Modal Frequencies Associations with Musculoskeletal Components of Human Legs for Extracorporeal Bone Healing Assessment Based on a Vibration Analysis Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prototype Design
2.2. Clinical Trial
2.3. Data Preprocessing
2.3.1. Descriptive Statistics of Participants
2.3.2. Clustering of Modal Parameters
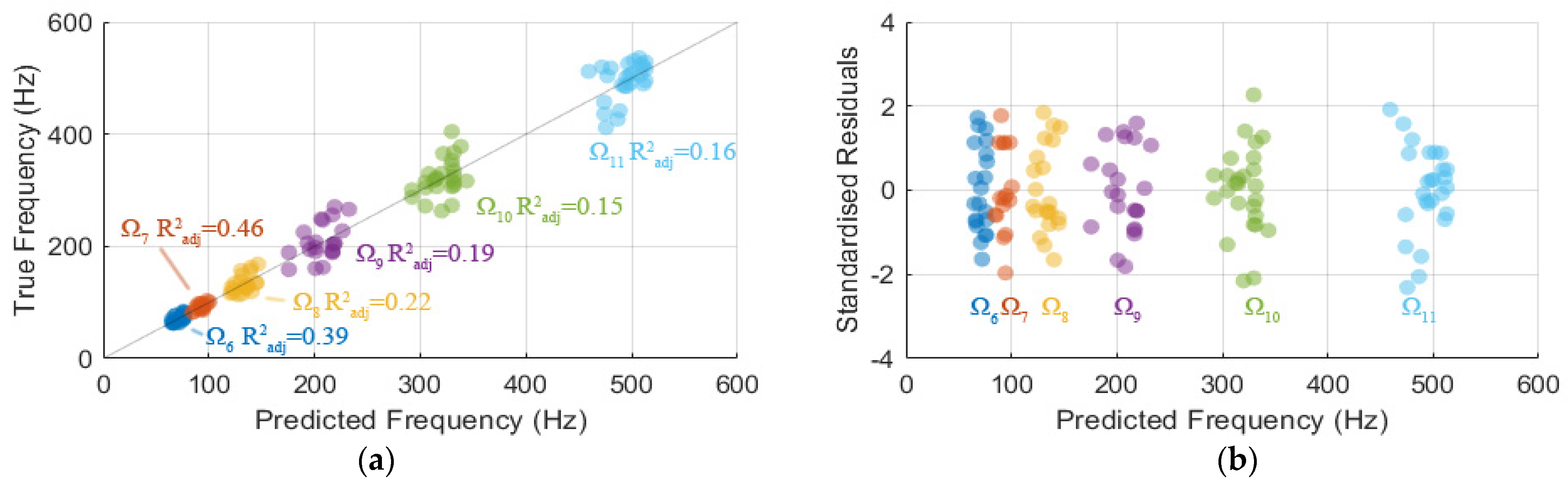
2.4. Model Formulation Using Stepwise Regression
3. Results
3.1. Modal Parameters
3.2. Association Analysis
3.3. Modal Frequency Association with Body Components
- has a weak negative (coefficient of −0.37) correlation with the lower thigh girth.
- have no correlation with the body components. There is a weak (coefficient of 0.38) correlation between and .
- has a moderate negative correlation (very high statistical significance of ) with height and leg length. Furthermore, also have fair negative correlations with weight and segmental lean leg mass, which is likely due to their correlation with leg length. Thereby, is considered the first OOP mode associated with the femur length.
- also has moderate negative correlations with height and leg length. is considered the first coupled mode associated with the femur length.
- has a moderate negative correlation with segmental lean leg mass. also has negative and positive correlations with and , respectively.
- and have a moderate negative correlation with segmental fat leg mass.
- has a moderate negative correlation with leg length.
- Though there is no correlation with statistical significance between and the body components, a further investigation found that has a weak negative correlation (coefficient of −0.33) with segmental fat leg mass with significance .
