1. Introduction
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder, affecting 2–3% of the senior population (age ≥ 65 years) [
1]. The most prominent motor symptoms of PD are tremor (i.e., involuntary movement of body parts), rigidity (i.e., resistance to externally imposed movements), bradykinesia (i.e., slowness of movement), and postural instability [
2]. As the pathological hallmark of PD is the loss of dopaminergic neurons in the substantia nigra pars compacta, the current treatment of PD is based on the replacement of dopamine. The available treatments can reduce the motor symptoms; however, due to the fluctuations of dopamine levels in the brain, the severity of the symptoms can drastically fluctuate and negatively influence the lives of people living with PD (PwP) [
3].
Among PD symptoms, hand tremor is very common. It is estimated that between 79% and 90% of PwP suffer from hand tremors [
2]. The two main classes of tremors are rest tremor and action tremor. Rest tremor occurs when relevant muscles are at rest, while action tremor occurs when relevant muscles are activated. Action tremor includes postural, kinetic, intention and specific tremor [
4,
5]. For many PwP, hand tremor is highly disabling and treatment is needed. Even though a few medications are available, no solid evidence exists regarding the effectiveness of most recommended treatments [
6,
7]. Thus, the treatment of hand tremors remains limited, such that one-third of patients discontinue taking their tremor medications [
8]. Hence, alternative methods to assess and manage the tremor should be considered. Recently, wearable robotic systems have shown promising results in providing rehabilitation and assistance in movement problems [
9]. Wearable devices have shown high accuracy in assessing the severity of tremor [
10]. However, tremor management can be more challenging. For example, the mechanical suppression of tremor has been used to improve voluntary motions [
11]. However, as the time evolution of tremor follows non-linear dynamics and can significantly vary from one person to another [
12], modeling tremor and distinguishing the voluntary motion from tremor can be challenging. Moreover, most of the current approaches are focused on predicting the tremor [
13,
14]. However, strong evidence of the existence of low dimensional chaotic structures in the dynamics of cortical activity of PwP has been observed [
15]. The consequence of dealing with a chaotic system is that even infinitesimal errors in the initial condition of a predictor model will grow exponentially fast. Consequently, the prediction horizon of tremor estimation is extremely limited in time. An alternative solution was proposed by Taheri et al. [
11], in which instead of estimating tremor, the muscle torque that produced the tremor was estimated, and then an equal and opposite torque would be applied to suppress tremor. The main challenge of this approach is the inability to attenuate tremor without interfering with voluntary motion. Hence, as motion of PwP are combinations of their voluntary movements and tremor, it is essential that the voluntary movement not be taken as tremor [
16]. Thus, identifying the task (gesture) and the tremor type (resting, postural, or action) can provide crucial information for a control system. Furthermore, as many PwP experience muscle weakness [
17], they require assistance with their voluntary movements, thus identifying the voluntary movement is deemed necessary.
Currently, surface electromyography (sEMG) sensors are one of the most common technologies that are used for controlling wearable devices or for hand gesture recognition [
18]. For example, in the rehabilitation sciences, sEMG is frequently used to examine voluntary muscle contractions [
19]. In sports physiology, sEMG signals are routinely used to coordinate the movement optimization and estimate muscle fatigue [
20]. The possibility of using sEMG to study muscle behaviours has been investigated in the field of clinical neurophysiology [
21,
22]. Moreover, sEMG has proven beneficial in differentiating between muscle movement characteristics of PD and healthy motion [
23,
24,
25]. Although, sEMG data have shown promising results in the mentioned research areas, their potential to identify parkinsonian hand tremor type and gesture classification has remained understudied. For example, in a small study with only one PwP participant, sEMG detectors were placed on specific muscles to determine their voluntarily movements [
26]. The participant was not performing any real task, and they just moved their hand to engage the target muscle. Investigating the possibility of using sEMG data to identify voluntary hand motions during task performance can be extremely important to help PwP with task performance and to suppress tremor.
In many wearable robotic tremor suppression devices, IMUs are being used [
27,
28,
29]. Alternatively, using sEMG data means that parts of the sensing system can be moved from the hands onto the forearms to reduce the required hardware within the device. Furthermore, as the orientation of the electrode does not change the output data, the calibration process of the tremor suppression devices could be simpler, and the devices could become more user friendly.
