A Comparative Study between Scanning Devices for 3D Printing of Personalized Ostomy Patches
Abstract
:1. Introduction
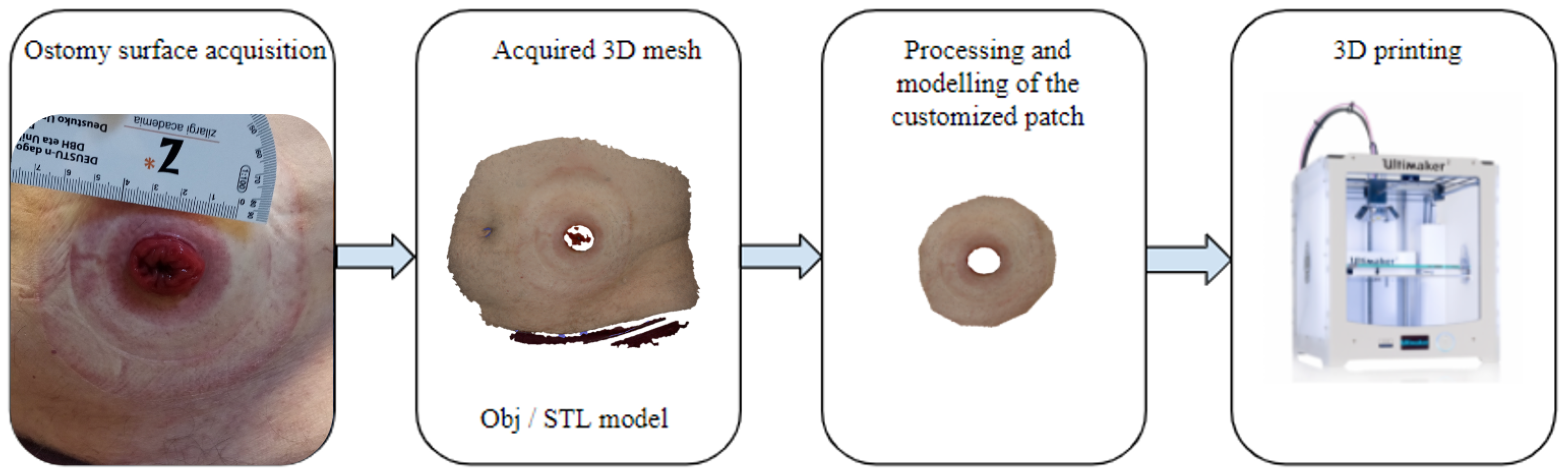
2. Materials and Methods
2.1. Participants
2.2. Hospital Visits and Scanning Protocol