3.4. Dependency of Modes Appearances
3.5. Model of Modal Frequencies Associated with Leg Components
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Harwood, P.J.; Newman, J.B.; Michael, A.L. (ii) An update on fracture healing and non-union. Orthop. Trauma 2010, 24, 9–23. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Comiskey, D.; MacDonald, B.; McCartney, W.; Synnott, K.; O’Byrne, J. The role of interfragmentary strain on the rate of bone healing—A new interpretation and mathematical model. J. Biomech. 2010, 43, 2830–2834. [Google Scholar] [CrossRef] [PubMed]
- Alierta, J.; Pérez, M.; Seral, B.; García-Aznar, J. Biomechanical assessment and clinical analysis of different intramedullary nailing systems for oblique fractures. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 1266–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, R.; Hammerby, S.; Lindholm, B. Accuracy of radiologic assessment of tibial shaft fracture union in humans. Clin. Orthop. Relat. Res. 1985, 199, 233–238. [Google Scholar] [CrossRef]
- Blokhuis, T.; De Bruine, J.; Bramer, J.; Den Boer, F.; Bakker, F.; Patka, P.; Haarman, H.T.M.; Manoliu, R. The reliability of plain radiography in experimental fracture healing. Skelet. Radiol. 2001, 30, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M.; Lindsey, R.W.; Walter, S.D. The clinician’s ability to evaluate the strength of healing fractures from plain radiographs. J. Orthop. Trauma 1989, 3, 29–32. [Google Scholar] [CrossRef]
- Brumback, R.J.; Jones, A.L. Interobserver agreement in the classification of open fractures of the tibia. The results of a survey of two hundred and forty-five orthopaedic surgeons. J. Bone Jt. Surg. 1994, 76, 1162–1166. [Google Scholar] [CrossRef]
- Corrales, L.A.; Morshed, S.; Bhandari, M.; Miclau, T., III. Variability in the assessment of fracture-healing in orthopaedic trauma studies. J. Bone Jt. Surg. Am. Vol. 2008, 90, 1862. [Google Scholar] [CrossRef]
- Morshed, S. Current options for determining fracture union. Adv. Med. 2014, 2014, 708574. [Google Scholar] [CrossRef] [Green Version]
- Morshed, S.; Corrales, L.; Genant, H.; Miclau, T., III. Outcome assessment in clinical trials of fracture-healing. J. Bone Jt. Surg. 2008, 90, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.E.; Cunningham, J.L. Monitoring the Mechanical Properties of Healing Bone. Clin. Orthop. Relat. Res. 2009, 467, 1964–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, M.; Guyatt, G.H.; Swiontkowski, M.F.; Tornetta Iii, P.; Sprague, S.; Schemitsch, E.H. A lack of consensus in the assessment of fracture healing among orthopaedic surgeons. J. Orthop. Trauma 2002, 16, 562–566. [Google Scholar] [CrossRef]
- Lu, S.; Vien, B.; Russ, M.; Fitzgerald, M.; Chiu, W. Non-radiative healing assessment techniques for fractured long bones and osseointegrated implant. Biomed. Eng. Lett. 2019, 10, 63–81. [Google Scholar] [CrossRef]
- Cunningham, B.P.; Brazina, S.; Morshed, S.; Miclau, T., III. Fracture healing: A review of clinical, imaging and laboratory diagnostic options. Injury 2017, 48, S69–S75. [Google Scholar] [CrossRef] [PubMed]
- Perren, S. Physical and biological aspects of fracture healing with special reference to internal fixation. Clin. Orthop. Relat. Res. 1979, 138, 175–196. [Google Scholar]
- Mora-Macías, J.; Reina-Romo, E.; López-Pliego, M.; Giráldez-Sánchez, M.; Domínguez, J. In vivo mechanical characterization of the distraction callus during bone consolidation. Ann. Biomed. Eng. 2015, 43, 2663–2674. [Google Scholar] [CrossRef]
- Lacroix, D.; Prendergast, P. A mechano-regulation model for tissue differentiation during fracture healing: Analysis of gap size and loading. J. Biomech. 2002, 35, 1163–1171. [Google Scholar] [CrossRef]
- Richardson, J.; Cunningham, J.; Goodship, A.; O’connor, B.; Kenwright, J. Measuring stiffness can define healing of tibial fractures. Bone Jt. J. 1994, 76, 389–394. [Google Scholar] [CrossRef]
