A Simple Method for the Detection of Biofilms Using a Heatable Capacitive Sensor Structure (CSS): Description, Proof of Concept, and Some Technical Improvements
Abstract
:1. Introduction
- The bacteria must be able to stick to the specific surface and produce an extracellular polymer matrix.
- The conditions must be suitable for bacteria to thrive. For example, there must be an adequate supply of oxygen and nutrients, especially carbon compounds.
- The ambient moisture must be sufficiently high as a result of humidity and/or condensation, regular wetting or flooding, or the ecosystem being permanently underwater.
- The sensors must not hydrodynamically obstruct flowing liquids. They should therefore be seamlessly integrated into smooth surfaces.
- To allow free surface water to be removed, the installation point must not be constantly underwater. Instead, the majority of the free water must be able to drain off freely before measurement takes place.
2. Materials and Methods
2.1. Sensor Structures
2.1.1. Modulated Sensor Structures
2.1.2. Flexible Sensor Structures
2.1.3. Rigid Sensor Structure
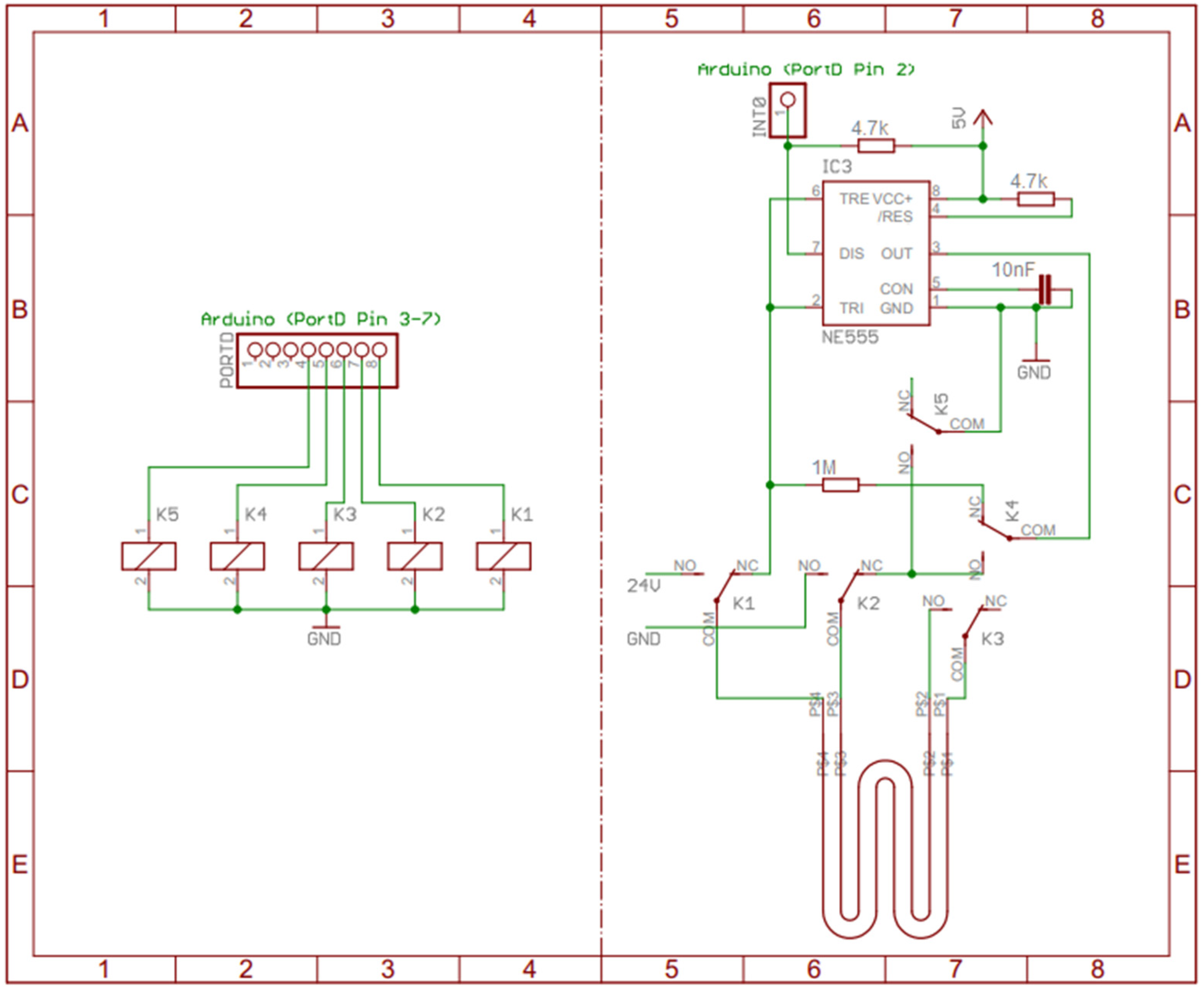
2.2. Heating Process
2.3. Capacitance Measurement
2.4. Temperature Measurement
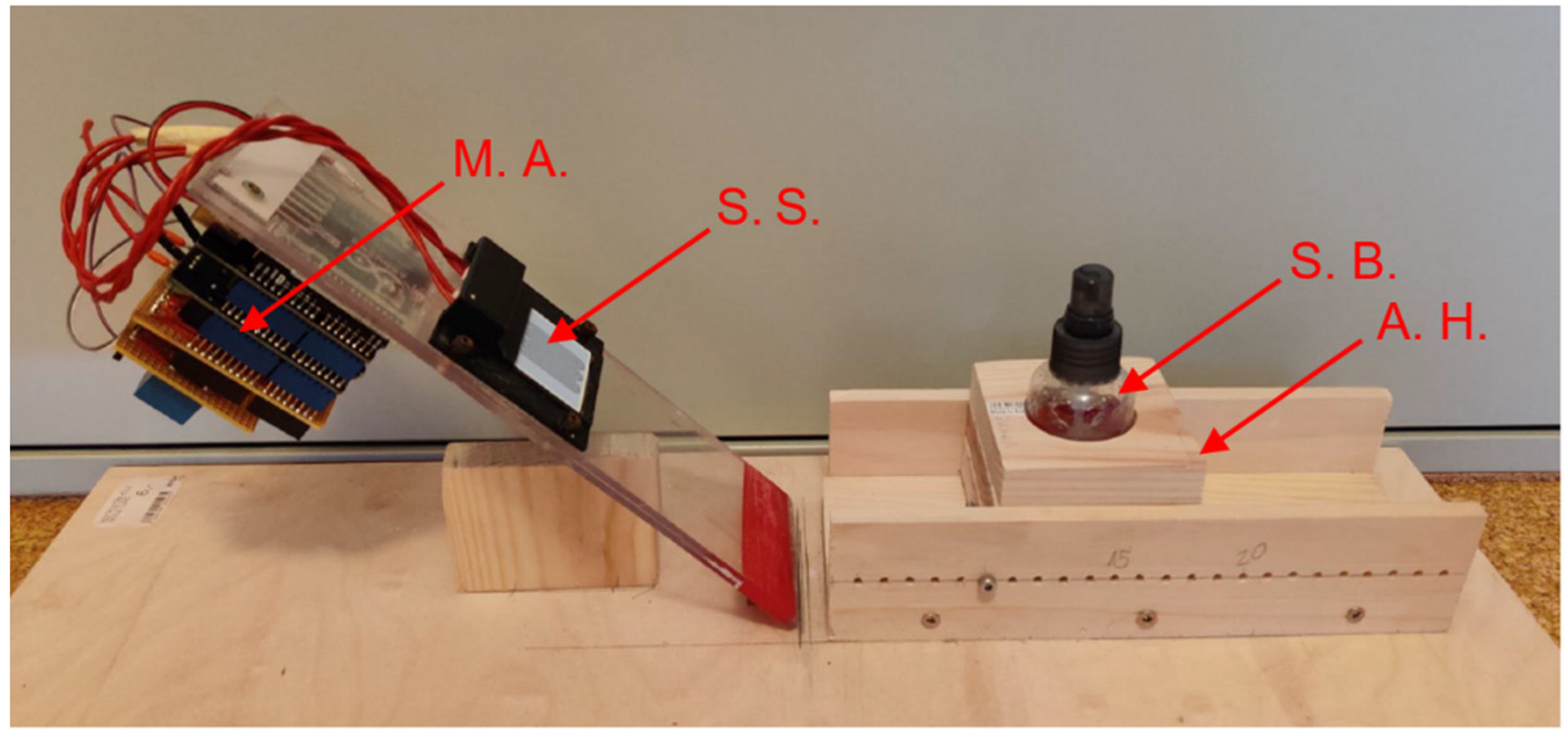
2.5. Internally Built Measuring Apparatus
2.6. Hydrogel as a Biofilm Model
2.7. Proof of Concept: Experiments
2.7.1. Flexible Sensor Structures (Polyimide PCBs)
2.7.2. Rigid Sensor Structures (Ceramic PCBs)
3. Results
3.1. Flexible Sensor Structures (Polyimide PCBs)
3.2. Rigid Sensor Structures (Ceramic PCBs)
4. Discussion
4.1. Flexible Sensor Structures (Polyimide PCBs)
- The increase in capacitance as the temperature rises can only be explained by an increase in the relative permittivity of the insulating material of the PCB and the heating foil. The electrical conductors cannot affect permittivity because the rise in temperature only increases the resistance of the copper.
