Supervisory Algorithm for Autonomous Hemodynamic Management Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development of the Fluid Resuscitation Adaptive Controller
2.2. Development of the Supervisory Algorithm for Casualty Management
2.3. PhysioVessel Flow Loop Test Platform
2.4. Adaptive Resuscitation Controller Experimental Design
2.5. Supervisory Algorithm Casualty Management (SACM) Scenario Testing
2.6. Data Analysis
3. Results
3.1. Experimental Evaluation of the Adaptive Resuscitative Controller (ARC)
3.2. Overview of SACM Algorithm and Its Utility for Initial Hemodynamic Stabilization
3.3. SACM Utility for Extended Patient Management
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
DoD Disclaimer
References
- Bitterman, D.S.; Aerts, H.J.W.L.; Mak, R.H. Approaching Autonomy in Medical Artificial Intelligence. Lancet Digit. Health 2020, 2, e447–e449. [Google Scholar] [CrossRef]
- Blobel, B.; Ruotsalainen, P.; Brochhausen, M.; Oemig, F.; Uribe, G.A. Autonomous Systems and Artificial Intelligence in Healthcare Transformation to 5P Medicine-Ethical Challenges. Stud. Health Technol. Inf. 2020, 270, 1089–1093. [Google Scholar] [CrossRef]
- Moustris, G.P.; Hiridis, S.C.; Deliparaschos, K.M.; Konstantinidis, K.M. Evolution of Autonomous and Semi-autonomous Robotic Surgical Systems: A Review of the Literature. Int. J. Med. Robot. Comput. Assist. Surg. 2011, 7, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Von Platen, P.; Pomprapa, A.; Lachmann, B.; Leonhardt, S. The Dawn of Physiological Closed-Loop Ventilation—A Review. Crit. Care 2020, 24, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, R.; Abbas, J.J.; Fuller, D.D.; Gomes, J.; Renaud, S.; Jung, R. Autonomous Control of Ventilation through Closed-Loop Adaptive Respiratory Pacing. Sci. Rep. 2020, 10, 21903. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, M.; Brunner, J.X. Closed-Loop Ventilation: An Emerging Standard of Care? Crit. Care Clin. 2007, 23, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Libert, N.; Chenegros, G.; Harrois, A.; Baudry, N.; Cordurie, G.; Benosman, R.; Vicaut, E.; Duranteau, J. Performance of Closed-Loop Resuscitation of Haemorrhagic Shock with Fluid Alone or in Combination with Norepinephrine: An Experimental Study. Ann. Intensive Care 2018, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Gholami, B.; Haddad, W.M.; Bailey, J.M.; Muir, W.W. Closed-Loop Control for Fluid Resuscitation: Recent Advances and Future Challenges. Front. Vet. Sci. 2021, 8, 145. [Google Scholar] [CrossRef]
