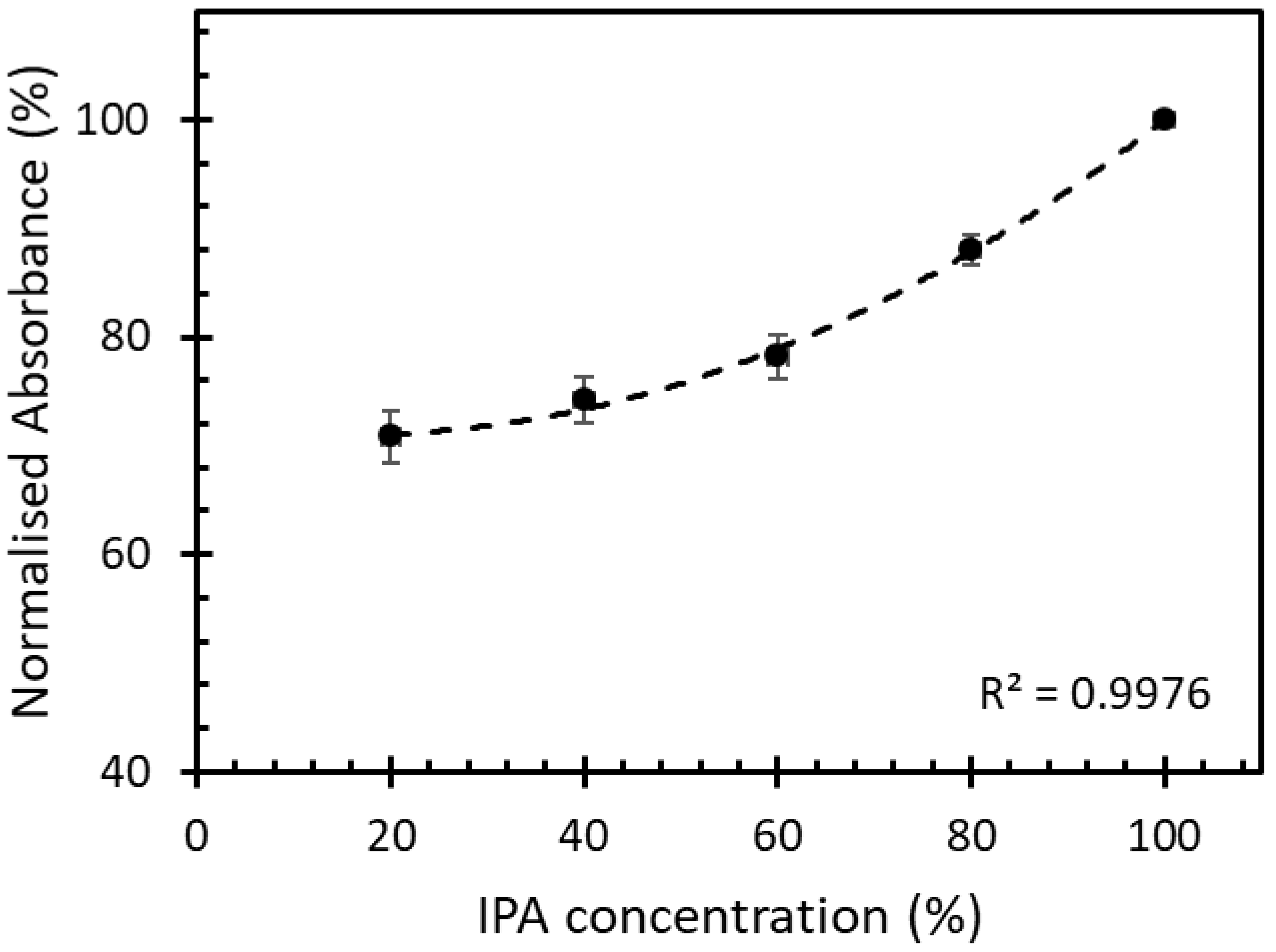
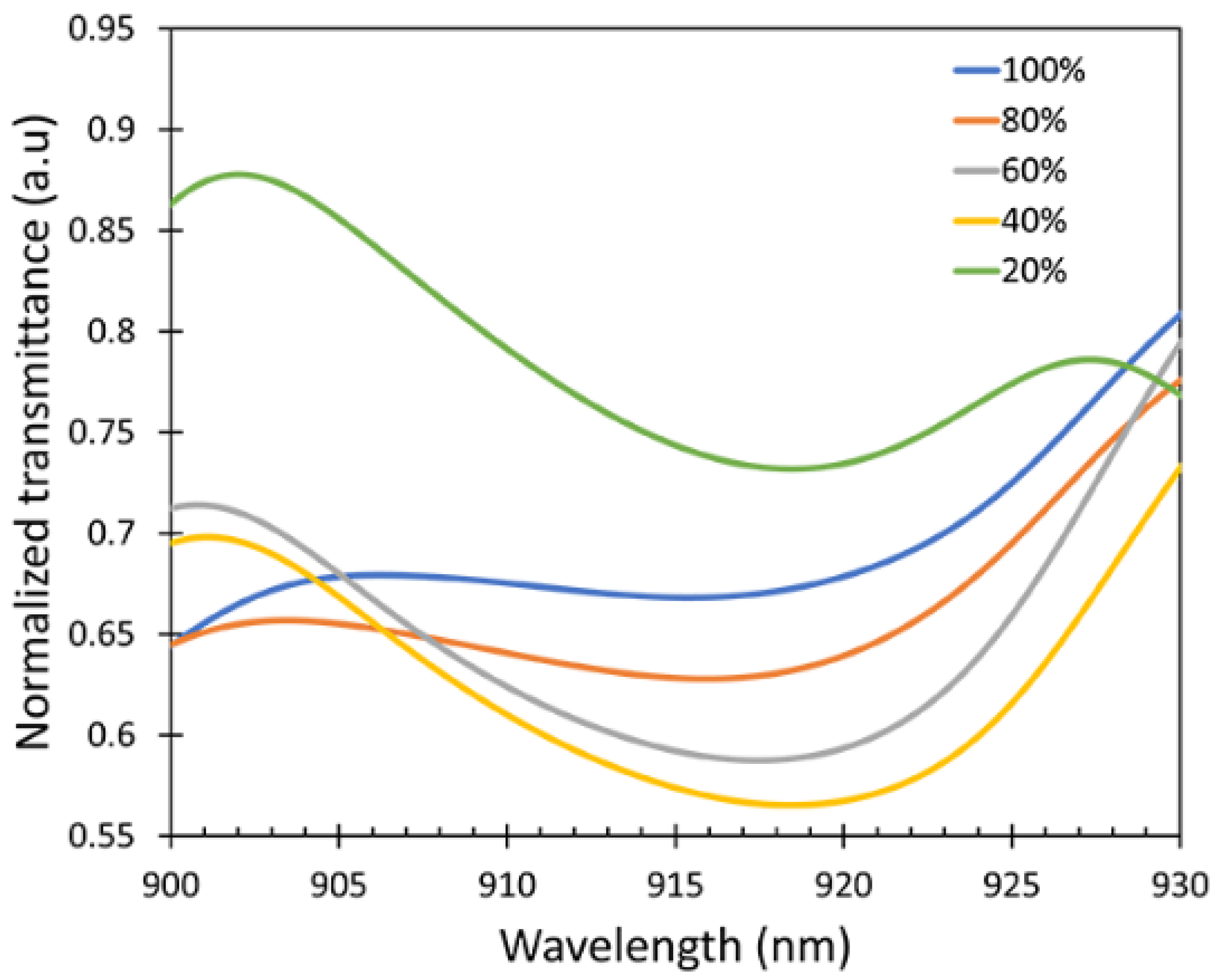
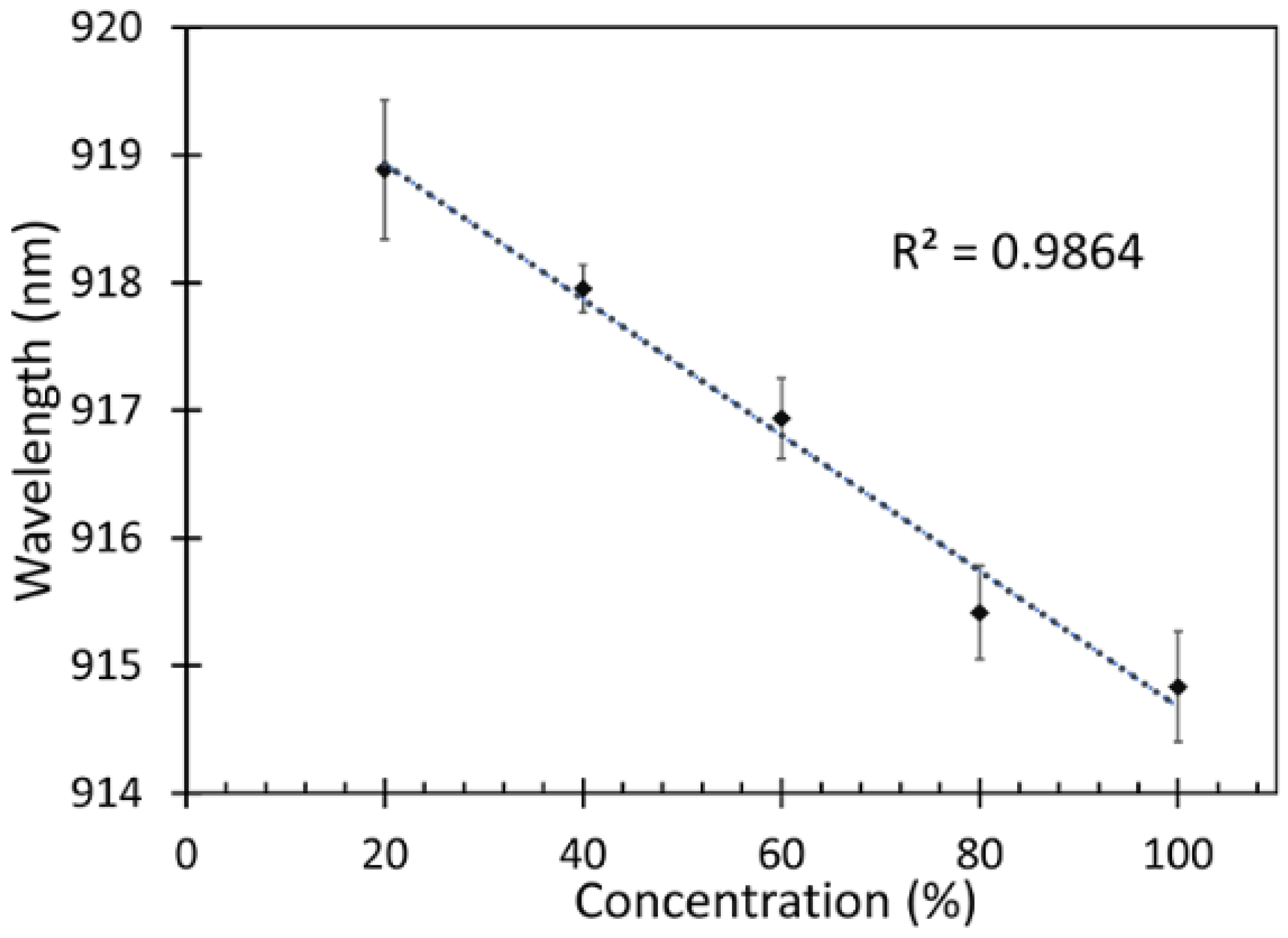
3.2. Sensitivity Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Elosua, C.; Matias, I.; Bariain, C.; Arregui, F. Volatile Organic Compound Optical Fiber Sensors: A Review. Sensors 2006, 6, 1440–1465. [Google Scholar] [CrossRef] [Green Version]
- Spadavecchia, J.; Ciccarella, G.; Rella, R.; Capone, S.; Siciliano, P. Metallophthalocyanines thin films in array configuration for electronic optical nose applications. Sens. Actuators B Chem. 2003, 96, 489–497. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.-H.; Brown, R.J.C. Coordination polymers: Opportunities and challenges for monitoring volatile organic compounds. Prog. Polym. Sci. 2015, 45, 102–118. [Google Scholar] [CrossRef]
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2014, 43, 1423–1449. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, C. Is breath acetone a biomarker of diabetes? A historical review on breath acetone measurements. J. Breath Res. 2013, 7, 37109. [Google Scholar] [CrossRef] [Green Version]
- Rudnicka, J.; Kowalkowski, T.; Ligor, T.; Buszewski, B. Determination of volatile organic compounds as biomarkers of lung cancer by SPME–GC–TOF/MS and chemometrics. J. Chromatogr. B 2011, 879, 3360–3366. [Google Scholar] [CrossRef]
- Teixeira, L.S.G.; Leão, E.S.; Dantas, A.F.; Pinheiro, H.L.C.; Costa, A.C.S.; de Andrade, J.B. Determination of formaldehyde in Brazilian alcohol fuels by flow-injection solid phase spectrophotometry. Talanta 2004, 64, 711–715. [Google Scholar] [CrossRef]
- Vesely, P.; Lusk, L.; Basarova, G.; Seabrooks, J.; Ryder, D. Analysis of Aldehydes in Beer Using Solid-Phase Microextraction with On-Fiber Derivatization and Gas Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 6941–6944. [Google Scholar] [CrossRef]
