Digital Pregnancy Test Powered by an Air-Breathing Paper-Based Microfluidic Fuel Cell Stack Using Human Urine as Fuel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Air-Breathing Paper-Based Microfluidic Fuel Cell Assembly Process
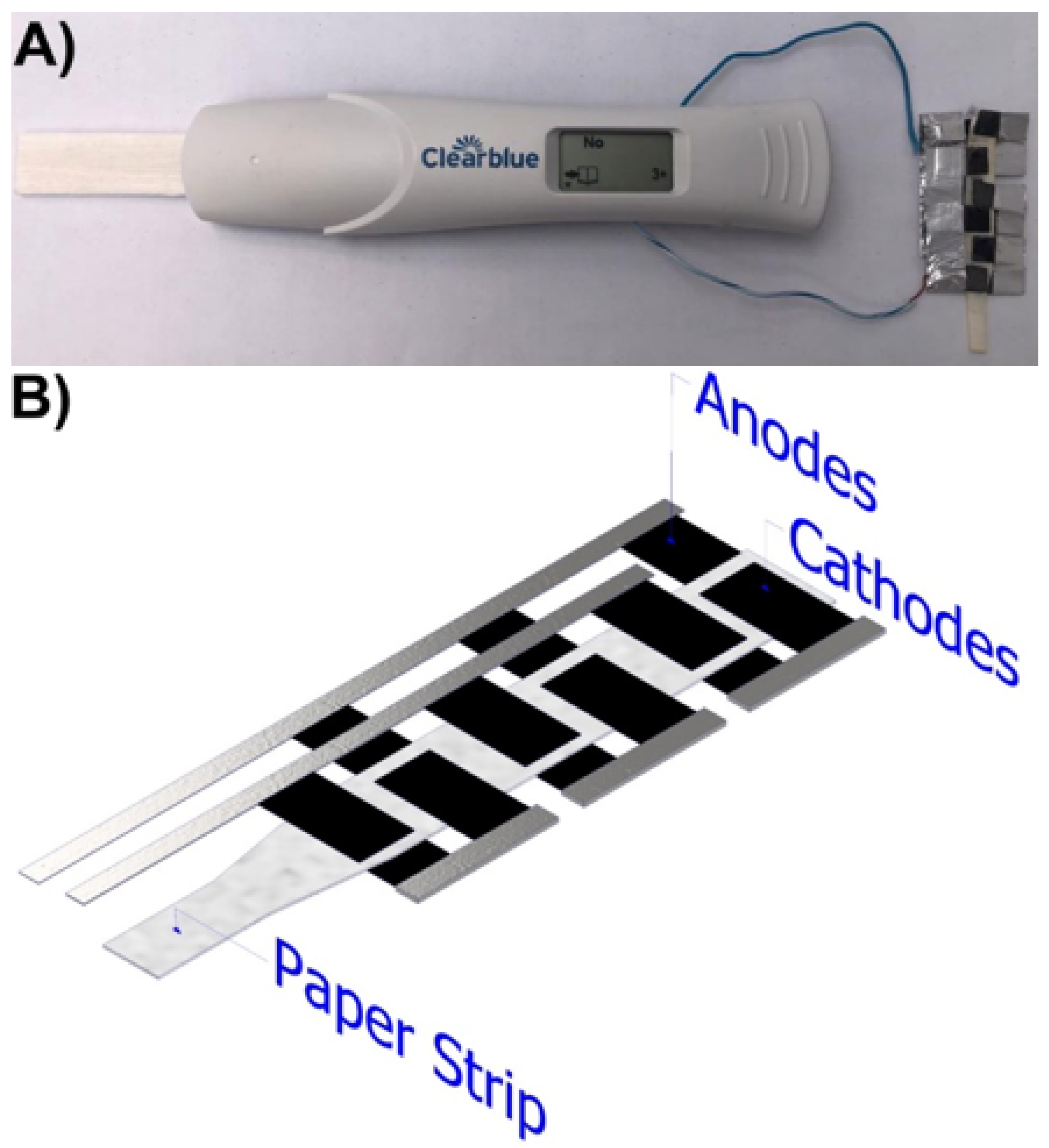
2.1.1. Air-Breathing Paper-Based µFC’s Placed Inside the Qualitative Pregnancy Test
2.1.2. Air-Breathing Paper-Based µFC’s Placed Outside the Quantitative Pregnancy Test
2.2. Performance of the Air-Breathing Paper-Based Microfluidic Fuel Cells
Air-Breathing Paper-Based µFC’s Placed Outside the Quantitative Pregnancy Test
3. Results and Discussion
3.1. Performance Measurement of the Air-Breathing Paper-Based Microfluidic Fuel Cell
| = −0.746 V (NHE) | |
| = 0.4 V (NHE) | |
| = 1.146 V (NHE) |
3.2. Application-Performance Measurement of the Air-Breathing Paper-Based Microfluidic Fuel Cells Stack
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Kawde, A.N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Sadeghi, P.; Sohrabi, H.; Hejazi, M.; Jahanban-Esfahlan, A.; Baradaran, B.; Tohidast, M.; Majidi, M.R.; Mokhtarzadeh, A.; Tavangar, S.M.; de la Guardia, M. Lateral flow assays (LFA) as an alternative medical diagnosis method for detection of virus species: The intertwine of nanotechnology with sensing strategies. TrAC Trends Anal. Chem. 2021, 145, 116460. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Handique, F. Utility of a lateral flow assay for culture confirmation of Mycobacterium tuberculosis complex. Med J. Armed Forces India 2016, 72, 41–44. [Google Scholar] [CrossRef]
- Domingues, R.; Silva, T. PMD37 Lateral Flow Urine Lipoarabinomannan Assay For Diagnosis of Active Tuberculosis in People Living with HIV: A Systematic Review and Meta-Analysis. Value Health 2020, 23, S195. [Google Scholar] [CrossRef]
- Akyar, I.; Kocagoz, T.; Sinik, G.; Oktem, S.; Aytekin, N.; Kocagoz, S. Lateral flow assay for rapid differentiation of Mycobacterium tuberculosis complex and 97 species of mycobacteria other than tuberculosis grown in Löwenstein-Jensen and TK-SLC medium. Indian J. Med. Microbiol. 2010, 28, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhong, H.; Huang, W.; Zhan, W.; Yang, X.; Tang, B.; Chen, K.; Wang, J.; Hu, T.; Zhang, C.; et al. Rapid and visual detection of Group B streptococcus using recombinase polymerase amplification combined with lateral flow strips. Diagn. Microbiol. Infect. Dis. 2019, 93, 9–13. [Google Scholar] [CrossRef]
