Enhanced Photoacoustic Visualisation of Clinical Needles by Combining Interstitial and Extracorporeal Illumination of Elastomeric Nanocomposite Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photoacoustic-Ultrasound Imaging System with Interstitial and Extracorporeal Illumination
2.2. Coating Optical Fibres and Medical Needles
2.2.1. Coating Fabrication
2.2.2. Coating Examination
2.2.3. Optical Characterisation
2.3. Imaging with Needle Insertions into Ex Vivo Tissue
3. Results
3.1. Coating Examination
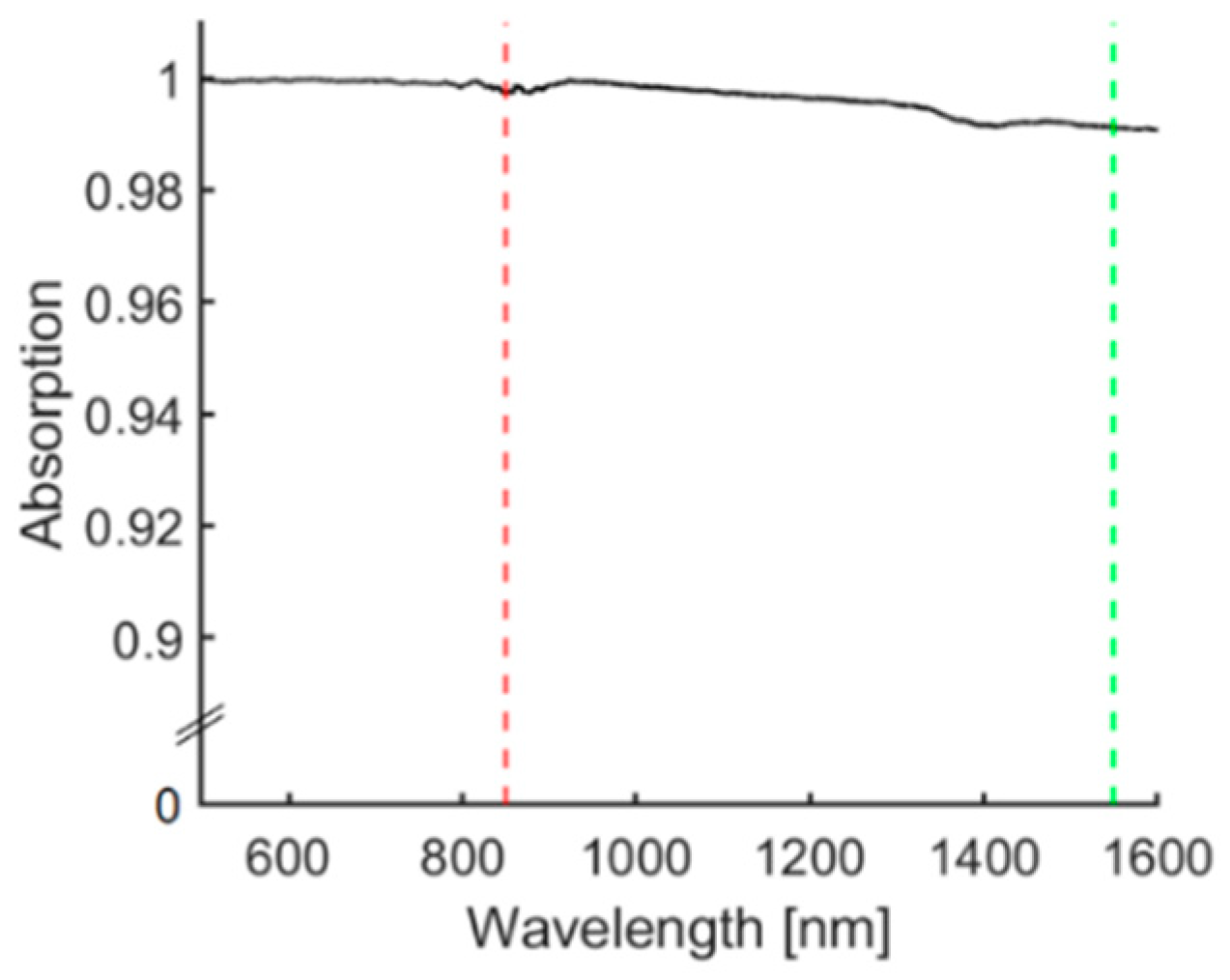
3.2. Optical Characterisation
3.3. Out-of-Plane Insertions
3.4. In-Plane Insertions
3.5. SNR Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chin, K.J.; Perlas, A.; Chan, V.W.S.; Brull, R. Needle Visualization in Ultrasound-Guided Regional Anesthesia: Challenges and Solutions. Reg. Anesth. Pain Med. 2008, 33, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Helbich, T.H.; Matzek, W.; Fuchsjäger, M.H. Stereotactic and Ultrasound-Guided Breast Biopsy. Eur. Radiol. 2004, 14, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Daffos, F.; Capella-Pavlovsky, M.; Forestier, F. Fetal Blood Sampling during Pregnancy with Use of a Needle Guided by Ultrasound: A Study of 606 Consecutive Cases. Am. J. Obstet. Gynecol. 1985, 153, 655–660. [Google Scholar] [CrossRef]
- Agarwal, K.; Alfirevic, Z. Pregnancy Loss after Chorionic Villus Sampling and Genetic Amniocentesis in Twin Pregnancies: A Systematic Review. Ultrasound Obstet. Gynecol. 2012, 40, 128–134. [Google Scholar] [CrossRef]
- Hebard, S.; Hocking, G. Echogenic Technology Can Improve Needle Visibility during Ultrasound-Guided Regional Anesthesia. Reg. Anesth. Pain Med. 2011, 36, 185–189. [Google Scholar] [CrossRef]
- Hocking, G.; Mitchell, C.H. Optimizing the Safety and Practice of Ultrasound-Guided Regional Anesthesia: The Role of Echogenic Technology. Curr. Opin. Anaesthesiol. 2012, 25, 603–609. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kamiya, T.; Arakawa, K.; Akiyama, S.; Sakai, K. Objective and Subjective Comparison of the Visibility of Three Echogenic Needles and a Nonechogenic Needle on Older Ultrasound Devices. Acta Anaesthesiol. Taiwan. 2015, 53, 1–6. [Google Scholar] [CrossRef]
- Chan, C.; Lam, F.; Rohling, R. A Needle Tracking Device for Ultrasound Guided Percutaneous Procedures. Ultrasound Med. Biol. 2005, 31, 1469–1483. [Google Scholar] [CrossRef]
- Kim, C.; Chang, D.; Petrisor, D.; Chirikjian, G.; Han, M.; Stoianovici, D. Ultrasound Probe and Needle-Guide Calibration for Robotic Ultrasound Scanning and Needle Targeting. IEEE Trans. Biomed. Eng. 2013, 60, 1728–1734. [Google Scholar] [CrossRef]