- Jernberger, A. Measurement of stability of tibial fractures: A mechanical method. Acta Orthop. Scand. 1970, 41, 1–88. [Google Scholar] [CrossRef]
- Protopappas, V.C.; Vavva, M.G.; Fotiadis, D.I.; Malizos, K.N. Ultrasonic monitoring of bone fracture healing. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2008, 55, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Protopappas, V.C.; Fotiadis, D.I.; Malizos, K.N. Guided ultrasound wave propagation in intact and healing long bones. Ultrasound Med. Biol. 2006, 32, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.; Kenwright, J.; Kershaw, C. Biomechanical measurement of fracture healing. J. Med. Eng. Technol. 1990, 14, 92–101. [Google Scholar] [CrossRef]
- Tower, S.S.; Beals, R.K.; Duwelius, P.J. Resonant frequency analysis of the tibia as a measure of fracture healing. J. Orthop. Trauma 1993, 7, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Benirschke, S.; Mirels, H.; Jones, D.; Tencer, A. The use of resonant frequency measurements for the noninvasive assessment of mechanical stiffness of the healing tibia. J. Orthop. Trauma 1993, 7, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuchi, Y.; Tsuchikane, A.; Nomura, A. The vibrational mode of the tibia and assessment of bone union in experimental fracture healing using the impulse response method. Med. Eng. Phys. 1996, 18, 575–583. [Google Scholar] [CrossRef]
- Inman, D.J. Engineering Vibration; Prentice Hall: Englewood Cliffs, NJ, USA; Hoboken, NJ, USA, 1994; Volume 3. [Google Scholar]
- Sekiguchi, T.; Hirayama, T. Assessment of fracture healing by vibration. Acta Orthop. Scand. 1979, 50, 391–398. [Google Scholar] [CrossRef]
- Cornelissen, M.; Cornelissen, P.; Van der Perre, G.; Christensen, A.; Ammitzboll, F.; Dyrbye, C. Assessment of tibial stiffness by vibration testing in situ—III. Sensitivity of different modes and interpretation of vibration measurements. J. Biomech. 1987, 20, 333–342. [Google Scholar] [CrossRef]
- Bediz, B.; Özgüven, H.N.; Korkusuz, F. Vibration measurements predict the mechanical properties of human tibia. Clin. Biomech. 2010, 25, 365–371. [Google Scholar] [CrossRef]
- Cornelissen, P.; Cornelissen, M.; Van der Perre, G.; Christensen, A.; Ammitzbøll, F.; Dyrbye, C. Assessment of tibial stiffness by vibration testing in situ—II. Influence of soft tissues, joints and fibula. J. Biomech. 1986, 19, 551–561. [Google Scholar] [CrossRef]
- Ong, W.; Chiu, W.; Russ, M.; Chiu, Z. Extending structural health monitoring concepts for bone healing assessment. Fatigue Fract. Eng. Mater. Struct. 2016, 39, 491–501. [Google Scholar] [CrossRef]
- Tsuchikane, A.; Nakatsuchi, Y.; Nomura, A. The influence of joints and soft tissue on the natural frequency of the human tibia using the impulse response method. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1995, 209, 149–155. [Google Scholar] [CrossRef]
- Chiu, W.K.; Vien, B.S.; Russ, M.; Fitzgerald, M. Towards a Non-Invasive Technique for Healing Assessment of Internally Fixated Femur. Sensors 2019, 19, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Lakes, R.S. The effect of soft tissue on wave-propagation and vibration tests for determining the in vivo properties of bone. J. Biomech. 1977, 10, 393–401. [Google Scholar] [CrossRef]
- Wakeling, J.M.; Nigg, B.M. Modification of soft tissue vibrations in the leg by muscular activity. J. Appl. Physiol. 2001, 90, 412–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nokes, L. The use of low-frequency vibration measurement in orthopaedics. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1999, 213, 271–290. [Google Scholar] [CrossRef]
- Chiu, W.K.; Vien, B.S.; Russ, M.; Fitzgerald, M. Healing assessment of fractured femur treated with an intramedullary nail. Struct. Health Monit. 2019, 20, 782–790. [Google Scholar] [CrossRef]