- Hundeshagen, G.; Kramer, G.C.; Ribeiro, N.M.; Salter, M.; Koutrouvelis, A.K.; Li, H.; Solanki, D.; Indrikovs, A.; Seeton, R.; Henkel, S.N.; et al. Closed-Loop and Decision-Assist Guided Fluid Therapy of Human Hemorrhage. Crit. Care Med. 2017, 45, e1068–e1074. [Google Scholar] [CrossRef]
- Salinas, J.; Drew, G.; Gallagher, J.; Cancio, L.C.; Wolf, S.E.; Wade, C.E.; Holcomb, J.B.; Herndon, D.N.; Kramer, G.C. Closed-Loop and Decision-Assist Resuscitation of Burn Patients. J. Trauma Inj. Infect. Crit. Care 2008, 64, S321–S332. [Google Scholar] [CrossRef] [Green Version]
- Mekhail, N.; Levy, R.M.; Deer, T.R.; Kapural, L.; Li, S.; Amirdelfan, K.; Hunter, C.W.; Rosen, S.M.; Costandi, S.J.; Falowski, S.M.; et al. Long-Term Safety and Efficacy of Closed-Loop Spinal Cord Stimulation to Treat Chronic Back and Leg Pain (Evoke): A Double-Blind, Randomised, Controlled Trial. Lancet Neurol. 2020, 19, 123–134. [Google Scholar] [CrossRef]
- Kong, E.; Nicolaou, N.; Vizcaychipi, M.P. Hemodynamic Stability of Closed-Loop Anesthesia Systems: A Systematic Review. Minerva Anestesiol. 2020, 86, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Vieira, S.M.; Leite, F.; Palos, C.; Finkelstein, S.; Sousa, J.M.C. Clinical Decision Support Systems for Triage in the Emergency Department Using Intelligent Systems: A Review. Artif. Intell. Med. 2020, 102, 101762. [Google Scholar] [CrossRef] [PubMed]
- Nates, J.L.; Nunnally, M.; Kleinpell, R.; Blosser, S.; Goldner, J.; Birriel, B.; Fowler, C.S.; Byrum, D.; Miles, W.S.; Bailey, H.; et al. ICU Admission, Discharge, and Triage Guidelines: A Framework to Enhance Clinical Operations, Development of Institutional Policies, and Further Research. Crit. Care Med. 2016, 44, 1553–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edbrooke, D.L.; Minelli, C.; Mills, G.H.; Iapichino, G.; Pezzi, A.; Corbella, D.; Jacobs, P.; Lippert, A.; Wiis, J.; Pesenti, A.; et al. Implications of ICU Triage Decisions on Patient Mortality: A Cost-Effectiveness Analysis. Crit. Care 2011, 15, R56. [Google Scholar] [CrossRef] [Green Version]
- Vega, S.; Hernandez-Torres, S.; Berard, D.; Boice, E.; Snider, E.J. Development and Characterization of a Self-Tightening Tourniquet System. Sensors, 2021; submitted. [Google Scholar]