- A substantial difference in capacitance between dry sensor structures and those wetted with water was to be expected with thicker layers of copper because there are more electric field lines in the area between the conductors where the dielectrics are air or water. Due to the higher absolute differences in capacitance, thicker conductors should be used (Table 1). However, the resulting greater unevenness has a negative impact, since soiling is more likely to stick. Thicker conductors also have lower ohmic resistance, which impedes heating.
- The differences between the average electrical capacitances of the double spiral structure S1 and the double meander structure M2 can be explained by the inhomogeneity of the functional structure (i.e., the structure’s sensitivity). In the double meander structures, the electrical conductors do not directly alternate; instead, the same conductor always lies next to itself in a series of pairs (see Figure 2, left). As a result, every other pair of conductors is at the same electrical potential and does not form an electrical field ‘with itself’. If there is a dielectric in these sections that can be distinguished from air, there is no local change in capacitance there. Due to this effect, only small differences in capacitance can be achieved with meandering structures compared to non-meandering structures with the same area.
- The capacitances of the sensor structures nearly returned to their initial value after being sprayed with water. The fact that the initial value wasn’t quite reached is probably due to the increased permittivity of the film, which is still warmer, and to structural moisture absorption by the polyimide, which is probably not reversible within the time considered. The sharp, rapid changes in capacitance over time can be logically explained by small drops suddenly flowing off. Having formed on the surface during spraying, on reaching a critical size these drops flowed down the inclined PCB and dropped off again (Figure 4).
4.2. Rigid Sensor Structures (Ceramic PCBs)
- Non-polar surfaces such as metal or glass tend to be less susceptible to biofouling than polymers [1,14]. However, an essential requirement for a sensor structure is that it should have similar growth dynamics for biofilms to its surroundings. Therefore, a sensor structure with a glass surface is probably far more suitable than a sensor with a polyimide surface for infrastructure systems with predominantly metallic surfaces.