- Chiu, W.K.; Vien, B.S.; Russ, M.; Fitzgerald, M. Vibration-based healing assessment of an internally fixated femur. J. Nondestruct. Eval. Diagn. Progn. Eng. Syst. 2019, 2, 021003. [Google Scholar] [CrossRef]
- Chiu, W.; Ong, W.; Russ, M.; Fitzgerald, M. Simulated vibrational analysis of internally fixated femur to monitor healing at various fracture angles. Procedia Eng. 2017, 188, 408–414. [Google Scholar] [CrossRef]
- Ong, W.; Chiu, W.; Russ, M.; Chiu, Z. Integrating sensing elements on external fixators for healing assessment of fractured femur. Struct. Control Health Monit. 2016, 23, 1388–1404. [Google Scholar] [CrossRef]
- Ong, W.; Chiu, W.; Russ, M.; Chiu, Z. Healing Assessment of Fractured Femur: Orthopaedic SHM. Struct. Health Monit. 2015, 2, 3139–3146. [Google Scholar]
- Mattei, L.; Di Fonzo, M.; Marchetti, S.; Di Puccio, F. A quantitative and non-invasive vibrational method to assess bone fracture healing: A clinical case study. Int. Biomech. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Mattei, L.; Di Puccio, F.; Marchetti, S. Fracture healing monitoring by impact tests: Single case study of a fractured tibia with external fixator. IEEE J. Transl. Eng. Health Med. 2019, 7, 2100206. [Google Scholar] [CrossRef]
- Mattei, L.; Di Puccio, F.; Marchetti, S. In vivo impact testing on a lengthened femur with external fixation: A future option for the non-invasive monitoring of fracture healing? J. R. Soc. Interface 2018, 15, 20180068. [Google Scholar] [CrossRef] [Green Version]
- Mattei, L.; Longo, A.; Di Puccio, F.; Ciulli, E.; Marchetti, S. Vibration Testing Procedures for Bone Stiffness Assessment in Fractures Treated with External Fixation. Ann. Biomed. Eng. 2017, 45, 1111–1121. [Google Scholar] [CrossRef]
- Di Puccio, F.; Mattei, L.; Longo, A.; Marchetti, S. Fracture healing assessment based on impact testing: In vitro simulation and monitoring of the healing process of a tibial fracture with external fixator. Int. J. Appl. Mech. 2017, 9, 1750098. [Google Scholar] [CrossRef]
- Vien, B.S.; Chiu, W.K.; Russ, M.; Fitzgerald, M. A vibration analysis strategy for quantitative fracture healing assessment of an internally fixated femur with mass-loading effect of soft tissue. Struct. Health Monit. 2020, 20, 1475921720978224. [Google Scholar] [CrossRef]
- Chiu, W.K.; Russ, M.; Fitzgerald, M. Method and System for Assessing the State of Healing of a Fractured Long Bone. Australian Patent 2019417983, 2019. Available online: http://pericles.ipaustralia.gov.au/ols/auspat/applicationDetails.do?applicationNo=2019417983 (accessed on 1 March 2021).
- Botanlioglu, H.; Kantarci, F.; Kaynak, G.; Unal, Y.; Ertan, S.; Aydingoz, O.; Erginer, R.; Unlu, M.C.; Mihmanli, I.; Babacan, M. Shear wave elastography properties of vastus lateralis and vastus medialis obliquus muscles in normal subjects and female patients with patellofemoral pain syndrome. Skelet. Radiol. 2013, 42, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, A.A.; Gumussoy, S. Transfer function estimation in system identification toolbox via vector fitting. IFAC-Pap. 2017, 50, 6232–6237. [Google Scholar] [CrossRef]
- Antonio, J.; Kenyon, M.; Ellerbroek, A.; Carson, C.; Burgess, V.; Tyler-Palmer, D.; Mike, J.; Roberts, J.; Angeli, G.; Peacock, C. Comparison of Dual-Energy X-Ray Absorptiometry (DXA) versus a Multi-frequency Bioelectrical Impedance (InBody 770) device for body composition assessment after a 4-week hypoenergetic diet. J. Funct. Morphol. Kinesiol. 2019, 4, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLester, C.N.; Nickerson, B.S.; Kliszczewicz, B.M.; McLester, J.R. Reliability and agreement of various InBody body composition analyzers as compared to dual-energy X-ray absorptiometry in healthy men and women. J. Clin. Densitom. 2020, 23, 443–450. [Google Scholar] [CrossRef]
- InBody 770 Information Pack. Available online: https://inbody.net.au/wp-content/uploads/2020/05/InBody_770_Information_pack_06.pdf (accessed on 1 March 2021).