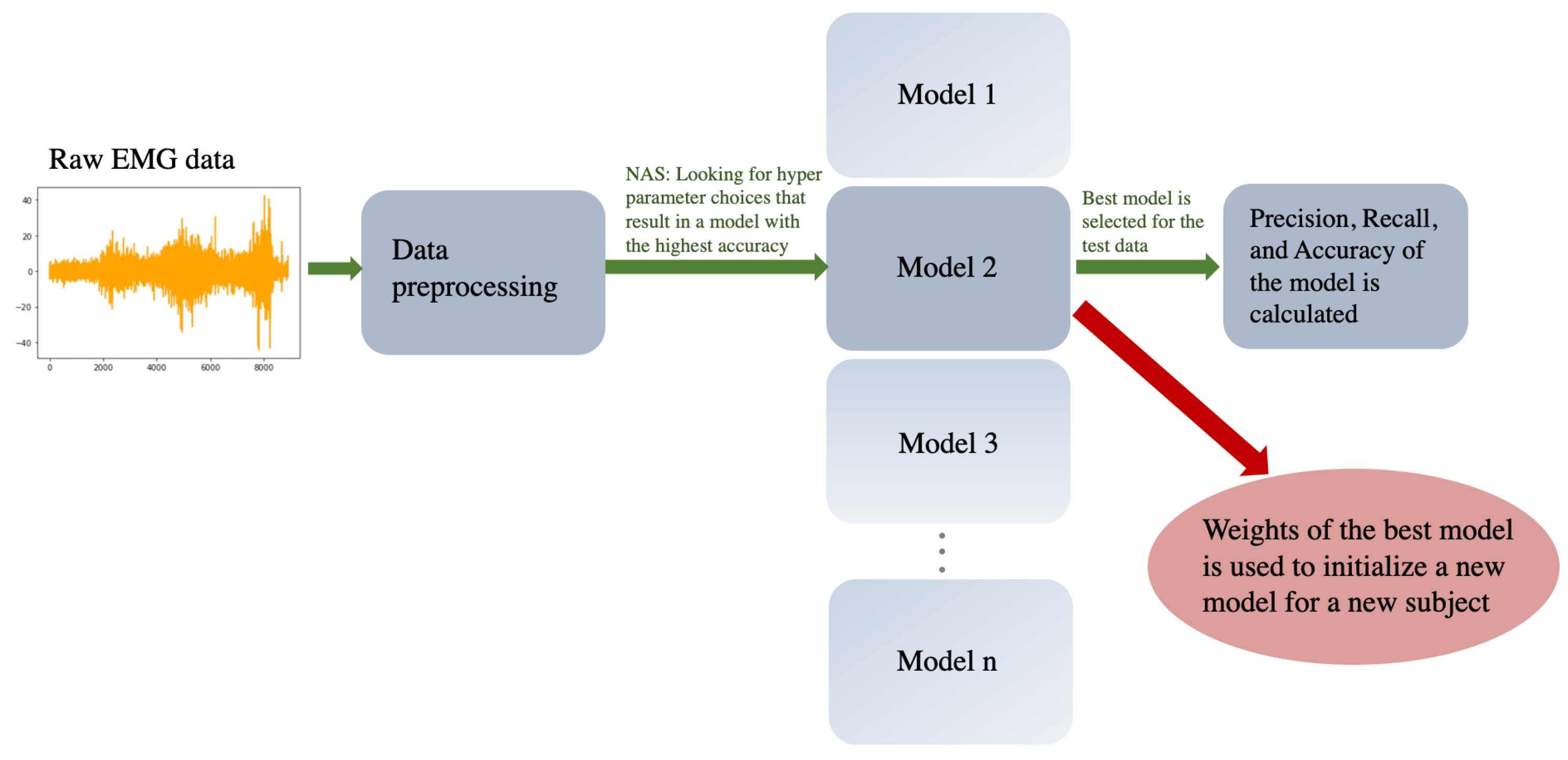
The contribution of this work is that it is the first implementation of a machine learning (ML) algorithm that can successfully identify motions of PwP, as well as the tremor type, purely from sEMG signals. When performing a task in the presence of tremor, accurate identification of the voluntary motions and distinguishing the type of tremor can significantly improve the design of wearable robotic tremor suppression devices, which could lead to improving the lives of PwP. Recently, ML methods have been used for both the identification and prediction of bio signal from sensors. As these algorithms have been shown to be able to find patterns in complex data, they were selected as the main statistical tool to identify the tremor types as well as intended voluntary motions of the people in the current study. A brief review of the proposed ML method is presented in
Section 2.