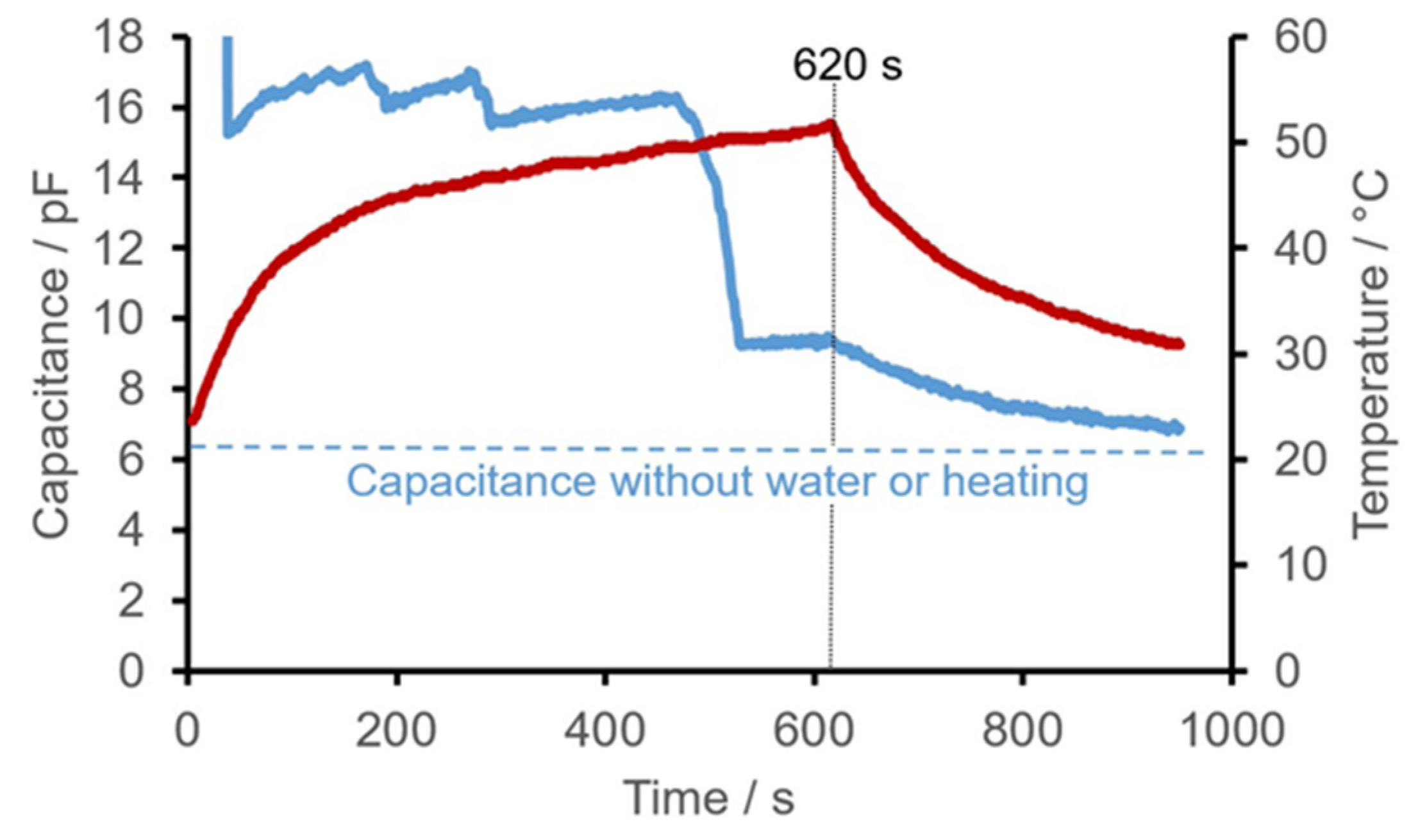
- The much smaller increase in capacitance with temperature in rigid PCBs compared to flexible PCBs can be well explained by the temperature dependence of the relative permittivity of the different materials. The relative permittivity of polyimide () [25] shows a much stronger temperature dependence than that of ceramics and glass (Alumina Ceramic: [26].
- With the ceramic PCBs, greater differences in capacitance between the measurements in air (dry) and in water were to be expected, because, compared to the polyimide PCBs, they were not just wetted with water during measurement but were completely immersed in water. As a result, the maximum capacitance change with the structure used was approximately 800%, roughly corresponding to the theoretical maximum possible change.