- Stüber, V.; Suero, E.M.; Hüfner, T.; Wiewiorski, M.; Krettek, C.; Citak, M. Linear Bearing Device as a Solution for Optical Navigation of Fine Needle Procedures. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2010, 18, 267–273. [Google Scholar] [CrossRef]
- Poulin, F.; Amiot, L.-P. Interference during the Use of an Electromagnetic Tracking System under OR Conditions. J. Biomech. 2002, 35, 733–737. [Google Scholar] [CrossRef]
- Breyer, B.; Cikeš, I. Ultrasonically Marked Catheter—a Method for Positive Echographic Catheter Position Identification. Med. Biol. Eng. Comput. 1984, 22, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Winsberg, F.; Mitty, H.A.; Shapiro, R.S.; Yeh, H.C. Use of an Acoustic Transponder for US Visualization of Biopsy Needles. Radiology 1991, 180, 877–878. [Google Scholar] [CrossRef]
- Xia, W.; Mari, J.M.; West, S.J.; Ginsberg, Y.; David, A.L.; Ourselin, S.; Desjardins, A.E. In-Plane Ultrasonic Needle Tracking Using a Fiber-Optic Hydrophone. Med. Phys. 2015, 42, 5983–5991. [Google Scholar] [CrossRef]
- Xia, W.; Ginsberg, Y.; West, S.J.; Nikitichev, D.I.; Ourselin, S.; David, A.L.; Desjardins, A.E. Coded Excitation Ultrasonic Needle Tracking: An in Vivo Study. Med. Phys. 2016, 43, 4065. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; West, S.J.; Mari, J.-M.; Ourselin, S.; David, A.L.; Desjardins, A.E. 3D Ultrasonic Needle Tracking with a 1.5D Transducer Array for Guidance of Fetal Interventions. Med. Image Comput. Comput.-Assist. Interv. MICCAI Int. Conf. Med. Image Comput. Comput.-Assist. Interv. 2016, 9900, 353–361. [Google Scholar] [CrossRef]
- Xia, W.; West, S.J.; Finlay, M.C.; Mari, J.-M.; Ourselin, S.; David, A.L.; Desjardins, A.E. Looking beyond the Imaging Plane: 3D Needle Tracking with a Linear Array Ultrasound Probe. Sci. Rep. 2017, 7, 3674. [Google Scholar] [CrossRef]
- Mung, J.; Han, S.; Yen, J.T. Design and in Vitro Evaluation of a Real-Time Catheter Localization System Using Time of Flight Measurements from Seven 3.5 MHz Single Element Ultrasound Transducers towards Abdominal Aortic Aneurysm Procedures. Ultrasonics 2011, 51, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Mung, J.; Vignon, F.; Erkamp, R.; Stanton, D.; Jain, A. Ultrasonically Marked Instruments for Ultrasound-Guided Interventions. In Proceedings of the 2013 IEEE International Ultrasonics Symposium (IUS), Prague, Czech Republic, 21–25 July 2013; pp. 2053–2056. [Google Scholar]
- Colchester, R.J.; Alles, E.J.; Desjardins, A.E. A Directional Fibre Optic Ultrasound Transmitter Based on a Reduced Graphene Oxide and Polydimethylsiloxane Composite. Appl. Phys. Lett. 2019, 114, 113505. [Google Scholar] [CrossRef]