- Woerman, A.L.; Binder-Macleod, S.A. Leg length discrepancy assessment: Accuracy and precision in five clinical methods of evaluation. J. Orthop. Sports Phys. Ther. 1984, 5, 230–239. [Google Scholar] [CrossRef]
- Klein, K.F.; Hu, J.; Reed, M.P.; Hoff, C.N.; Rupp, J.D. Development and validation of statistical models of femur geometry for use with parametric finite element models. Ann. Biomed. Eng. 2015, 43, 2503–2514. [Google Scholar] [CrossRef]
- Weinberg, D.S.; Liu, R.W. The association of tibia femur ratio and degenerative disease of the spine, hips, and knees. J. Pediatric Orthop. 2017, 37, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Ester, M.; Kriegel, H.-P.; Sander, J.; Xu, X. A density-based algorithm for discovering clusters in large spatial databases with noise. In Proceedings of the Second International Conference on Knowledge Discovery and Data Mining, Portland, OR, USA, 2–4 August 1996; AAAI Press: Palo Alto, CA, USA, 1996; Volume 96, pp. 226–231. [Google Scholar]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Conventional Radiography | Computed Tomography | Bone Scintigraphy | Direct Biomechanical Testing | Indirect Biomechanical Testing | Vibration Analysis Method | Quantitative Ultrasound/Guided Wave | |
|---|---|---|---|---|---|---|---|
| Strategy | Imaging Measures | Imaging Measures | Imaging Measures | Mechanical Properties Testing | Mechanical Properties Testing | Mechanical Properties Testing | Mechanical Properties Testing |
| Clinical practice | Y | Y | Y | N | N | N | N |
| Non-destructive/ Non-invasive | Y | Y | Y | N | Y | Y | Y |
| Non-radiative | N | N | N | Y | Y | Y | Y |
| Qualitative/Quantitative | Qualitative/ Relative quantitative | Quantitative | Qualitative | Quantitative | Quantitative | Quantitative | Quantitative |
| Principle of Evaluation | Relative bone mineral density | Callus volume and bone mineral density | Radioactivity in tissues | Deflection under certain load | Strain | Resonant frequency | Wave velocity and modes |
| Earliest Stage of Healing Detection | Reparative | Inflammatory | Inflammatory | Reparative | Inflammatory | Inflammatory | Inflammatory |
| Other Limitations |
|
|
|
|
| Surrounding soft tissue and joints significantly obscure the readings. | Surrounding soft tissue and joints significantly obscure the readings. |
| Preliminary Stage | Device Testing Stage | |||
|---|---|---|---|---|
| InBody Scan | Manual Measurements | Installation of Device | Testing of Device | |
| Participant maintains test posture on InBody Body Scan to obtain body parameters. | Participant stands upright to acquire thigh circumferences. | Participant lays (supine position) on medical examination bed to measure leg lengths. | Whilst the participant is in supine position, the device is installed on participant’s knee and inflated to 180 mmHg. | Extension rod is struck to obtain 10-spectrum averaging. The pressure cuff is then deflated and removed. This process is repeated 10 times for each leg. |
| Height | Lower Thigh Girth | Upper Thigh Girth | Leg Length | Weight | Segmental Lean Leg Mass | Segmental Fat Leg Mass | |
|---|---|---|---|---|---|---|---|
| Units | cm | cm | cm | cm | kg | kg | kg |
| Mean | 173.32 | 42.36 | 50.30 | 93.48 | 71.62 | 8.49 | 2.69 |
| Minimum | 163.50 | 33.70 | 43.20 | 87.00 | 51.70 | 5.94 | 1.20 |
| Maximum | 189.20 | 53.00 | 64.00 | 104.00 | 117.20 | 11.89 | 6.10 |