2.3. Data Acquisition
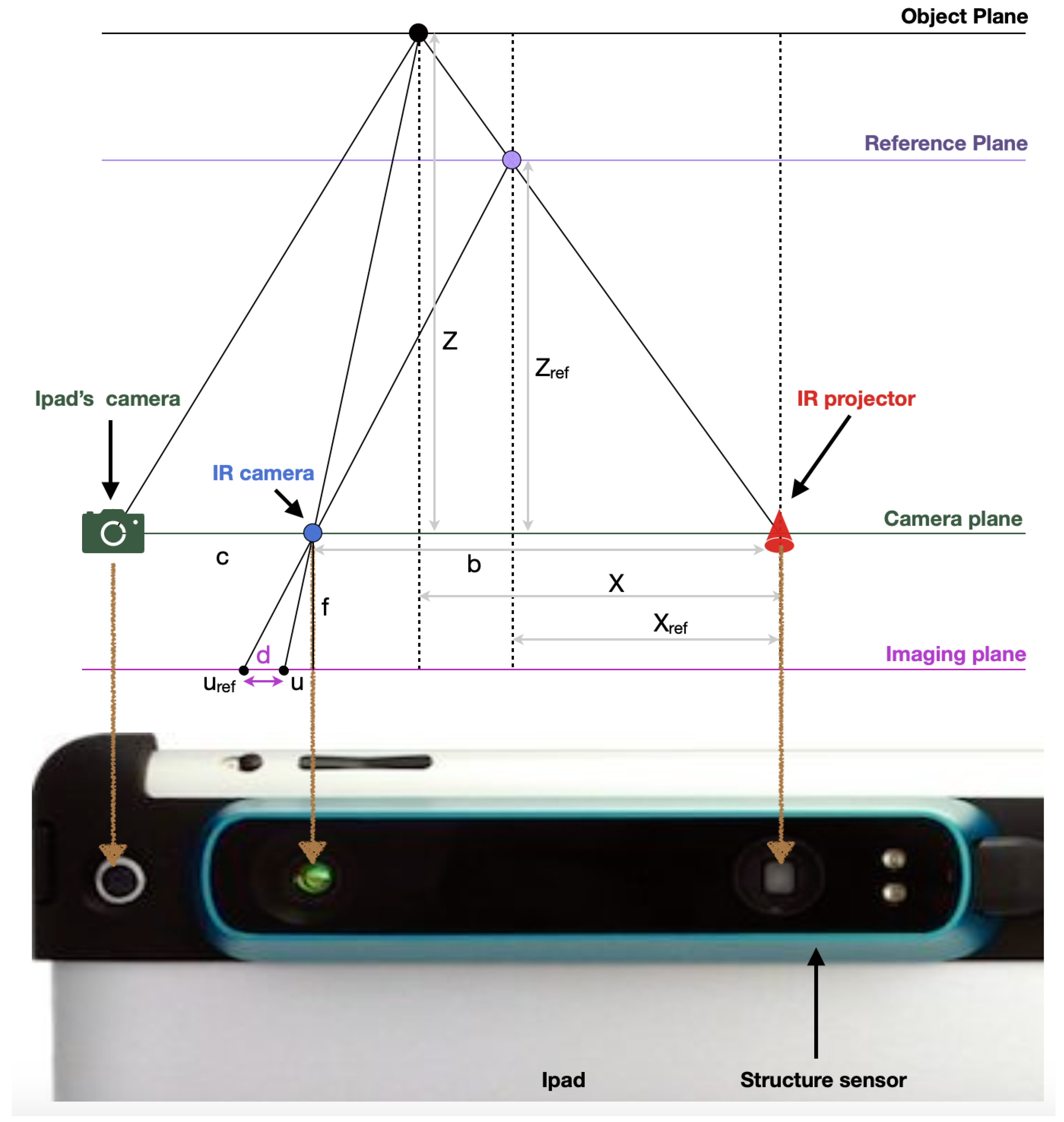
2.3.1. Occipital Inc.: Structure Sensor
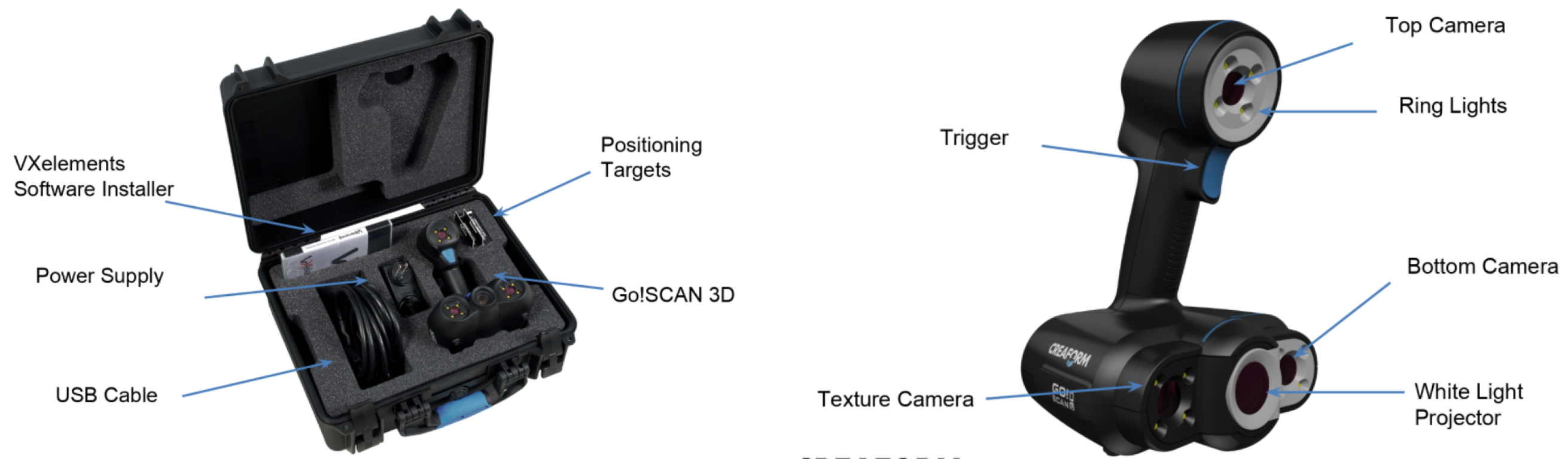
2.3.2. Creaform Inc. (Academia): Go!Scan 20
2.3.3. Technical Comparison between the Structure Sensor and Go!Scan 20