- Wei, C.-W.; Nguyen, T.-M.; Xia, J.; Arnal, B.; Wong, E.Y.; Pelivanov, I.M.; O’Donnell, M. Real-Time Integrated Photoacoustic and Ultrasound (PAUS) Imaging System to Guide Interventional Procedures: Ex Vivo Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Noimark, S.; Ourselin, S.; West, S.J.; Finlay, M.C.; David, A.L.; Desjardins, A.E. Ultrasonic Needle Tracking with a Fibre-Optic Ultrasound Transmitter for Guidance of Minimally Invasive Fetal Surgery. Med. Image Comput. Comput.-Assist. Interv. MICCAI Int. Conf. Med. Image Comput. Comput.-Assist. Interv. 2017, 10434, 637–645. [Google Scholar] [CrossRef]
- Lediju Bell, M.A.; Shubert, J. Photoacoustic-Based Visual Servoing of a Needle Tip. Sci. Rep. 2018, 8, 15519. [Google Scholar] [CrossRef]
- Cheng, A.; Itsarachaiyot, Y.; Kim, Y.; Zhang, H.K.; Taylor, R.H.; Boctor, E.M. Catheter Tracking in an Interventional Photoacoustic Surgical System. In Proceedings of the Medical Imaging 2017: Image-Guided Procedures, Robotic Interventions, and Modeling, Orlando, FL, USA, 3 March 2017; Volume 10135, pp. 580–587. [Google Scholar]
- Takeshima, H.; Tanaka, T.; Imai, R. Position Detection of Guidewire Tip Emitting Ultrasound by Using a Kalman Filter. Jpn. J. Appl. Phys. 2021, 60, 087002. [Google Scholar] [CrossRef]
- Beard, P. Biomedical Photoacoustic Imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Desjardins, A.E.; Ourselin, S.; Vercauteren, T.; Xia, W. Minimally Invasive Photoacoustic Imaging: Current Status and Future Perspectives. Photoacoustics 2019, 16, 100146. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Yao, J. A Practical Guide to Photoacoustic Tomography in the Life Sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Kuniyil Ajith Singh, M.; Maneas, E.; Sato, N.; Shigeta, Y.; Agano, T.; Ourselin, S.; West, S.J.; Desjardins, A.E. Handheld Real-Time LED-Based Photoacoustic and Ultrasound Imaging System for Accurate Visualization of Clinical Metal Needles and Superficial Vasculature to Guide Minimally Invasive Procedures. Sensors 2018, 18, 1394. [Google Scholar] [CrossRef] [PubMed]
- Kuniyil Ajith Singh, M.; Xia, W. Portable and Affordable Light Source-Based Photoacoustic Tomography. Sensors 2020, 20, 6173. [Google Scholar] [CrossRef]
- Singh, M.K.A. LED-Based Photoacoustic Imaging: From Bench to Bedside; Springer Nature: Berlin/Heidelberg, Germany, 2020; ISBN 9789811539848. [Google Scholar]
- Xia, W.; Noimark, S.; Maneas, E.; Brown, N.M.; Singh, M.K.A.; Ourselin, S.; West, S.J.; Desjardins, A.E. Enhancing Photoacoustic Visualization of Medical Devices with Elastomeric Nanocomposite Coatings. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2019, San Francisco, CA, USA, 27 February 2019; Volume 10878, pp. 299–303. [Google Scholar]
- Jaeger, M.; Schüpbach, S.; Gertsch, A.; Kitz, M.; Frenz, M. Fourier Reconstruction in Optoacoustic Imaging Using Truncated Regularized Inverse k -Space Interpolation. Inverse Probl. 2007, 23, S51–S63. [Google Scholar] [CrossRef]