- Sabourin, P.J.; Bechtold, W.E.; Henderson, R.F. A high pressure liquid chromatographic method for the separation and quantitation of water-soluble radiolabeled benzene metabolites. Anal. Biochem. 1988, 170, 316–327. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, T.; Wang, Z.; Tsukimoto, S.; Saito, M.; Ikuhara, Y. Selective Detection of Formaldehyde Gas Using a Cd-Doped TiO(2)-SnO(2) Sensor. Sensors 2009, 9, 9029–9038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantalei, S.; Zampetti, E.; Bearzotti, A.; De Cesare, F.; Macagnano, A. Improving sensing features of a nanocomposite PEDOT:PSS sensor for NO breath monitoring. Sens. Actuators B Chem. 2013, 179, 87–94. [Google Scholar] [CrossRef]
- Komkova, M.A.; Karyakina, E.E.; Marken, F.; Karyakin, A.A. Hydrogen Peroxide Detection in Wet Air with a Prussian Blue Based Solid Salt Bridged Three Electrode System. Anal. Chem. 2013, 85, 2574–2577. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, M.R.; Bakhirkin, Y.A.; Tittel, F.K. Quantum cascade laser-based integrated cavity output spectroscopy of exhaled nitric oxide. Appl. Phys. B 2006, 85, 445–452. [Google Scholar] [CrossRef]
- Mitsubayashi, K.; Minamide, T.; Otsuka, K.; Kudo, H.; Saito, H. Optical bio-sniffer for methyl mercaptan in halitosis. Anal. Chim. Acta 2006, 573–574, 75–80. [Google Scholar] [CrossRef]
- Pathak, A.K.; Viphavakit, C. VOC biomarker monitoring for diabetes through exhaled breath using Ag/P-TiO 2 composite plasmonic sensor. IEEE Sens. J. 2021, 21, 22631–22637. [Google Scholar] [CrossRef]
- Pathak, A.K.; Limprapassorn, P.; Kongruttanachok, N.; Viphavakit, C. Molecularly Imprinted Polymer-Based Optical Sensor for Isopropanol Vapor. J. Sens. Actuator Netw. 2022, 11, 46. [Google Scholar] [CrossRef]
- Swargiary, K.; Jolivot, R.; Mohammed, W.S. Demonstration of a Polymer-Based Single Step Waveguide by 3D Printing Digital Light Processing Technology for Isopropanol Alcohol-Concentration Sensor. Photonic Sens. 2022, 12, 10–12. [Google Scholar] [CrossRef]