2.3.4. CT Scans
2.3.5. Processing of CT Scans and Design of the Ostomy Patch
2.4. Data Processing
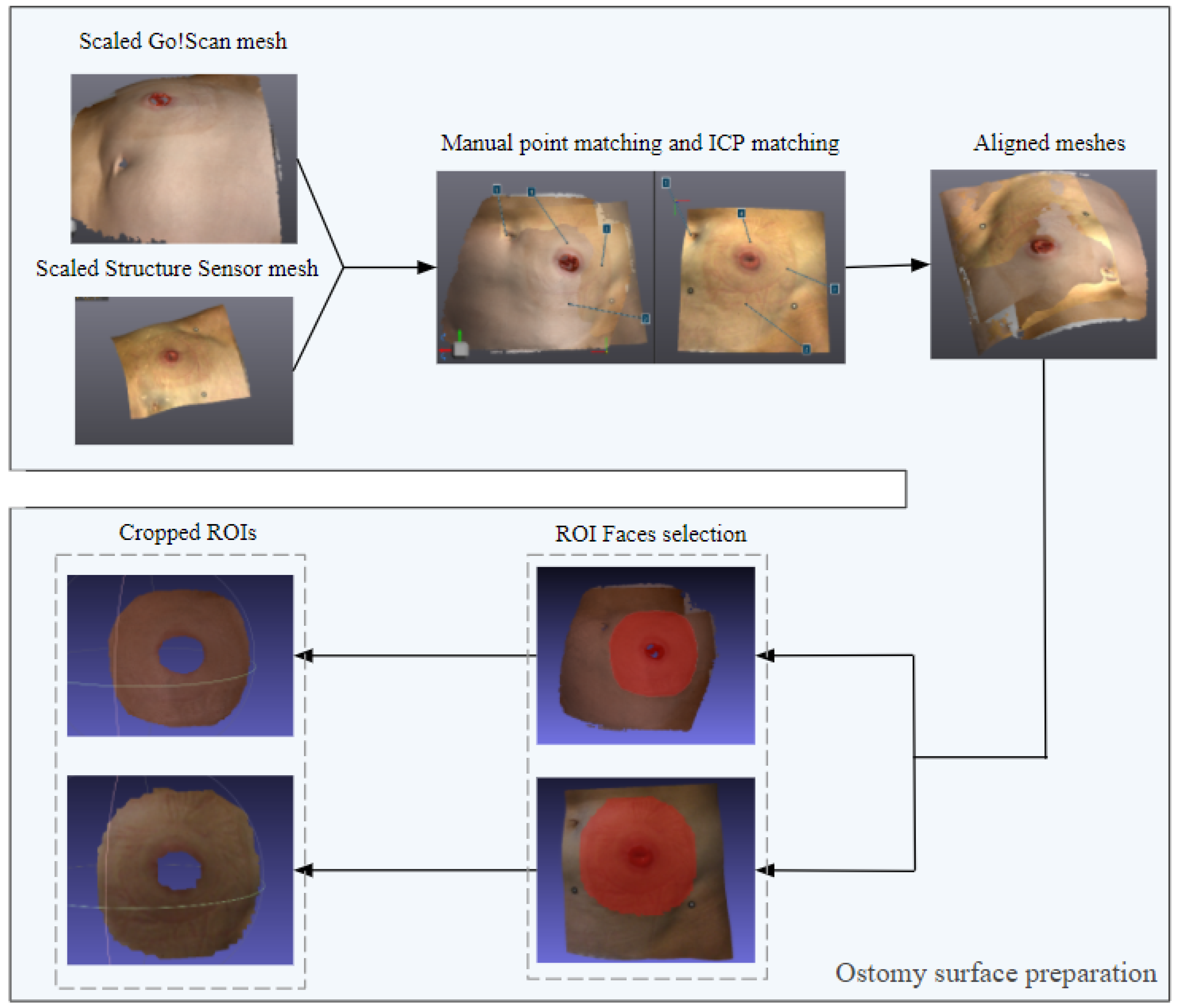
2.4.1. Ostomy Surface Preparation
- Finding the nearest point for each point pair on the reference model.
