Validity of Ultra-Short-Term HRV Analysis Using PPG—A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
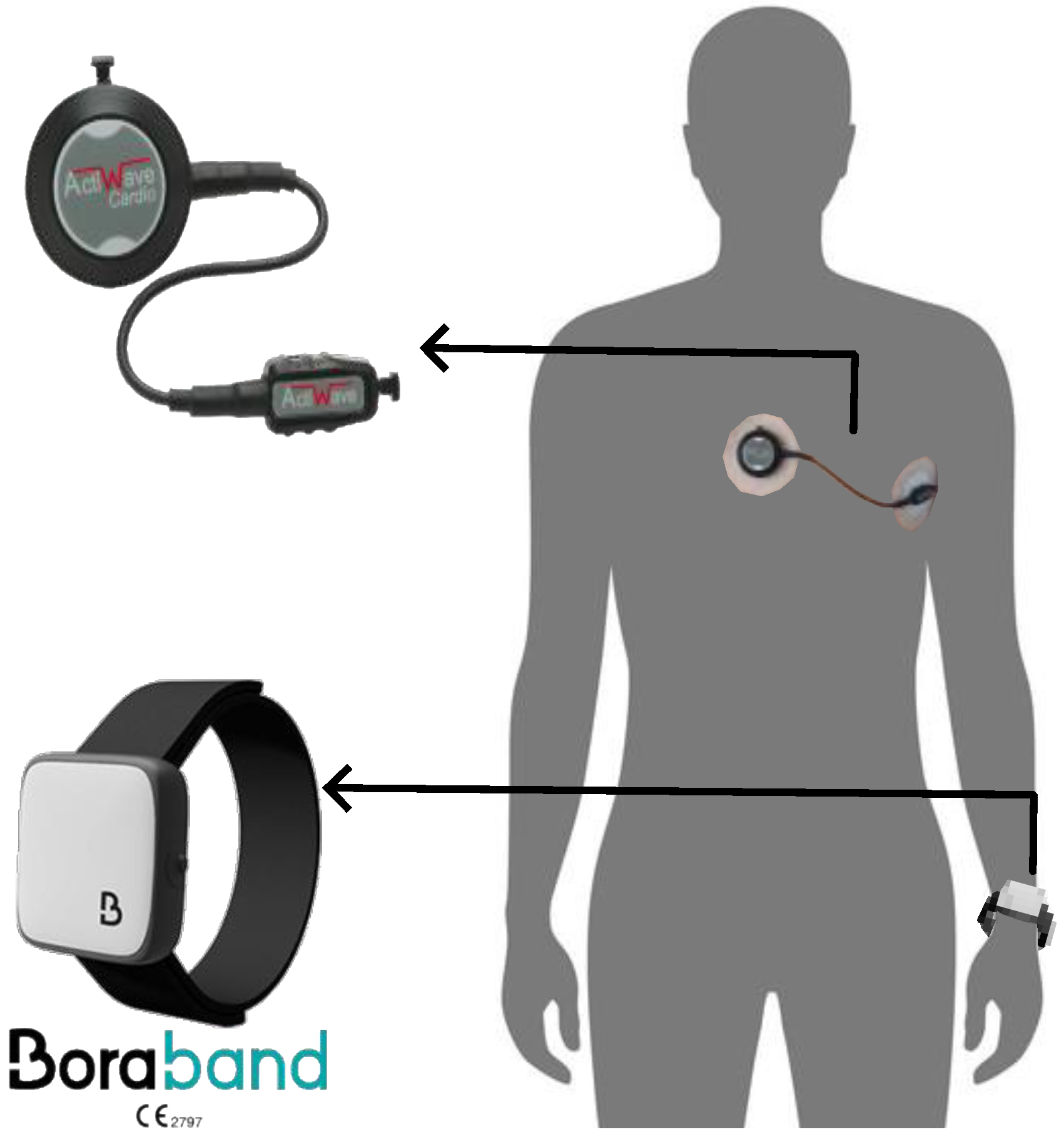
2.1. Data Collection
2.2. Data Processing
- Recording duration: two recording durations are tested—the default duration of 5 min 30 s and 1 min 30 s. The first 90 s of each recording was considered to obtain the shorter recordings of 1 min 30 s.