- Lan, X.; Huang, J.; Han, Q.; Wei, T.; Gao, Z.; Jiang, H.; Dong, J.; Xiao, H. Fiber ring laser interrogated zeolite-coated singlemode-multimode-singlemode structure for trace chemical detection. Opt. Lett. 2012, 37, 1998. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes; Flavourings and Processing Aids. Scientific Opinion on the safety evaluation of the substance zinc oxide, nanoparticles, uncoated and coated with [3- (methacryloxy)propyl] trimethoxysilane, for use in food contact materials. EFSA J. 2015, 13, 4063–4072. [Google Scholar]
- Qi, Q.; Zhang, T.; Yu, Q.; Wang, R.; Zeng, Y.; Liu, L.; Yang, H. Properties of humidity sensing ZnO nanorods-base sensor fabricated by screen-printing. Sens. Actuators B Chem. 2008, 133, 638–643. [Google Scholar] [CrossRef]
- Renganathan, B.; Sastikumar, D.; Gobi, G.; Rajeswari Yogamalar, N.; Chandra Bose, A. Nanocrystalline ZnO coated fiber optic sensor for ammonia gas detection. Opt. Laser Technol. 2011, 43, 1398–1404. [Google Scholar] [CrossRef]
- Wen, X.; Huang, J.; Xiao, H.; Yang, M.H. ZnO-coated SMS structure interrogated by a fiber ring laser for chemical sensing. Meas. Sci. Technol. 2014, 25, 114002. [Google Scholar] [CrossRef]
- Rahim, H.R.A.; Thokchom, S.; Mohammed, W.S.; Dutta, J.; Harun, S.W. Optical fiber coated with zinc oxide nanorods toward light side coupling for sensing application. In Nano-Optics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 293–304. [Google Scholar]
- Pathak, A.K.; Chaudhary, D.K.; Singh, V.K. Broad range and highly sensitive optical pH sensor based on Hierarchical ZnO microflowers over tapered silica fiber. Sens. Actuators A Phys. 2018, 280, 399–405. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Lei, H.; Song, J.; Chen, H.; Li, B. Growth of well-arrayed ZnO nanorods on thinned silica fiber and application for humidity sensing. Opt. Express 2012, 20, 19404. [Google Scholar] [CrossRef]