- Estimating the rotation and translation matrices R and t such that .
- Iterating through the previous steps until the error is less than a given threshold value or the maximum number of iterations is reached [35].
2.4.2. Hausdorff Distance Measurement
2.4.3. Gaussian Curvature Calculation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLS | Structured Light System |
| SOC | System On a Chip |
| IR | Infrared |
| CT | Computed Tomography |
References
- Ecancer. Pesquisa de Cáncer Colorrectal: Perspectiva de la Agencia Internacional de Investigación del Cáncer (OMS)-Ecancer. Available online: http://ecancer.org/es/news/13590-pesquisa-de-cancer-colorrectal-perspectiva-de-la-agencia-internacional-de-investigacion-del-cancer-oms (accessed on 10 March 2021).
- Cataldo, P.A.; MacKeigan, J.M. Intestinal Stomas. In Principles, Techniques and Management; Marcel Dekker, Inc.: New York, NY, USA, 2004; ISBN 0-8247-4707-0. [Google Scholar]
- Husain, S.G.; Cataldo, T.E. Late stomal complications. Clin. Colon Rectal. Surg. 2008, 21, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Park, J.J.; del Pino, A.; Orsay, C.P.; Nelson, R.L.; Pearl, R.K.; Cintron, J.R. Stoma complications: The Cook County Hospital experience. Dis. Colon. Rectum. 1999, 42, 1575–1580. [Google Scholar] [CrossRef]
- Nastro, P.; Knowles, C.H.; McGrath, A.; Heyman, B.; Porrett, T.R.; Lunnis, P.J. Complications of intestinal stomas. Br. J. Surg. 2010, 97, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Cottam, J.; Richards, K.; Hasted, A.; Blackman, A. Results of a nationwide prospective audit of stoma complications within 3 weeks of surgery. Colorectal. Dis. 2007, 9, 834–838. [Google Scholar] [CrossRef]
- Carne, P.W.; Robertson, G.M.; Frizelle, F.A. Parastomal hernia. Br. J. Surg. 2003, 90, 784–793. [Google Scholar] [CrossRef]
- Pilgrim, C.H.; McIntyre, R.; Bailey, M. Prospective audit of parastomal hernia: Prevalence and associated comorbidities. Dis. Colon. Rectum. 2010, 53, 71–76. [Google Scholar] [CrossRef]
- Available online: https://www.euskadi.eus/ayuda_subvencion/2019/elkartek-2019-convocatoria-fase-i/web01-tramite/es/ (accessed on 10 March 2021).