- Sampling rate: As mentioned earlier, the nominal sampling rate for the Bora Band is 25 Hz. However, the temporal resolution over this sampling rate is low compared to ECG. Therefore, the PPG recordings are resampled to 200 Hz using the fast Fourier transform.
2.2.1. Pre-Processing
2.2.2. R-Peak Detection from the ECG
2.2.3. P-Peak Detection from the PPG
2.2.4. R-R and P-P Intervals Computation
2.2.5. HRV Features Extraction
2.3. Statistical Analysis
3. Results
3.1. Comparison between ECG and PPG Measurements
3.2. Effect of the Duration of the Recordings
3.3. Effect of the Sampling Rate
4. Discussion
5. Clinical Interest
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosoli, G.; Scalise, L.; Poli, A.; Spinsante, S. Wearable Devices as a Valid Support for Diagnostic Excellence: Lessons from a Pandemic Going Forward. Health Technol. 2021, 11, 673–675. [Google Scholar] [CrossRef]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef] [Green Version]
- Draghici, A.E.; Taylor, J.A. The Physiological Basis and Measurement of Heart Rate Variability in Humans. J. Physiol. Anthropol. 2016, 35, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricca-Mallada, R.; Migliaro, E.R.; Piskorski, J.; Guzik, P. Exercise Training Slows down Heart Rate and Improves Deceleration and Acceleration Capacity in Patients with Heart Failure. J. Electrocardiol. 2012, 45, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Kabbach, E.Z.; Mazzuco, A.; Borghi-Silva, A.; Cabiddu, R.; Agnoleto, A.G.; Barbosa, J.F.; de Carvalho Junior, L.C.S.; Mendes, R.G. Increased Parasympathetic Cardiac Modulation in Patients with Acute Exacerbation of COPD: How Should We Interpret It? Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2221–2230. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.-Y.; Chang, J.C.-Y.; Chen, Y.-C.; Huang, H.-H.; Lin, C.-S.; How, C.-K.; Yen, D.H.-T. Changes of Heart Rate Variability Predicting Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease Requiring Hospitalization after Emergency Department Treatment. J. Chin. Med. Assoc. JCMA 2018, 81, 47–52. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Diseases and Injuries Collaborators Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Gutiérrez Villegas, C.; Paz-Zulueta, M.; Herrero-Montes, M.; Parás-Bravo, P.; Madrazo Pérez, M. Cost Analysis of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review. Health Econ. Rev. 2021, 11, 31. [Google Scholar] [CrossRef]
- Shaffer, F.; Meehan, Z.M.; Zerr, C.L. A Critical Review of Ultra-Short-Term Heart Rate Variability Norms Research. Front. Neurosci. 2020, 14, 594880. [Google Scholar] [CrossRef]
- Moraes, J.L.; Rocha, M.X.; Vasconcelos, G.G.; Vasconcelos Filho, J.E.; de Albuquerque, V.H.C.; Alexandria, A.R. Advances in Photopletysmography Signal Analysis for Biomedical Applications. Sensors 2018, 18, E1894. [Google Scholar] [CrossRef] [PubMed]
- Allen, J. Photoplethysmography and Its Application in Clinical Physiological Measurement. Physiol. Meas. 2007, 28, R1-39. [Google Scholar] [CrossRef] [Green Version]