- Chang, W.-Y.; Huang, W.; Kim, J.; Li, S.; Jiang, X. Candle Soot Nanoparticles-Polydimethylsiloxane Composites for Laser Ultrasound Transducers. Appl. Phys. Lett. 2015, 107, 161903. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Zhao, T.; West, S.J.; Desjardins, A.E.; Vercauteren, T.; Xia, W. Improving Needle Visibility in LED-Based Photoacoustic Imaging Using Deep Learning with Semi-Synthetic Datasets. Photoacoustics 2022, 26, 100351. [Google Scholar] [CrossRef] [PubMed]
- Noimark, S.; Colchester, R.J.; Blackburn, B.J.; Zhang, E.Z.; Alles, E.J.; Ourselin, S.; Beard, P.C.; Papakonstantinou, I.; Parkin, I.P.; Desjardins, A.E. Carbon-Nanotube–PDMS Composite Coatings on Optical Fibers for All-Optical Ultrasound Imaging. Adv. Funct. Mater. 2016, 26, 8390–8396. [Google Scholar] [CrossRef]
- Noimark, S.; Colchester, R.J.; Poduval, R.K.; Maneas, E.; Alles, E.J.; Zhao, T.; Zhang, E.Z.; Ashworth, M.; Tsolaki, E.; Chester, A.H.; et al. Polydimethylsiloxane Composites for Optical Ultrasound Generation and Multimodality Imaging. Adv. Funct. Mater. 2018, 28, 1704919. [Google Scholar] [CrossRef]
- Bodian, S.; Colchester, R.J.; Macdonald, T.J.; Ambroz, F.; Briceno de Gutierrez, M.; Mathews, S.J.; Fong, Y.M.M.; Maneas, E.; Welsby, K.A.; Gordon, R.J.; et al. CuInS2 Quantum Dot and Polydimethylsiloxane Nanocomposites for All-Optical Ultrasound and Photoacoustic Imaging. Adv. Mater. Interfaces 2021, 8, 2100518. [Google Scholar] [CrossRef]
- Chang, W.-Y.; Jiang, X. A Fiber Optic Laser Ultrasound Transducer Using Candle Soot Nanoparticles/PDMS Composites. In Proceedings of the 2018 IEEE 18th International Conference on Nanotechnology (IEEE-NANO), Cork, Ireland, 23–26 July 2018; pp. 1–2. [Google Scholar]
- Chen, S.-L. Review of Laser-Generated Ultrasound Transmitters and Their Applications to All-Optical Ultrasound Transducers and Imaging. Appl. Sci. 2016, 7, 25. [Google Scholar] [CrossRef]
- Aytac-Kipergil, E.; Alles, E.J.; Pauw, H.C.; Karia, J.; Noimark, S.; Desjardins, A.E. Versatile and Scalable Fabrication Method for Laser-Generated Focused Ultrasound Transducers. Opt. Lett. 2019, 44, 6005. [Google Scholar] [CrossRef]
- Dromi, S.; Frenkel, V.; Luk, A.; Traughber, B.; Angstadt, M.; Bur, M.; Poff, J.; Xie, J.; Libutti, S.K.; Li, K.C.P.; et al. Pulsed-High Intensity Focused Ultrasound and Low Temperature–Sensitive Liposomes for Enhanced Targeted Drug Delivery and Antitumor Effect. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 2722–2727. [Google Scholar] [CrossRef]