- Herrero, D.R.; Soler-Palacin, P.; Cibrian, J.B.; Ferrer, V.F.; Anton Pagarolas, A.; Martin-Gomez, M.T. Detection of Streptococcus pneumoniae antigen in pleural fluid: Usefulness of an immunofluorescence-based lateral flow assay for the diagnosis of pneumococcal pneumonia. Diagn. Microbiol. Infect. Dis. 2020, 98, 115162. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Guo, T.; Xiao, C.; Liu, L.; Zhang, X.; Liu, B.; Li, P.; Liu, A.; Li, B.; et al. A rapid point-of-care test for dengue virus-1 based on a lateral flow assay with a near-infrared fluorescent dye. J. Immunol. Methods 2018, 456, 23–27. [Google Scholar] [CrossRef]
- Lee, J.H.; Seo, H.S.; Kwon, J.H.; Kim, H.T.; Kwon, K.C.; Sim, S.J.; Cha, Y.J.; Lee, J. Multiplex diagnosis of viral infectious diseases (AIDS, hepatitis C, and hepatitis A) based on point of care lateral flow assay using engineered proteinticles. Biosens. Bioelectron. 2015, 69, 213–225. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Famulok, M.; Mayer, G.; Blind, M. Nucleic Acid AptamersFrom Selection In Vitro to Applications In Vivo. Acc. Chem. Res. 2000, 33, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wu, W.; Lu, X.; Zeng, L. Lateral flow biosensor for DNA extraction-free detection of salmonella based on aptamer mediated strand displacement amplification. Biosens. Bioelectron. 2014, 56, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Dector, A.; de-la Rosa, J.G.; Amaya-Cruz, D.; Ortíz-Verdín, A.; Guerra-Balcázar, M.; Olivares-Ramírez, J.; Arriaga, L.; Ledesma-García, J. Towards autonomous lateral flow assays: Paper-based microfluidic fuel cell inside an HIV-test using a blood sample as fuel. Int. J. Hydrogen Energy 2017, 42, 27979–27986. [Google Scholar] [CrossRef]
- Chino, I.; Muneeb, O.; Do, E.; Ho, V.; Haan, J.L. A paper microfluidic fuel cell powered by urea. J. Power Sources 2018, 396, 710–714. [Google Scholar] [CrossRef]
- Esquivel, J.P.; Del Campo, F.J.; Gómez de la Fuente, J.L.; Rojas, S.; Sabaté, N. Microfluidic fuel cells on paper: Meeting the power needs of next generation lateral flow devices. Energy Environ. Sci. 2014, 7, 1744–1749. [Google Scholar] [CrossRef]
- González-Guerrero, M.J.; del Campo, F.J.; Esquivel, J.P.; Leech, D.; Sabaté, N. Paper-based microfluidic biofuel cell operating under glucose concentrations within physiological range. Biosens. Bioelectron. 2017, 90, 475–480. [Google Scholar] [CrossRef]