- Georgiou, K.; Larentzakis, A.V.; Khamis, N.N.; Alsuhaibani, G.I.; Alaska, Y.A.; Giallafos, E.J. Can Wearable Devices Accurately Measure Heart Rate Variability? A Systematic Review. Folia Med. 2018, 60, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Gil, E.; Orini, M.; Bailón, R.; Vergara, J.M.; Mainardi, L.; Laguna, P. Photoplethysmography Pulse Rate Variability as a Surrogate Measurement of Heart Rate Variability during Non-Stationary Conditions. Physiol. Meas. 2010, 31, 1271–1290. [Google Scholar] [CrossRef]
- Maeda, Y.; Sekine, M.; Tamura, T. Relationship between Measurement Site and Motion Artifacts in Wearable Reflected Photoplethysmography. J. Med. Syst. 2011, 35, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Pecchia, L.; Castaldo, R.; Montesinos, L.; Melillo, P. Are Ultra-Short Heart Rate Variability Features Good Surrogates of Short-Term Ones? State-of-the-Art Review and Recommendations. Healthc. Technol. Lett. 2018, 5, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Phuc Thu, T.; Hernández, A.I.; Costet, N.; Patural, H.; Pichot, V.; Carrault, G.; Beuchée, A. Improving Methodology in Heart Rate Variability Analysis for the Premature Infants: Impact of the Time Length. PLoS ONE 2019, 14, e0220692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobbs, W.C.; Fedewa, M.V.; MacDonald, H.V.; Holmes, C.J.; Cicone, Z.S.; Plews, D.J.; Esco, M.R. The Accuracy of Acquiring Heart Rate Variability from Portable Devices: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Leon, C.; Carrault, G.; Pladys, P.; Beuchee, A. Early Detection of Late Onset Sepsis in Premature Infants Using Visibility Graph Analysis of Heart Rate Variability. IEEE J. Biomed. Health Inform. 2021, 25, 1006–1017. [Google Scholar] [CrossRef]
- Qin, Q.; Li, J.; Yue, Y.; Liu, C. An Adaptive and Time-Efficient ECG R-Peak Detection Algorithm. J. Healthc. Eng. 2017, 2017, 5980541. [Google Scholar] [CrossRef] [Green Version]
- Navarro, X.; Porée, F.; Beuchée, A.; Carrault, G. Artifact Rejection and Cycle Detection in Immature Breathing: Application to the Early Detection of Neonatal Sepsis. Biomed. Signal Process. Control 2015, 16, 9–16. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomb, N.R. Least-Squares Frequency Analysis of Unequally Spaced Data. Astrophys. Space Sci. 1976, 39, 447–462. [Google Scholar] [CrossRef]
- Hernando, A.; Lazaro, J.; Gil, E.; Arza, A.; Garzon, J.M.; Lopez-Anton, R.; de la Camara, C.; Laguna, P.; Aguilo, J.; Bailon, R. Inclusion of Respiratory Frequency Information in Heart Rate Variability Analysis for Stress Assessment. IEEE J. Biomed. Health Inform. 2016, 20, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Toichi, M.; Sugiura, T.; Murai, T.; Sengoku, A. A New Method of Assessing Cardiac Autonomic Function and Its Comparison with Spectral Analysis and Coefficient of Variation of R-R Interval. J. Auton. Nerv. Syst. 1997, 62, 79–84. [Google Scholar] [CrossRef]
- Bauer, A.; Kantelhardt, J.W.; Barthel, P.; Schneider, R.; Mäkikallio, T.; Ulm, K.; Hnatkova, K.; Schömig, A.; Huikuri, H.; Bunde, A.; et al. Deceleration Capacity of Heart Rate as a Predictor of Mortality after Myocardial Infarction: Cohort Study. Lancet 2006, 367, 1674–1681. [Google Scholar] [CrossRef]
- Piskorski, J.; Guzik, P. Asymmetric Properties of Long-Term and Total Heart Rate Variability. Med. Biol. Eng. Comput. 2011, 49, 1289–1297. [Google Scholar] [CrossRef] [Green Version]