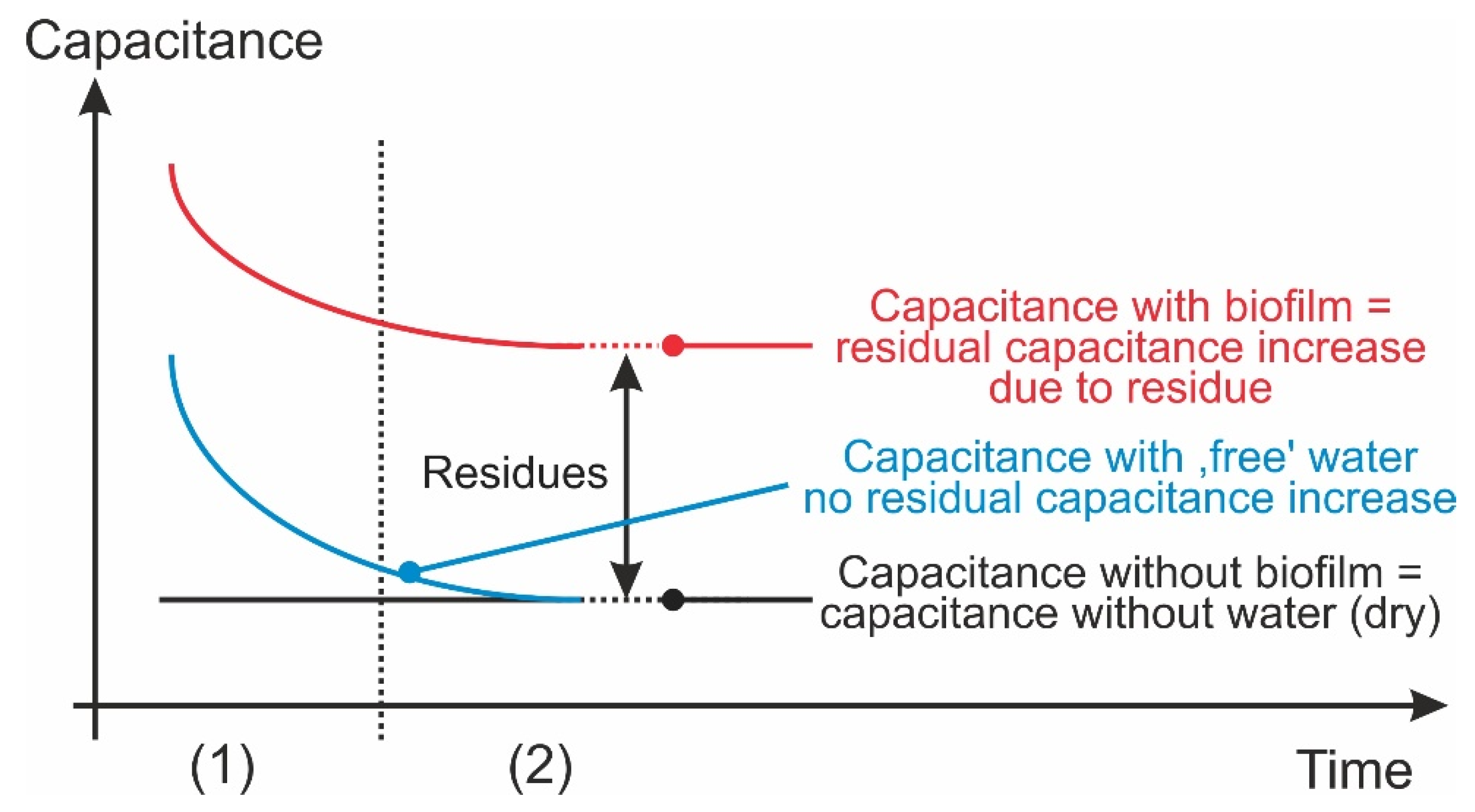
- The non-uniform layer thickness of the hydrogel (Figure 5), which was found in experiments to be dependent on the spraying distance, is not thought to present a problem because in order to detect a biofilm, only a reliable detection threshold needs to be determined above which a biofilm can be identified, and no quantification (such as the thickness or the water content of the biofilm, etc.) is required.
- If only water is applied to a sensor structure, the maximum difference in capacitance is recorded immediately afterwards, as is to be expected. However, when measured again, large differences in the temporal course occur despite the identical number of sprays, which is almost certainly due to drops of water on the sensor surface randomly converging and then draining off. If water is sprayed more than 10 times, drops form immediately and then converge on the underside of the PCB due to its inclined position. However, all the measurements carried out indicated a decrease in capacitance down to the initial value, which was reached at the end of the observation period (apart from scatter).