- Yusof, H.H.M.; Harun, S.W.; Dimyati, K.; Bora, T.; Mohammed, W.S.; Dutta, J. Optical dynamic range maximization for humidity sensing by controlling growth of zinc oxide nanorods. Photonics Nanostruct.-Fundam. Appl. 2018, 30, 57–64. [Google Scholar] [CrossRef]
- Rahim, H.R.B.A.; Lokman, M.Q.B.; Harun, S.W.; Dutta, J.; Mohammed, W.S. Temperature Sensing by Side Coupling of Light through Zinc Oxide Nanorods on Optical Fibers. Sens. Actuators A Phys. 2017, 257, 15–19. [Google Scholar] [CrossRef]
- Narasimman, S.; Balakrishnan, L.; Alex, Z.C. Fiber-Optic Ammonia Sensor Based on Amine Functionalized ZnO Nanoflakes. IEEE Sens. J. 2018, 18, 201–208. [Google Scholar] [CrossRef]
- Azad, S.; Sadeghi, E.; Parvizi, R.; Mazaheri, A. Fast response relative humidity clad-modified multimode optical fiber sensor with hydrothermally dimension controlled ZnO nanorods. Mater. Sci. Semicond. Processing 2017, 66, 200–206. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Effect of seeded substrates on hydrothermally grown ZnO nanorods. J. Sol-Gel Sci. Technol. 2009, 50, 456–464. [Google Scholar] [CrossRef]
- Fallah, H.; Harun, S.W.; Mohammed, W.S.; Dutta, J. Excitation of core modes through side coupling to multimode optical fiber by hydrothermal growth of ZnO nanorods for wide angle optical reception. J. Opt. Soc. Am. B 2014, 31, 2232–2238. [Google Scholar] [CrossRef]
- Mohammed, W.S.; Smith, P.W.E.; Gu, X. All-fiber multimode interference bandpass filter. Opt. Lett. 2006, 31, 2547. [Google Scholar] [CrossRef] [PubMed]
- Bora, T.; Fallah, H.; Chaudhari, M.; Apiwattanadej, T.; Harun, S.W.; Mohammed, W.S.; Dutta, J. Controlled side coupling of light to cladding mode of ZnO nanorod coated optical fibers and its implications for chemical vapor sensing. Sens. Actuators B Chem. 2014, 202, 543–550. [Google Scholar] [CrossRef]