- Liaw, C.Y.; Murat, G. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef] [PubMed]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Toth, L.; Schiffer, A.; Nyitrai, M.; Pentek, A.; Told, R.; Maroti, P. Developing an anti-spastic orthosis for daily home-use of stroke patients using smart memory alloys and 3D printing technologies. Mater. Des. 2020, 195, 109029. [Google Scholar] [CrossRef]
- Wang, K.; Ho, C.C.; Zhang, C.; Wang, B. A review on the 3D printing of functional structures for medical phantoms and regenerated tissue and organ applications. Engineering 2017, 3, 653–662. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Arif, U.; Haider, S.; Haider, A.; Khan, N.; Alghyamah, A.A.; Jamila, N.; Kang, I.K. Biocompatible polymers and their potential biomedical applications: A review. Curr. Pharm. Des. 2019, 25, 3608–3619. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef]
- Arefin, A.M.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Crowe, S.; Luscombe, J.; Maxwell, S.; Simpson-Page, E.; Poroa, T.; Wilks, R.; Li, W.; Cleland, S.; Chan, P.; Lin, C.; et al. Evaluation of optical 3D scanning system for radiotherapy use. J. Med. Radiat. Sci. 2021, 1–9. [Google Scholar] [CrossRef]
- Knoops, P.G.; Beaumont, C.A.; Borghi, A.; Rodriguez-Florez, N.; Breakey, R.W.; Rodgers, W.; Angullia, F.; Jeelani, N.O.; Schievano, S.; Dunaway, D.J. Comparison of three-dimensional scanner systems for craniomaxillofacial imaging. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 441–449. [Google Scholar] [CrossRef]
- Petrides, G.; Clark, J.R.; Low, H.; Lovell, N.; Eviston, T.J. Three-dimensional scanners for soft-tissue facial assessment in clinical practice. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 605–614. [Google Scholar] [CrossRef]
- Shah, P.B.; Luximon, Y. Review on 3D scanners for head and face modeling. In Proceedings of the InInternational Conference on Digital Human Modeling and Applications in Health, Safety, Ergonomics and Risk Management, Las Vegas, NV, USA, 7 July 2017; pp. 47–56. [Google Scholar]
- Zhao, Y.J.; Xiong, Y.X.; Wang, Y. Three-dimensional accuracy of facial scan for facial deformities in clinics: A new evaluation method for facial scanner accuracy. PLoS ONE 2017, 12, e0169402. [Google Scholar] [CrossRef] [PubMed]
- Modabber, A.; Peters, F.; Kniha, K.; Goloborodko, E.; Ghassemi, A.; Lethaus, B.; Hölzle, F.; Möhlhenrich, S.C. Evaluation of the accuracy of a mobile and a stationary system for three-dimensional facial scanning. J. Craniomaxillofac. Surg. 2016, 44, 1719–1724. [Google Scholar] [CrossRef]
- ter Braak, T.P.; Nguyen, H.C.; van Silfhout, L.; ter Gunne, A.F.P.; Hekma, E.J. Accuracy of forearm dimensions obtained with a three-dimensional surface scanner. J. Surg. Surg. Technol. 2020, 2, 21–26. [Google Scholar]
- Secher, J.J.; Darvann, T.A.; Pinholt, E.M. Accuracy and reproducibility of the DAVID SLS-2 scanner in three-dimensional facial imaging. J. Craniomaxillofac. Surg. 2017, 45, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://structure.io/ (accessed on 11 January 2021).
- Kalantari, M.; Nechifor, M. Accuracy and utility of the Structure Sensor for collecting 3D indoor information. Geo-Spat. Inf. Sci. 2016, 19, 202–209. [Google Scholar] [CrossRef]
- Khoshelham, K.; Elberink, S.O. Accuracy and resolution of kinect depth data for indoor mapping applications. Sensors 2012, 12, 1437–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.creaform3d.com/sites/default/files/assets/brochures/files/goscan/2016/goscan3d_industrial_brochure_en_hq_21032016.pdf (accessed on 15 December 2021).
- Patient Page | ARRT-The American Registry of Radiologic Technologists. 9 November 2014. Available online: https://web.archive.org/web/20141109192141/https://www.arrt.org/Patient-Public/Patient-Page (accessed on 17 March 2021).
- CT Scan-Mayo Clinic. Available online: https://www.mayoclinic.org/tests-procedures/ct-scan/about/pac-20393675 (accessed on 10 March 2021).
- Available online: www.slicer.org (accessed on 20 February 2021).
- Available online: https://www.ptc.com/en/products/creo/parametric (accessed on 20 February 2021).