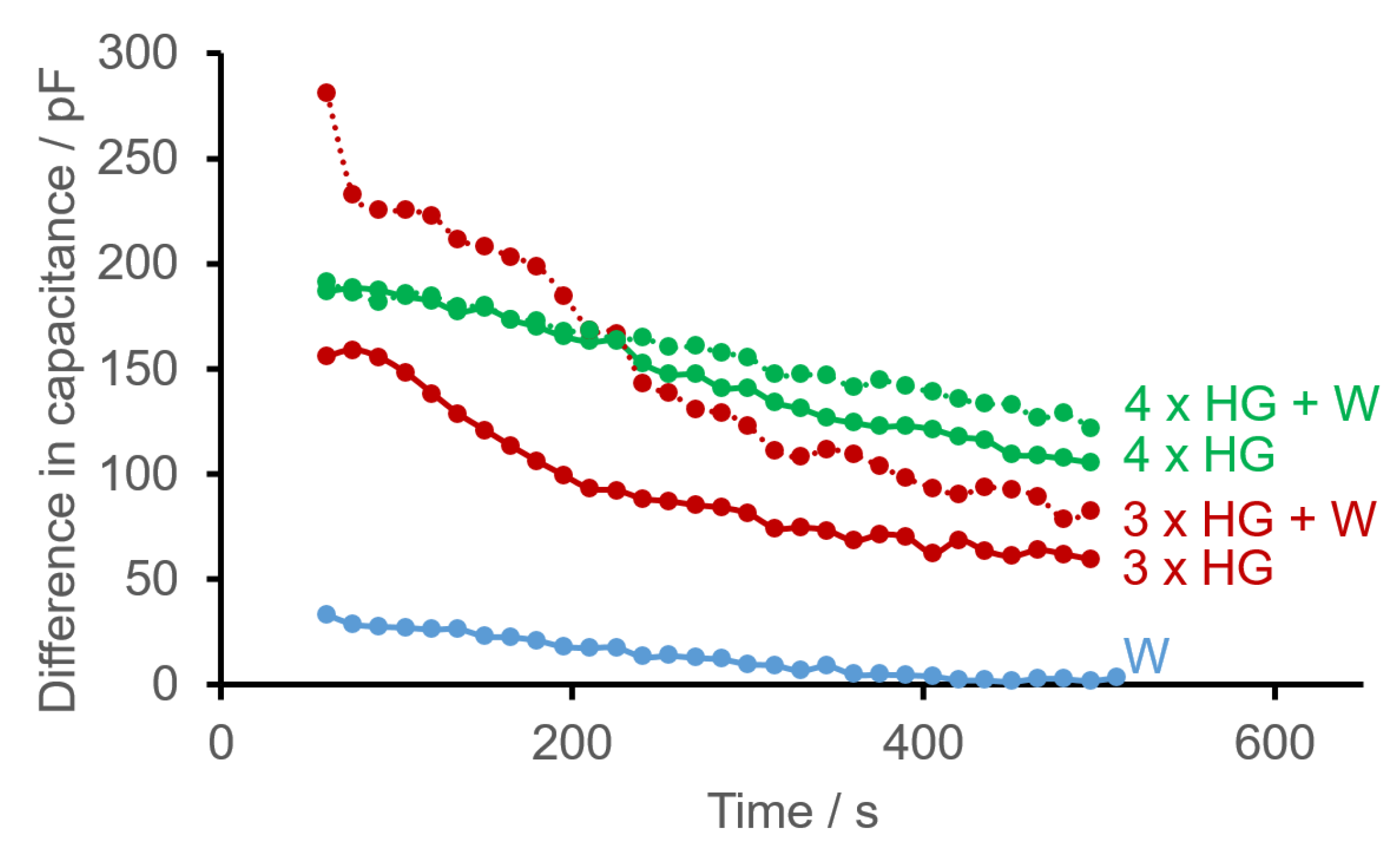
- If hydrogel is sprayed onto the surface of a sensor structure multiple times, the maximum values of the current differences in capacitance are also reached relatively quickly. Deviations also exist in the time courses. Again, a maximum value correlating with the number of spray bursts cannot be observed initially because—like water—homogeneous application with hydrogel was not possible either. However, it is clearly apparent (Figure 6) that as the thickness of the layer of hydrogel increases (due to more spray bursts), there is a higher difference in capacitance on average after 550 s.
- If water and hydrogel are applied to the surface, the initial values differ even more clearly, albeit again without a recognizable pattern, which is probably also due to inhomogeneous application.