- Metem, P.; Patchoo, W.; Mohammed, W.S.; Viphavakit, C. Numerical investigation of Au-silane functionalised optical fibre sensor for volatile organic compounds biomarker (VOCs) detection. In Proceedings of the Optical Fibers and Sensors for Medical Diagnostics, Treatment and Environmental Applications XXI, Online, 5 March 2021. [Google Scholar]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 13001. [Google Scholar] [CrossRef]
- Kyaw, H.H.; Bora, T.; Dutta, J. One-Diode Model Equivalent Circuit Analysis for ZnO Nanorod-Based Dye-Sensitized Solar Cells: Effects of Annealing and Active Area. IEEE Trans. Nanotechnol. 2012, 11, 763–768. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. pH-dependent growth of zinc oxide nanorods. J. Cryst. Growth 2009, 311, 2549–2554. [Google Scholar] [CrossRef]
- Promnimit, S.; Baruah, S.; Lamdu, U.; Dutta, J. Hydrothermal growth of ZnO hexagonal nanocrystals: Effect of growth conditions. J. Nano Res. 2013, 21, 57–63. [Google Scholar] [CrossRef]
- Yusof, H.H.M.; Harun, S.W.; Dimyati, K.; Bora, T.; Sterckx, K.; Mohammed, W.S.; Dutta, J. Low-Cost Integrated Zinc Oxide Nanorod-Based Humidity Sensors for Arduino Platform. IEEE Sens. J. 2019, 19, 2442–2449. [Google Scholar] [CrossRef]
- Cai, X.; Hu, D.; Deng, S.; Han, B.; Wang, Y.; Wu, J.; Wang, Y. Isopropanol sensing properties of coral-like ZnO–CdO composites by flash preparation via self-sustained decomposition of metal–organic complexes. Sens. Actuators B Chem. 2014, 198, 402–410. [Google Scholar] [CrossRef]
- Luo, Y.; Ly, A.; Lahem, D.; Zhang, C.; Debliquy, M. A novel low-concentration isopropanol gas sensor based on Fe-doped ZnO nanoneedles and its gas sensing mechanism. J. Mater. Sci. 2020, 56, 3230–3245. [Google Scholar] [CrossRef]

| Structures | Parameter for Detection | Sensitivity | References |
|---|---|---|---|
| Glass substrate + ZnO-coated | Relative humidity | RH from 35% to 55% = −4.4 mV/% RH from 55% and 70% = 10 mV/% RH between 70% and 90% = 24.6 mV/% | [40] |
| SMS + ZnO-coated | Relative humidity | 0.06 nm/%RH | [22] |