Section 3 provides details about the experimental setup for the training of the ML algorithms. In
Section 4, the results of the study are shared, and
Section 5 provides a discussion related to the results of the study.
5. Discussion
The focus of this study was to investigate if low-cost sEMG devices could be used with deep learning models to identify hand tremor types in PwP, as well as the voluntary motions performed. In the present study for tremor type classification and voluntary motion identification, a short sequence of data was used and fed into a network. Thus, a natural choice for the ML algorithm was a BiLSTM algorithm that is a sequence-aware algorithm. As a result of employing a BiLSTM algorithm, crucial information related to the time sequence of motions was conserved. The accuracy of task classification stood at , and the accuracy of tremor classification was . Of note, both models performed significantly above the chance levels (20% and 33% for task and tremor classification, respectively) which indicates that the models could successfully identify the task and tremor types. The results of the study clearly indicate that sEMG signals can be used to identify voluntary motions and thus they can be used in tremor suppression devices. This study was also the first attempt to use sEMG data to identify PwP voluntary motions during the performance of daily tasks (such as grabbing objects). The potential of using sEMG for identifying voluntary motions has been understudied. The only similar study was conducted on a single participant and rather than performing common tasks, the participant was performing eight movements targeting each muscle individually. Thus, their result could not be generalized to PwP performing activities of daily living, such as grabbing objects or drawing. Furthermore, the results from a single participant could not be extrapolated to a larger population. Although the present study is based on a small data set, it could still be used to provide an analysis of the accuracy of the trained models among the participants.
Moreover, using the warm initialization approach, the average accuracy of the model among the participants remained the same. Modest improvements in a few tasks were observed for only two participants; therefore, no conclusive statements related to the benefits of using the warm initialization approach can be presented. Nevertheless, as the warm-start could be closer to the optimal solution [
44], it would be a better initialization option. Thus, the warm initialization remained part of the proposed algorithm.
Another focus of the present study was to automate the hyperparameter tuning process. Hyperparameter tuning is an important yet challenging task that typically requires hours of time from an expert. In this paper, by implementing a regularized evolutionary algorithm, the search for the optimal neural architecture became fully automated. Thus, several parameters, including the number of neurons, learning rate, number of epochs, batch size, activation function, and optimization algorithms, were considered, and the best neural architecture for each subject was built. This can be an important step toward building practical tremor suppression devices, as the optimal hyperparameters can be found automatically, and accurate models can be trained.
The present study provides proof that the BiLSTM algorithm can identify a user’s tremor type, as well as their intended motion, from sEMG data alone. However, the experiments were limited to a few minutes of data, which was one of the main limitations of the current study. One of the major goals of implementing personalized ML models is to adapt the tremor suppression devices to the progression of the disease. Thus, going forward, it would be worthwhile to expand the duration of the data collection experiments to several hours or even several days, so that all possible motion and pattern variations of PwP can be recorded and fed into a classification model. Having access to longer periods of data would make it possible to examine the accuracy of models trained based on earlier data, and to explore the extent of accuracy in time. For example, having access to data collected every minute during a month would allow an examination would allow an examination into whether training the models based on the first week of data would result in accurate classifications in the last few days of the month, that would allow an assessment of whether the generalization holds over time and over more people. Another important aspect of conducting longer experiments is that the model could be trained and evaluated based on data collected outside of the laboratory. As reported in other studies, models trained using outside data had higher uncertainties [
59], thus it is important to evaluate the models under more realistic circumstances than within a lab setting. Of note, in the present study, the number of participants was limited to 15, thus the average accuracy among all of the participants had a higher standard deviation. One future direction is to conduct the experiment with a larger population of participants, as it is expected that repeating the experiment with an increased number of participants and longer duration experiments will minimize the effect of outliers.
It is also worth mentioning that the recruitment of participants for the current study was limited to PwP attending the local clinic, thus recruiting an equal number of males and females was challenging. The participants included nine male and six female participants, which was a balanced representation of the population, as Parkinson’s Disease affects more males than females. Of note, the evaluations did not indicate that the sex of the patients had a statistical effect on the results.
Furthermore, the present study used sEMG data to train the model to investigate whether the sEMG data alone could be used for task and tremor classification. Since the IMU data for the experiments were also recorded, it would be interesting in future work to train models using the IMU data, and to compare the accuracy of ML models that are trained using each of the data sets and their combination. Finally, another direction for future work will explore embedding this algorithm into the control system of a wearable device, and evaluate the accuracy of classifications in real time.