- By estimating the layer thickness of hydrogel applied per spray burst at a given distance, even layer thicknesses below 20 µm can be detected very well with the setup used.
4.3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Costerton, J.W.; Geesey, G.; Cheng, K.J. How bacteria stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef]
- Donlan, R. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Hassan, A.; Usman, J.; Kaleem, F.; Omair, M.; Khalid, A.; Iqbal, M. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz. J. Infect. Dis. 2011, 15, 305–311. [Google Scholar] [CrossRef]
- Reyes-Romero, D.F.; Behrmann, O.; Dame, G.; Urban, G.A. Dynamic thermal sensor for biofilm monitoring. Sens. Actuators A Phys. 2014, 213, 43–51. [Google Scholar] [CrossRef]
- Gula, G.; Szymanowska, P.; Piasecki, T.; Góras, S.; Gotszalk, T.; Drulis-Kawa, Z. The application of impedance spectroscopy for pseudomonas biofilm monitoring during phage infection. Viruses 2020, 12, 407. [Google Scholar] [CrossRef]
- Gutierrez, D.E.; Hidalgo Cantabrana, C.; Rodriguez, A.; Garca, P.; Ruas-Madiedo, P. Monitoring in real time the formation and removal of biofilms from clinical related pathogens using an impedance-based technology. PLoS ONE 2016, 11, e0163966. [Google Scholar] [CrossRef]
- Harris, C.; Todd, R.; Bungard, S.; Lovitt, R.B.; Morris, J.G.; Kell, D. Dielectric permittivity of microbial suspensions at radio frequencies: A novel method for the real-time estimation of microbial biomass. Enzym. Microb. Technol. 1987, 9, 181–186. [Google Scholar] [CrossRef]
- Freeman, D.J.; Falkiner, F.; Keane, C. New method for detecting slime producing by coagulase negative staphylococci. J. Clin. Pathol. 1989, 42, 872–874. [Google Scholar] [CrossRef]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef]
- Lewandowski, Z.; Beyenal, H. Biofilm monitoring: A perfect solution in search of a problem. Water Sci. Technol. 2003, 47, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wimpenny, J.W.T.; Colasanti, R. A unifying hypothesis for the structure of microbial biofilms based on cellular automaton models. FEMS Microbiol. Ecol. 1997, 22, 1–16. [Google Scholar] [CrossRef]
- Sarkar, S.; Mazumder, D. Development of a simplified biofilm model. Appl. Water Sci. 2017, 7, 1799–1806. [Google Scholar] [CrossRef]
- Živković, V.; Kurevija, T.; Drenjančěvić, I.H.; Bogdan, M.; Paradžik, M.T.; Talapko, J.; Drenjančěvić, D. To biofilm or not to biofilm? Southeast. Eur. Med. J. 2018, 2, 12–19. [Google Scholar]
- Donlan, R. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Zhang, X.; Bishop, P.; Kupferle, M. Measurement of polysaccharides and proteins in biofilm extracellular polymers. Water Sci. Technol. 1998, 37, 345–348. [Google Scholar] [CrossRef]
- Vasiljevic, D.; Kojic, S.; Radovanovic, M.; Pivas, B.; Tadic, A.; Mirkovic, S. Cost-effective interdigitated capacitive sensors for efficient bacteria detection. Infoteh-Jahorina. 2016. Available online: https://infoteh.etf.ues.rs.ba/zbornik/2016/radovi/ELS/ELS-3.pdf (accessed on 2 March 2021).
- Boudaden, J.; Steinmaßl, M.; Endres, H.-E.; Drost, A.; Eisele, I.; Kutter, C.; Müller-Buschbaum, P. Polyimide-based capacitive humidity sensor. Sensors 2018, 18, 1516. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneyra, A.; Fernández-Salmerón, J.; Agudo-Acemel, M.; López-Villanueva, J.A.; Capitan-Vallvey, L.F.; Palma, A.J. Printed electrodes structures as capacitive humidity sensors: A comparison. Sens. Actuators A Phys. 2016, 244, 56–65. [Google Scholar] [CrossRef]
- Žuk, S.; Pietrikova, A.; Vehec, I. Possibilities of planar capacitive rain sensor manufacturing by thick film technology. Acta Electrotech. Inform. 2018, 18, 11–16. [Google Scholar] [CrossRef]
- LeitOn GmbH: Wasseraufnahme fr4-Material (German), Produkt-Information. Available online: https://www.leiton.de/leiterplatten-lexikon-1-wasseraufnahme.html (accessed on 21 December 2021).