- Berard, D.; Vega, S.; Hernandez-Torres, S.; Polykratis, I.A.; Salinas, J.; Ross, E.; Boice, E.; Snider, E.J. Development of the PhysioVessel: A Customizable Platform for Simulating Physiological Fluid Resuscitation. J. Physiol. Meas. 2021; submitted. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Masri, B.A.; Eisen, A.; Duncan, C.P.; McEwen, J.A. Tourniquet-Induced Nerve Compression Injuries Are Caused by High Pressure Levels and Gradients—A Review of the Evidence to Guide Safe Surgical, Pre-Hospital and Blood Flow Restriction Usage. BMC Biomed. Eng. 2020, 2, 7. [Google Scholar] [CrossRef]
- Husted, H.; Toftgaard Jensen, T. Influence of the Pneumatic Tourniquet on Patella Tracking in Total Knee Arthroplasty: A Prospective Randomized Study in 100 Patients. J. Arthroplast. 2005, 20, 694–697. [Google Scholar] [CrossRef]
- Mayer, C.; Franz, A.; Harmsen, J.-F.; Queitsch, F.; Behringer, M.; Beckmann, J.; Krauspe, R.; Zilkens, C. Soft-Tissue Damage during Total Knee Arthroplasty. J. Orthop. 2017, 14, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Koons, N.J. The Compensatory Reserve: Potential for Accurate Individualized Goal-Directed Whole Blood Resuscitation. Transfusion 2020, 60, S150–S157. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Schiller, A.M. Measuring the Compensatory Reserve to Identify Shock. J. Trauma Acute Care Surg. 2017, 82, S57. [Google Scholar] [CrossRef] [PubMed]
- Wenner, M.M.; Hinds, K.A.; Howard, J.T.; Nawn, C.D.; Stachenfeld, N.S.; Convertino, V.A. Measurement of Compensatory Reserve Predicts Racial Differences in Tolerance to Simulated Hemorrhage in Women. J. Trauma Acute Care Surg. 2018, 85, S77–S83. [Google Scholar] [CrossRef] [PubMed]
- Kamišalić, A.; Fister, I.; Turkanović, M.; Karakatič, S. Sensors and Functionalities of Non-Invasive Wrist-Wearable Devices: A Review. Sensors 2018, 18, 1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjahjadi, H.; Ramli, K. Review of Photoplethysmography Based Non-Invasive Continuous Blood Pressure Methods. In Proceedings of the 2017 15th International Conference on Quality in Research (QiR): International Symposium on Electrical and Computer Engineering, Nusa Dua, Bali, Indonesia, 24–27 July 2017; pp. 173–178. [Google Scholar]
- Mukherjee, R.; Ghosh, S.; Gupta, B.; Chakravarty, T. A Literature Review on Current and Proposed Technologies of Noninvasive Blood Pressure Measurement. Telemed. E-Health 2018, 24, 185–193. [Google Scholar] [CrossRef] [PubMed]