- Rusinkiewicz, S.; Levoy, M. Efficient variants of the ICP algorithm. In Proceedings of the third International Conference on 3-D Digital Imaging and Modeling, Quebec City, QC, Canada, 28 May–1 June 2001; pp. 145–152. [Google Scholar]
- Liang, L. Precise iterative closest point algorithm for RGB-D data registration with noise and outliers. Neurocomputing 2020, 399, 361–368. [Google Scholar] [CrossRef]
- Kang, Y.; Kyung, M.H.; Yoon, S.H.; Kim, M.S. Fast and robust Hausdorff distance computation from triangle mesh to quad mesh in near-zero cases. Comput. Aided Geom. Des. 2018, 62, 91–103. [Google Scholar] [CrossRef]
- Garland, M.; Heckbert, P.S. Surface simplification using quadric error metrics. In Proceedings of the 24th Annual Conference on Computer Graphics and Interactive Techniques, Anaheim, CA, USA, 24–28 July 1997; pp. 209–216. [Google Scholar]
- Taubin, G. Curve and surface smoothing without shrinkage. In Proceedings of the IEEE International Conference on Computer Vision, Cambridge, MA, USA, 20–23 June 1995; pp. 852–857. [Google Scholar]
- Rusinkiewicz, S. Estimating curvatures and their derivatives on triangle meshes. In Proceedings of the 2nd International Symposium on 3D Data Processing, Visualization and Transmission, Thessaloniki, Greece, 6–9 September 2004; pp. 486–493. [Google Scholar]




















| Characteristics | N | Parameter |
|---|---|---|
| Weight (kg) | 19 | - |
| Median (P25–P75) | 77 (69–84) | |
| Age (years) | 19 | - |
| <45 | 2 | - |
| 45–54 | 3 | - |
| 55–64 | 7 | - |
| 65–74 | 7 | - |
| Median (P25–P75) | 61 (53–67) | |
| Gender (Male) | 13 | 68.40% |
| Height (cm) | 18 | - |
| Median (P25–P75) | 168.5 (163.7–174.0) | |
| Pathology of origin | 19 | - |
| Rectal neoplasm | 6 | 31.60% |
| Colon cancer | 1 | 5.30% |
| Radical cystectomy for bladder tumor | 2 | 10.50% |
| Recurrent interstitial cystitis | 1 | 5.30% |
| Ulcerative colitis | 2 | 10.50% |
| Crohn’s disease | 4 | 21.10% |
| Anal malformation and Crohn’s disease | 1 | 5.30% |
| Bladder neoplasm | 2 | 10.50% |
| Structure Sensor | Go!Scan 20 | |
|---|---|---|
| Operating range (recommended) | 0.4–3.5 m | 0.05–0.5 m |
| Accuracy | 0.5 mm at 40 cm 30 mm at 3 m | Up to 0.1 mm |
| Mesh resolution | 1 mm | 0.100 mm |
| Weight | 95 g | 930 g |
| Dimensions (L × W × H) | 29 × 28 × 119.2 mm | 154 × 178 × 235 mm |
| Light source | Infrared LEDs | White LED |
| Output Formats | .obj | .dae, .fbx, .ma, .obj, .ply, .stl, .txt, .wrl, .x3d, .x3dz, .zpr |
| Price | $379 without mounting device | Around $10,000 with the scanning software only (VXmodel) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahia, S.; Garcia-Zapirain, B.; Anakabe, J.; Ander, J.; Jossa Bastidas, O.; Loizate Totoricagüena, A. A Comparative Study between Scanning Devices for 3D Printing of Personalized Ostomy Patches. Sensors 2022, 22, 560. https://doi.org/10.3390/s22020560
Zahia S, Garcia-Zapirain B, Anakabe J, Ander J, Jossa Bastidas O, Loizate Totoricagüena A. A Comparative Study between Scanning Devices for 3D Printing of Personalized Ostomy Patches. Sensors. 2022; 22(2):560. https://doi.org/10.3390/s22020560
Chicago/Turabian StyleZahia, Sofia, Begonya Garcia-Zapirain, Jon Anakabe, Joan Ander, Oscar Jossa Bastidas, and Alberto Loizate Totoricagüena. 2022. "A Comparative Study between Scanning Devices for 3D Printing of Personalized Ostomy Patches" Sensors 22, no. 2: 560. https://doi.org/10.3390/s22020560
APA StyleZahia, S., Garcia-Zapirain, B., Anakabe, J., Ander, J., Jossa Bastidas, O., & Loizate Totoricagüena, A. (2022). A Comparative Study between Scanning Devices for 3D Printing of Personalized Ostomy Patches. Sensors, 22(2), 560. https://doi.org/10.3390/s22020560