- Lacasa, L.; Luque, B.; Luque, J.; Nuño, J.C. The Visibility Graph: A New Method for Estimating the Hurst Exponent of Fractional Brownian Motion. EPL Europhys. Lett. 2009, 86, 30001. [Google Scholar] [CrossRef] [Green Version]
- Luque, B.; Lacasa, L.; Ballesteros, F.; Luque, J. Horizontal Visibility Graphs: Exact Results for Random Time Series. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2009, 80, 046103. [Google Scholar] [CrossRef] [Green Version]
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef] [Green Version]
- Vallat, R. Pingouin: Statistics in Python. J. Open Source Softw. 2018, 3, 1026. [Google Scholar] [CrossRef]
- Parker, R.A.; Weir, C.J.; Rubio, N.; Rabinovich, R.; Pinnock, H.; Hanley, J.; McCloughan, L.; Drost, E.M.; Mantoani, L.C.; MacNee, W.; et al. Application of Mixed Effects Limits of Agreement in the Presence of Multiple Sources of Variability: Exemplar from the Comparison of Several Devices to Measure Respiratory Rate in COPD Patients. PLoS ONE 2016, 11, e0168321. [Google Scholar] [CrossRef] [Green Version]
- Vargha, A.; Delaney, H.D. A Critique and Improvement of the CL Common Language Effect Size Statistics of McGraw and Wong. J. Educ. Behav. Stat. 2000, 25, 101–132. [Google Scholar] [CrossRef]
- Lakens, D. Equivalence Tests: A Practical Primer for t Tests, Correlations, and Meta-Analyses. Soc. Psychol. Personal. Sci. 2017, 8, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-S.; Lu, W.-A.; Pagaduan, J.C.; Kuo, C.-D. A Novel Smartphone App for the Measurement of Ultra-Short-Term and Short-Term Heart Rate Variability: Validity and Reliability Study. JMIR mHealth uHealth 2020, 8, e18761. [Google Scholar] [CrossRef] [PubMed]
- Wehler, D.; Jelinek, H.F.; Gronau, A.; Wessel, N.; Kraemer, J.F.; Krones, R.; Penzel, T. Reliability of Heart-Rate-Variability Features Derived from Ultra-Short ECG Recordings and Their Validity in the Assessment of Cardiac Autonomic Neuropathy. Biomed. Signal. Process. Control 2021, 68, 102651. [Google Scholar] [CrossRef]
- Moya-Ramon, M.; Mateo-March, M.; Peña-González, I.; Zabala, M.; Javaloyes, A. Validity and Reliability of Different Smartphones Applications to Measure HRV during Short and Ultra-Short Measurements in Elite Athletes. Comput. Methods Programs Biomed. 2022, 217, 106696. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.M.; Saint-Maurice, P.F.; Kim, Y.; Hibbing, P.; Bai, Y.; Welk, G.J. A Primer on the Use of Equivalence Testing for Evaluating Measurement Agreement. Med. Sci. Sports Exerc. 2018, 50, 837–845. [Google Scholar] [CrossRef] [Green Version]
- Castaldo, R.; Montesinos, L.; Melillo, P.; James, C.; Pecchia, L. Ultra-Short Term HRV Features as Surrogates of Short Term HRV: A Case Study on Mental Stress Detection in Real Life. BMC Med. Inform. Decis. Mak. 2019, 19, 12. [Google Scholar] [CrossRef] [Green Version]
- Munoz, M.L.; van Roon, A.; Riese, H.; Thio, C.; Oostenbroek, E.; Westrik, I.; de Geus, E.J.C.; Gansevoort, R.; Lefrandt, J.; Nolte, I.M.; et al. Validity of (Ultra-)Short Recordings for Heart Rate Variability Measurements. PLoS ONE 2015, 10, e0138921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaffer, F.; Shearman, S.; Meehan, Z.M. The Promise of Ultra-Short-Term (UST) Heart Rate Variability Measurements. Biofeedback 2016, 44, 229–233. [Google Scholar] [CrossRef]