- Illing, R.O.; Kennedy, J.E.; Wu, F.; ter Haar, G.R.; Protheroe, A.S.; Friend, P.J.; Gleeson, F.V.; Cranston, D.W.; Phillips, R.R.; Middleton, M.R. The Safety and Feasibility of Extracorporeal High-Intensity Focused Ultrasound (HIFU) for the Treatment of Liver and Kidney Tumours in a Western Population. Br. J. Cancer 2005, 93, 890–895. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Arias Moreno, A.J.; van Rietbergen, B.; ter Hoeve, N.D.; van Diest, P.J.; Grüll, H. Effects of Magnetic Resonance-Guided High-Intensity Focused Ultrasound Ablation on Bone Mechanical Properties and Modeling. J. Ther. Ultrasound 2015, 3, 13. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Chang, W.-Y.; Huang, W.; Jiang, X.; Dayton, P.A. Candle-Soot Carbon Nanoparticles in Photoacoustics: Advantages and Challenges for Laser Ultrasound Transmitters. IEEE Nanotechnol. Mag. 2019, 13, 13–28. [Google Scholar] [CrossRef]
- Mulay, M.R.; Chauhan, A.; Patel, S.; Balakrishnan, V.; Halder, A.; Vaish, R. Candle Soot: Journey from a Pollutant to a Functional Material. Carbon 2019, 144, 684–712. [Google Scholar] [CrossRef]
- Pandey, H.; Saini, S.; Singh, S.P.; Gautam, N.K.; Singh, S. Candle Soot Derived Carbon Nanoparticles: An Assessment of Cellular and Progressive Toxicity Using Drosophila Melanogaster Model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 228, 108646. [Google Scholar] [CrossRef] [PubMed]
- Esteves da Silva, J.C.G.; Gonçalves, H.M.R. Analytical and Bioanalytical Applications of Carbon Dots. TrAC Trends Anal. Chem. 2011, 30, 1327–1336. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Bodian, S.; West, S.J.; Sathasivam, S.; Gordon, R.J.; Collier, P.; Vercauteren, T.; Desjardins, A.E.; Noimark, S.; Xia, W. Enhanced Photoacoustic Visualisation of Clinical Needles by Combining Interstitial and Extracorporeal Illumination of Elastomeric Nanocomposite Coatings. Sensors 2022, 22, 6417. https://doi.org/10.3390/s22176417
Shi M, Bodian S, West SJ, Sathasivam S, Gordon RJ, Collier P, Vercauteren T, Desjardins AE, Noimark S, Xia W. Enhanced Photoacoustic Visualisation of Clinical Needles by Combining Interstitial and Extracorporeal Illumination of Elastomeric Nanocomposite Coatings. Sensors. 2022; 22(17):6417. https://doi.org/10.3390/s22176417
Chicago/Turabian StyleShi, Mengjie, Semyon Bodian, Simeon J. West, Sanjayan Sathasivam, Ross J. Gordon, Paul Collier, Tom Vercauteren, Adrien E. Desjardins, Sacha Noimark, and Wenfeng Xia. 2022. "Enhanced Photoacoustic Visualisation of Clinical Needles by Combining Interstitial and Extracorporeal Illumination of Elastomeric Nanocomposite Coatings" Sensors 22, no. 17: 6417. https://doi.org/10.3390/s22176417
APA StyleShi, M., Bodian, S., West, S. J., Sathasivam, S., Gordon, R. J., Collier, P., Vercauteren, T., Desjardins, A. E., Noimark, S., & Xia, W. (2022). Enhanced Photoacoustic Visualisation of Clinical Needles by Combining Interstitial and Extracorporeal Illumination of Elastomeric Nanocomposite Coatings. Sensors, 22(17), 6417. https://doi.org/10.3390/s22176417