| SMS + ZnO-coated | Ethanol concentrations | 50% of C2H5OH = −0.056 fitted response curve 60% of C2H5OH = −0.065 fitted response curve | [22] |
| ZnO-CdO composites on alumina tubes with Au electrodes and platinum wires | Isopropanol concentrations | 0.153CIPA CIPA = gas concentration of IPA at Temperature = 248 °C | [41] |
| Fe-doped ZnO nanoneedles | Isopropanol concentrations | 5 ppm IPA, response = 23.6 250 ppb IPA, response = 4.7 | [42] |
| SMS + ZnO-coated | Isopropanol concentrations | 0.053 nm/%IPA vapor (20%–100%) IPA vapor | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swargiary, K.; Metem, P.; Kulatumyotin, C.; Thaneerat, S.; Ajchareeyasoontorn, N.; Jitpratak, P.; Bora, T.; Mohammed, W.S.; Dutta, J.; Viphavakit, C. ZnO Nanorods Coated Single-Mode–Multimode–Single-Mode Optical Fiber Sensor for VOC Biomarker Detection. Sensors 2022, 22, 6273. https://doi.org/10.3390/s22166273
Swargiary K, Metem P, Kulatumyotin C, Thaneerat S, Ajchareeyasoontorn N, Jitpratak P, Bora T, Mohammed WS, Dutta J, Viphavakit C. ZnO Nanorods Coated Single-Mode–Multimode–Single-Mode Optical Fiber Sensor for VOC Biomarker Detection. Sensors. 2022; 22(16):6273. https://doi.org/10.3390/s22166273
Chicago/Turabian StyleSwargiary, Kankan, Prattakorn Metem, Chayapol Kulatumyotin, Suphavit Thaneerat, Noppasin Ajchareeyasoontorn, Pannathorn Jitpratak, Tanujjal Bora, Waleed S. Mohammed, Joydeep Dutta, and Charusluk Viphavakit. 2022. "ZnO Nanorods Coated Single-Mode–Multimode–Single-Mode Optical Fiber Sensor for VOC Biomarker Detection" Sensors 22, no. 16: 6273. https://doi.org/10.3390/s22166273
APA StyleSwargiary, K., Metem, P., Kulatumyotin, C., Thaneerat, S., Ajchareeyasoontorn, N., Jitpratak, P., Bora, T., Mohammed, W. S., Dutta, J., & Viphavakit, C. (2022). ZnO Nanorods Coated Single-Mode–Multimode–Single-Mode Optical Fiber Sensor for VOC Biomarker Detection. Sensors, 22(16), 6273. https://doi.org/10.3390/s22166273