- Lau, C.; Moehlenbrock, M.J.; Arechederra, R.L.; Falase, A.; Garcia, K.; Rincon, R.; Minteer, S.D.; Banta, S.; Gupta, G.; Babanova, S.; et al. Paper based biofuel cells: Incorporating enzymatic cascades for ethanol and methanol oxidation. Int. J. Hydrogen Energy 2015, 40, 14661–14666. [Google Scholar] [CrossRef]
- Copenhaver, T.S.; Purohit, K.H.; Domalaon, K.; Pham, L.; Burgess, B.J.; Manorothkul, N.; Galvan, V.; Sotez, S.; Gomez, F.A.; Haan, J.L. A microfluidic direct formate fuel cell on paper. Electrophoresis 2015, 36, 1825–1829. [Google Scholar] [CrossRef]
- Castillo-Martínez, L.C.; Amaya-Cruz, D.M.; Gachuz, J.; Ortega-Díaz, D.; Olivares-Ramírez, J.M.; Dector, D.; Duarte-Moller, A.; Villa, A.L.; Dector, A. Urea oxidation in a paper-based microfluidic fuel cell using Escherichia coli anode electrode. J. Phys. Conf. Ser. 2018, 1119, 012004. [Google Scholar] [CrossRef]
- González-Guerrero, M.J.; del Campo, F.J.; Esquivel, J.P.; Giroud, F.; Minteer, S.D.; Sabaté, N. Paper-based enzymatic microfluidic fuel cell: From a two-stream flow device to a single-stream lateral flow strip. J. Power Sources 2016, 326, 410–416. [Google Scholar] [CrossRef]
- Hernández Rivera, J.; Ortega Díaz, D.; Amaya Cruz, D.M.; Rodríguez-Reséndiz, J.; Olivares Ramírez, J.M.; Dector, A.; Dector, D.; Galindo, R.; Esparza Ponce, H.E. A Paper-Based Microfluidic Fuel Cell Using Soft Drinks as a Renewable Energy Source. Energies 2020, 13, 2443. [Google Scholar] [CrossRef]
- Gómez-Ramírez, M.; Dector, A.; Amaya-Cruz, D.M.; Alamilla-Martínez, D.G.; Olivares-Ramírez, J.M.; Rojas-Avelizapa, N.G.; Sosa-Domínguez, A.; Morales, J. Effect of the (AuNPs) Biosynthesized Used as Anodes on the Performance of a Human Blood Paper-Based Microfluidic Fuel Cell. ECS Trans. 2021, 100, 109–116. [Google Scholar] [CrossRef]
- Basumatary, P.; Konwar, D.; Yoon, Y.S. A novel NiCu/ZnO@MWCNT anode employed in urea fuel cell to attain superior performances. Electrochim. Acta 2018, 261, 78–85. [Google Scholar] [CrossRef]
- Sarigul, N.; Korkmaz, F.; Kurultak, İ. A new artificial urine protocol to better imitate human urine. Sci. Rep. 2019, 9, 20159. [Google Scholar] [CrossRef]
- Sayed, E.T.; Eisa, T.; Mohamed, H.O.; Abdelkareem, M.A.; Allagui, A.; Alawadhi, H.; Chae, K.J. Direct urea fuel cells: Challenges and opportunities. J. Power Sources 2019, 417, 159–175. [Google Scholar] [CrossRef]
- Schranck, A.; Marks, R.; Yates, E.; Doudrick, K. Effect of Urine Compounds on the Electrochemical Oxidation of Urea Using a Nickel Cobaltite Catalyst: An Electroanalytical and Spectroscopic Investigation. Environ. Sci. Technol. 2018, 52, 8638–8648. [Google Scholar] [CrossRef]