| Ω1 | Ω2 | Ω3 | Ω4 | Ω5 | Ω6 | Ω7 | Ω8 | Ω9 | Ω10 | Ω11 | Ω12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average f (Hz) | 4.28 | 7.61 | 13.15 | 21.98 | 56.70 | 71.59 | 92.71 | 133.73 | 208.79 | 322.24 | 494.74 | 664.02 |
| Minimum f (Hz) | 3.00 | 4.70 | 10.00 | 18.75 | 51.18 | 62.90 | 82.40 | 113.67 | 158.44 | 263.10 | 411.67 | 604.17 |
| Maximum f (Hz) | 5.08 | 11.00 | 16.70 | 26.50 | 66.70 | 84.00 | 103.50 | 168.50 | 270.67 | 404.63 | 536.25 | 741.00 |
| Standard deviation f (Hz) | 0.42 | 0.34 | 0.71 | 0.71 | 1.69 | 1.91 | 4.66 | 4.34 | 6.40 | 8.99 | 11.94 | 16.08 |
| Relative power (dB) | −12.5 | −13.8 | −12.4 | −11.7 | −0.3 | −0.8 | −1.4 | −5.3 | −10.1 | −14.9 | −17.8 | −18.8 |
| Amplitude cross-spectrum ((m/s2)2/Hz) | 3.71 × 10−3 | 2.88 × 10−3 | 3.48 × 10−3 | 1.92 × 10−3 | 3.44 × 10−2 | 2.53 × 10−2 | 1.85 × 10−2 | 1.04 × 10−2 | 4.11 × 10−3 | 1.23 × 10−3 | 7.31 × 10−4 | 5.31 × 10−4 |
| Phase (Radian) | 0.87π | 0.65π | 0.75π | 0.92π | 0.87π | 0.82π | 0.73π | 0.69π | 0.59π | 0.64π | 0.60π | 0.53π |
| Mode | OOP | Coupled | OOP | OOP | OOP | OOP | Coupled | Coupled | Coupled | Coupled | Coupled | Coupled |
| Coherence | 0.93 | 0.90 | 0.89 | 0.94 | 0.99 | 0.99 | 0.98 | 0.99 | 0.97 | 0.96 | 0.95 | 0.95 |
| Damping ratio | 0.22 | 0.17 | 0.17 | 0.14 | 0.19 | 0.21 | 0.18 | 0.14 | 0.13 | 0.12 | 0.11 | 0.09 |
| Number of samples | 33 | 35 | 36 | 7 | 32 | 21 | 16 | 21 | 22 | 26 | 26 | 30 |
| GROUP | h | tL | tU | l | w | sL | sF | |
|---|---|---|---|---|---|---|---|---|
| Ω7 | O | - | - | - | - | 66.3 * | 7.975 ** | - |
| N | - | - | - | - | 72.4 * | 8.77 ** | - | |
| Ω8 | O | 173.6 | 43.5 | 51.1 | 95 | 75.2 | 8.92 | 2.8 ^ |
| N | 169.9 | 42 | 49.1 | 92 | 61.8 | 7.2 | 1.8 ^ | |
| Ω10 | O | - | - | - | - | 64 | 7.975 | - |
| N | - | - | - | - | 73.85 | 8.875 | - |
| Modes | Equation | Coefficient | |||||
|---|---|---|---|---|---|---|---|
| Value | SE | Value | SE | ||||
| 9.12 | 1.30 | −1.23 | 0.33 | 0.001 | |||
| 10.08 | 1.49 | −1.41 | 0.38 | 0.002 | |||
| 8.72 | 1.06 | −0.64 | 0.27 | 0.026 | |||
| 5.70 | 0.32 | −0.37 | 0.14 | 0.019 | |||
| 5.48 | 0.07 | −0.17 | 0.07 | 0.024 | |||
| 5.86 | 0.04 | −0.10 | 0.04 | 0.030 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vien, B.S.; Chiu, W.K.; Russ, M.; Fitzgerald, M. Modal Frequencies Associations with Musculoskeletal Components of Human Legs for Extracorporeal Bone Healing Assessment Based on a Vibration Analysis Approach. Sensors 2022, 22, 670. https://doi.org/10.3390/s22020670
Vien BS, Chiu WK, Russ M, Fitzgerald M. Modal Frequencies Associations with Musculoskeletal Components of Human Legs for Extracorporeal Bone Healing Assessment Based on a Vibration Analysis Approach. Sensors. 2022; 22(2):670. https://doi.org/10.3390/s22020670
Chicago/Turabian StyleVien, Benjamin Steven, Wing Kong Chiu, Matthias Russ, and Mark Fitzgerald. 2022. "Modal Frequencies Associations with Musculoskeletal Components of Human Legs for Extracorporeal Bone Healing Assessment Based on a Vibration Analysis Approach" Sensors 22, no. 2: 670. https://doi.org/10.3390/s22020670
APA StyleVien, B. S., Chiu, W. K., Russ, M., & Fitzgerald, M. (2022). Modal Frequencies Associations with Musculoskeletal Components of Human Legs for Extracorporeal Bone Healing Assessment Based on a Vibration Analysis Approach. Sensors, 22(2), 670. https://doi.org/10.3390/s22020670