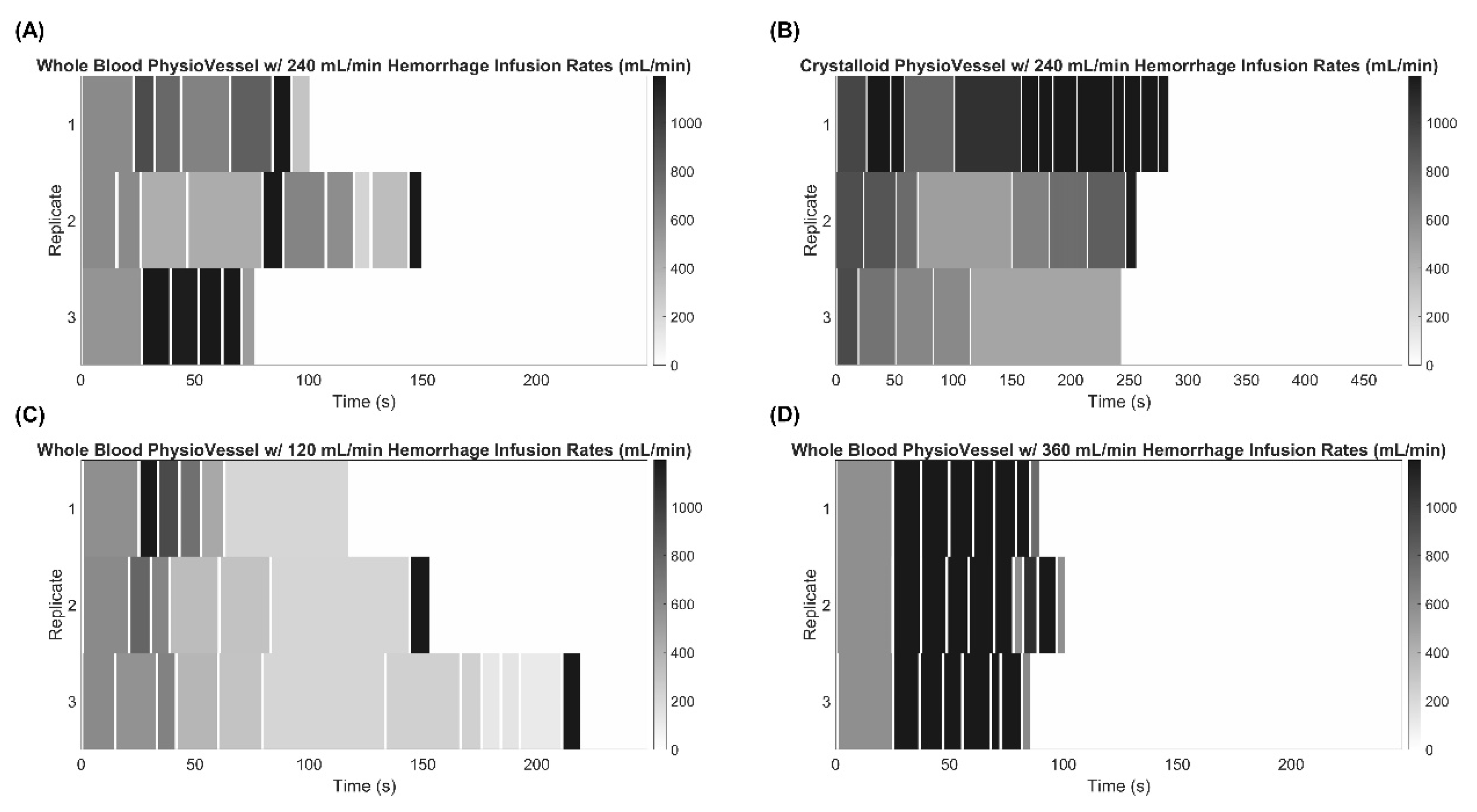





| Adaptive Resuscitation Controller (ARC) Testing Scenarios | ||
|---|---|---|
| Whole Blood PhysioVessel (PVWB) | Scaling factor adjustment | 0.5× Scaling Factor |
| Scaling Factor = 1 (Baseline) | ||
| 2× Scaling Factor | ||
| Baseline scaling factor with internal hemorrhage | 120 mL/min | |
| 240 mL/min | ||
| 360 mL/min | ||
| Crystalloid PhysioVessel (PVC) | Baseline scaling factor | |
| Internal Hemorrhage at 240 mL/min | ||
| Testing Scenario | Normalized Time Intervals for Each Scenario | ||||
|---|---|---|---|---|---|
| (0–1) | (1–2) | (2–3) | (3–4) | (4–5) | |
| #1 | Initial Hemorrhage | Extremity Bleed + aTKT | ARC Resuscitation | ||
| #2 | Initial Hemorrhage | Extremity Bleed + aTKT | ARC Resuscitation | Internal Hemorrhage + ARC Resuscitation | Internal Hemorrhage + ARC Resuscitation |
| #3 | Initial Hemorrhage | Extremity Bleed + aTKT | ARC Resuscitation | Loosen Tourniquet + aTKT | Loosen Tourniquet + aTKT |
| #4 | Initial Hemorrhage | Extremity Bleed + aTKT | ARC Resuscitation | Loosen Tourniquet + aTKT | Internal Hemorrhage + ARC Resuscitation |
| #5 | Initial Hemorrhage | Extremity Bleed + aTKT | ARC Resuscitation | Internal Hemorrhage + ARC Resuscitation | Loosen Tourniquet + aTKT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snider, E.J.; Vega, S.J.; Ross, E.; Berard, D.; Hernandez-Torres, S.I.; Salinas, J.; Boice, E.N. Supervisory Algorithm for Autonomous Hemodynamic Management Systems. Sensors 2022, 22, 529. https://doi.org/10.3390/s22020529
Snider EJ, Vega SJ, Ross E, Berard D, Hernandez-Torres SI, Salinas J, Boice EN. Supervisory Algorithm for Autonomous Hemodynamic Management Systems. Sensors. 2022; 22(2):529. https://doi.org/10.3390/s22020529
Chicago/Turabian StyleSnider, Eric J., Saul J. Vega, Evan Ross, David Berard, Sofia I. Hernandez-Torres, Jose Salinas, and Emily N. Boice. 2022. "Supervisory Algorithm for Autonomous Hemodynamic Management Systems" Sensors 22, no. 2: 529. https://doi.org/10.3390/s22020529
APA StyleSnider, E. J., Vega, S. J., Ross, E., Berard, D., Hernandez-Torres, S. I., Salinas, J., & Boice, E. N. (2022). Supervisory Algorithm for Autonomous Hemodynamic Management Systems. Sensors, 22(2), 529. https://doi.org/10.3390/s22020529