- Hon, E.H.; Lee, S.T. Electronic Evaluation of the Fetal Heart Rate. VIII. Patterns Preceding Fetal Death, Further Observations. Am. J. Obstet. Gynecol. 1963, 87, 814–826. [Google Scholar] [PubMed]
- Zamarrón, C.; Lado, M.J.; Teijeiro, T.; Morete, E.; Vila, X.A.; Lamas, P.F. Heart Rate Variability in Patients with Severe Chronic Obstructive Pulmonary Disease in a Home Care Program. Technol. Health Care 2014, 22, 91–98. [Google Scholar] [CrossRef] [PubMed]




| Correlation Coefficient | Equivalence Test p-Value | ||
|---|---|---|---|
| Time-domain features | |||
| MeanNN, MedianNN, SDNN, CVNN, IQRNN, pNN50 | >0.7 | < 0.11 | <0.05 |
| RMSSD, SDSD, CVSD | >0.7 | < 0.28 | ≥0.05 |
| Frequency-domain features | |||
| LF, LF (FR), LFnu, LFnu (FR), HFnu (FR), LF/HF (FR) | >0.7 | < 0.28 | <0.05 |
| HF, HF (FR), HFnu, LF/HF | >0.7 | < 0.28 | ≥0.05 |
| Nonlinear features | |||
| DFA-α1, SD2, CVI, SDNNa, SDNNd | >0.7 | < 0.28 | <0.05 |
| SD1, SD1/SD2, S, CSI | >0.7 | < 0.28 | ≥0.05 |
| Visibility graph features | |||
| C-VG | >0.7 | < 0.28 | <0.05 |
| Correlation Coefficient | Equivalence Test p-Value | ||
|---|---|---|---|
| Time-domain features | |||
| MeanNN, MedianNN, SDNN, pNN50 | >0.7 | < 0.11 | <0.05 |
| RMSSD, SDSD | >0.7 | 0.11 < < 0.28 | ≥0.05 |
| Frequency-domain features | |||
| LFnu (FR) | >0.7 | < 0.28 | <0.05 |
| HF (FR) | >0.7 | < 0.28 | ≥0.05 |
| Nonlinear features | |||
| SD2, S, CVI, SDNNa, SDNNd | >0.7 | < 0.28 | <0.05 |
| SD1 | >0.7 | < 0.28 | ≥0.05 |
| Agreements between HRV25P1 and HRV25P5 | |
|---|---|
| Time-domain features | MeanNN, MedianNN, SDNN, RMSSD, SDSD, CVSD, pNN20, pNN50 |
| Frequency-domain features | HF, HF (FR), LFnu (FR) |
| Nonlinear features | SD1, SD2, SD1/SD2, S, CVI, SDNNa, SDNNd |
| Features | 5 min 30 s | 1 min 30 s |
|---|---|---|
| Time-domain | MeanNN, MedianNN, SDNN, CVNN, IQRNN, RMSSD, SDSD, CVSD, pNN20, pNN50, kurtosis | MeanNN, MedianNN, SDNN, IQRNN, RMSSD, SDSD, pNN20, pNN50 |
| Frequency-domain | LF, LF (FR), HF, HF (FR), LFnu, LFnu (FR), HFnu, HFnu (FR), LF/HF, LF/HF (FR) | HF (FR), LFnu (FR) |
| Nonlinear | SampEn, DFA-α1, SD1, SD2, SD1/SD2, S, CSI, CVI, AC, DC, SDNNa, SDNNd | SD1, SD2, S, CVI, SDNNa, SDNNd |
| Visibility | MD-VG, C-VG, Tr-VG, Tr-HVG | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taoum, A.; Bisiaux, A.; Tilquin, F.; Le Guillou, Y.; Carrault, G. Validity of Ultra-Short-Term HRV Analysis Using PPG—A Preliminary Study. Sensors 2022, 22, 7995. https://doi.org/10.3390/s22207995
Taoum A, Bisiaux A, Tilquin F, Le Guillou Y, Carrault G. Validity of Ultra-Short-Term HRV Analysis Using PPG—A Preliminary Study. Sensors. 2022; 22(20):7995. https://doi.org/10.3390/s22207995
Chicago/Turabian StyleTaoum, Aline, Alexis Bisiaux, Florian Tilquin, Yann Le Guillou, and Guy Carrault. 2022. "Validity of Ultra-Short-Term HRV Analysis Using PPG—A Preliminary Study" Sensors 22, no. 20: 7995. https://doi.org/10.3390/s22207995
APA StyleTaoum, A., Bisiaux, A., Tilquin, F., Le Guillou, Y., & Carrault, G. (2022). Validity of Ultra-Short-Term HRV Analysis Using PPG—A Preliminary Study. Sensors, 22(20), 7995. https://doi.org/10.3390/s22207995