- Choi, W.S.; Lee, S.H.; Ko, J.W.; Park, C.B. Human Urine-Fueled Light-Driven NADH Regeneration for Redox Biocatalysis. ChemSusChem 2016, 9, 1559–1564. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Botte, G.G. Electrochemical decomposition of urea with Ni-based catalysts. Appl. Catal. B Environ. 2012, 127, 221–226. [Google Scholar] [CrossRef]
- Putnam, D.F. Composition and Concentrative Properties of Human Urine; Technical Report; NASA: Washington, DC, USA, 1971. [Google Scholar]
- Dector, D.; Ortega-Díaz, D.; Olivares-Ramírez, J.; Dector, A.; Pérez-Bueno, J.; Fernández, D.; Amaya-Cruz, D.; Reyes-Rojas, A. Harvesting energy from real human urine in a photo-microfluidic fuel cell using TiO2–Ni anode electrode. Int. J. Hydrogen Energy 2021, 46, 26163–26173. [Google Scholar] [CrossRef]
- Galindo-de-la Rosa, J.; Arriaga, L.G.; Álvarez, A.; Arjona, N.; Déctor, A.; Chavéz-Ramírez, A.U.; Vallejo-Becerra, V.; Ledesma-García, J. NiAl Layered Double Hydroxides and PdNiO as Multifunctional Anodes for Prospective Self-Powered Lab-on-a-Chip Dopamine Sensors. ChemNanoMat 2018, 4, 688–697. [Google Scholar] [CrossRef]
- Mankar, C.; Rewatkar, P.; Dhone, M.; Balpande, S.; Kalambe, J.; Pande, R.; Goel, S. Paper Based Microfluidic Microbial Fuel Cell to Harvest Energy from Urine. Sens. Lett. 2019, 17, 69–74. [Google Scholar] [CrossRef]
- Liu, L.; Mo, H.; Wei, S.; Raftery, D. Quantitative analysis of urea in human urine and serum by 1 H nuclear magnetic resonances. Analyst 2012, 3, 595–600. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.T.; Kim, J. Photocatalytic oxidation of urea on TiO2 in water and urine: Mechanism, product distribution, and effect of surface platinization. Environ. Sci. Pollut. Res. 2019, 26, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Ovando-Medina, V.; Dector, A.; Antonio-Carmona, I.; Romero-Galarza, A.; Martínez-Gutiérrez, H.; Olivares-Ramírez, J. A new type of air-breathing photo-microfluidic fuel cell based on ZnO/Au using human blood as energy source. Int. J. Hydrogen Energy 2019, 44, 31423–31433. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, C.; Wang, Y.; Wang, K. Advanced Nickel-Based Catalysts for Urea Oxidation Reaction: Challenges and Developments. Catalysts 2022, 12, 337. [Google Scholar] [CrossRef]





| Electrodes (Anode-Cathode) | Fuel | µFCs Type | OCV (V) | (mA cm) | (mW cm) | Reference |
|---|---|---|---|---|---|---|