- Walker, J.T.; Dowsett, A.B.; Dennis, P.J.K.; Keevil, C.W. Continuous culture studies of biofilm associated with copper corrosion. Int. Biodeterior. 1991, 27, 121–134. [Google Scholar] [CrossRef]
- Texas Instruments: NE555 Datasheet. 2021. Available online: https://www.ti.com/lit/ds/symlink/ne555.pdf (accessed on 12 December 2021).
- Stoodley, P.; Dodds, I.; Boyle, J.D.; Lappin-Scott, H.M. Influence of hydrodynamics and nutrients on biofilm structure. J. Appl. Microbiol. 1998, 85, 19–28. [Google Scholar] [CrossRef]
- James, G.A.; Beaudette, L.; Costerton, J.W. Interspecies bacterial interactions in biofilms. J. Ind. Microbiol. 1995, 15, 257–262. [Google Scholar] [CrossRef]
- DuPont Electronic Materials: DuPont Pyralux AP Polyimide Flexible Laminate, Technical Product-Information. 2009. Available online: https://www.multi-circuit-boards.eu/fileadmin/pdf/leiterplatten_material/e_dupont_pyralux-ap-polyimid_www.multi-circuit-boards.eu.pdf (accessed on 21 December 2021).
- CoorsTek, Inc. Thick Film Ceramic Substrates, Design Guide. 2021. Available online: https://www.coorstek.com/media/4221/thick-film-ceramic-substrates-design-guide.pdf (accessed on 21 December 2021).
- Wang, J. Electrical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]


| Structure | Thickness of Copper | Width of Copper | Spacing |
|---|---|---|---|
| S1 | 28 µm | 1.6 mm | 0.4 mm |
| K1 | 18 µm | 1.6 mm | 0.4 mm |
| M1 | 18 µm | 1.6 mm | 0.6 mm |
| M2 | 28 µm | 1.6 mm | 0.6 mm |
| Spray Distance in cm | 5 | 10 | 15 | 20 |
|---|---|---|---|---|
| Mean layer thickness (µm; SD: ±0.8 µm) | 44 | 18 | 8 | 4 |
| Structure | ||||
|---|---|---|---|---|
| S1 | 29 ± 1 | 90 ± 18 | 61 ± 19 | 3.1 ± 0.7 |
| K1 | 6 ± 1 | 17 ± 3 | 11 ± 4 | 3.0 ± 1.0 |
| M1 | 8 ± 1 | 34 ± 13 | 26 ± 14 | 4.5 ± 2.3 |
| M2 | 30 ± 5 | 58 ± 12 | 28 ± 17 | 2.1 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zirk, K.-U.; Olze, M.; Pötzschke, H. A Simple Method for the Detection of Biofilms Using a Heatable Capacitive Sensor Structure (CSS): Description, Proof of Concept, and Some Technical Improvements. Sensors 2022, 22, 445. https://doi.org/10.3390/s22020445
Zirk K-U, Olze M, Pötzschke H. A Simple Method for the Detection of Biofilms Using a Heatable Capacitive Sensor Structure (CSS): Description, Proof of Concept, and Some Technical Improvements. Sensors. 2022; 22(2):445. https://doi.org/10.3390/s22020445
Chicago/Turabian StyleZirk, Kai-Uwe, Manuel Olze, and Harald Pötzschke. 2022. "A Simple Method for the Detection of Biofilms Using a Heatable Capacitive Sensor Structure (CSS): Description, Proof of Concept, and Some Technical Improvements" Sensors 22, no. 2: 445. https://doi.org/10.3390/s22020445
APA StyleZirk, K.-U., Olze, M., & Pötzschke, H. (2022). A Simple Method for the Detection of Biofilms Using a Heatable Capacitive Sensor Structure (CSS): Description, Proof of Concept, and Some Technical Improvements. Sensors, 22(2), 445. https://doi.org/10.3390/s22020445