| Pt-C | Urea (0.3 M) in KOH (1 M) | Paper µFCs | 0.6 | 4.3 | 0.91 | [15] |
| Pt/C-E. coli | Urea (0.33 M) in KOH (0.3 M) | Paper-based bacterial µFCs | 0.83 | 3.253 | 0.608 | [20] |
| Ag-C | Human urine (pH = 6) in H2O2 (9.78 M) | Paper-based microfluidic microbial FCs | 0.5 | 0.56 | 0.1288 | [33] |
| NiAl–LDHs–Pt/C | Human urine (0.7973 M urea) | air breathing PMMA µFCs using pump | 1 | 122 | 50 | [32] |
| TiO-Ni–Pt/C | Human urine (0.366 M urea) | Photo PMMA µFCs using pump | 0.7 | 1.7 | 0.09 | [31] |
| TiO-Ni–Pt/C | Human urine (0.391 M urea) | Air-breathing paper-based µFCs inside pregnancy test | 0.96 | 1 | 0.23 | This work |
| TiO-Ni–Pt/C | Human urine (0.391 M urea) | Air-breathing paper-based µFCs stack outside pregnancy test | 1.89 | 2.77 | 1.38 | This work |
| LDHs: Layered Double Hydroxides | ||||||
| PMMA: Polymethylmethacrylate | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-Estrada, I.L.; Olivares-Ramírez, J.M.; Rodríguez-Reséndiz, J.; Dector, A.; Mendiola-Santibañez, J.D.; Amaya-Cruz, D.M.; Sosa-Domínguez, A.; Ortega-Díaz, D.; Dector, D.; Ovando-Medina, V.M.; et al. Digital Pregnancy Test Powered by an Air-Breathing Paper-Based Microfluidic Fuel Cell Stack Using Human Urine as Fuel. Sensors 2022, 22, 6641. https://doi.org/10.3390/s22176641
Vera-Estrada IL, Olivares-Ramírez JM, Rodríguez-Reséndiz J, Dector A, Mendiola-Santibañez JD, Amaya-Cruz DM, Sosa-Domínguez A, Ortega-Díaz D, Dector D, Ovando-Medina VM, et al. Digital Pregnancy Test Powered by an Air-Breathing Paper-Based Microfluidic Fuel Cell Stack Using Human Urine as Fuel. Sensors. 2022; 22(17):6641. https://doi.org/10.3390/s22176641
Chicago/Turabian StyleVera-Estrada, Irma Lucia, Juan Manuel Olivares-Ramírez, Juvenal Rodríguez-Reséndiz, Andrés Dector, Jorge Domingo Mendiola-Santibañez, Diana María Amaya-Cruz, Adrían Sosa-Domínguez, David Ortega-Díaz, Diana Dector, Victor Manuel Ovando-Medina, and et al. 2022. "Digital Pregnancy Test Powered by an Air-Breathing Paper-Based Microfluidic Fuel Cell Stack Using Human Urine as Fuel" Sensors 22, no. 17: 6641. https://doi.org/10.3390/s22176641
APA StyleVera-Estrada, I. L., Olivares-Ramírez, J. M., Rodríguez-Reséndiz, J., Dector, A., Mendiola-Santibañez, J. D., Amaya-Cruz, D. M., Sosa-Domínguez, A., Ortega-Díaz, D., Dector, D., Ovando-Medina, V. M., & Antonio-Carmona, I. D. (2022). Digital Pregnancy Test Powered by an Air-Breathing Paper-Based Microfluidic Fuel Cell Stack Using Human Urine as Fuel. Sensors, 22(17), 6641. https://doi.org/10.3390/s22176641








