Applying Artificial Intelligence to Wearable Sensor Data to Diagnose and Predict Cardiovascular Disease: A Review
Abstract
1. Introduction
2. Methodology
- (a)
- Current scientific advances in wearable device applications in CVD diagnosis and prediction with AI in the digital healthcare domain;
- (b)
- AI tools used in diagnostic practice; and
- (c)
- Current challenges and future developments of AI in cardiac disease management.
Search Strategy
3. Results
3.1. Types of Sensor Data
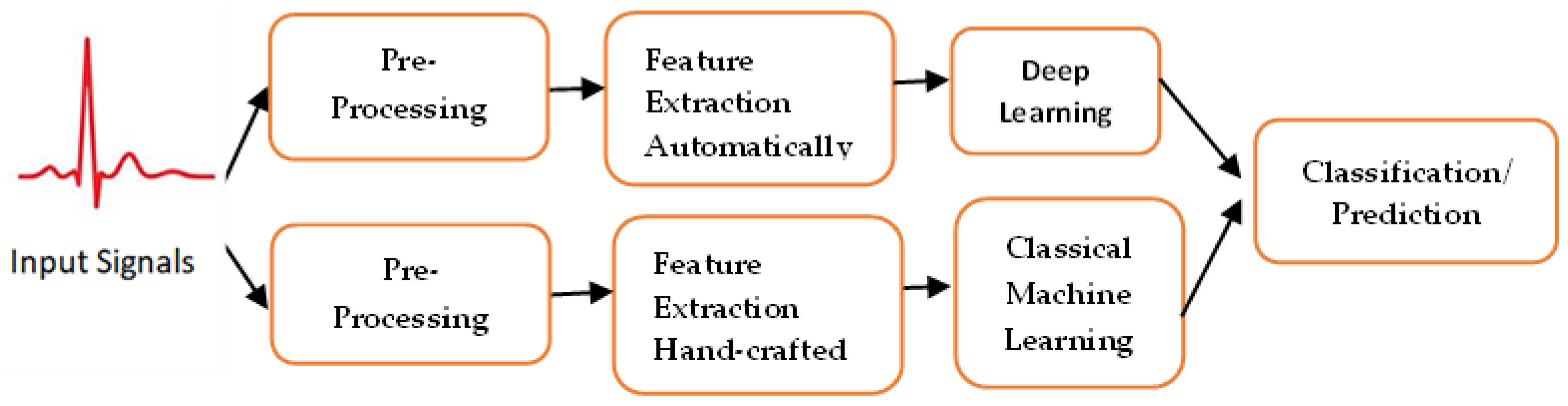
3.2. Artificial Intelligence Tools used for Cardiovascular Disease Diagnosis/Prediction, Especially for ECG
- (1)
- Preprocessing
- (2)
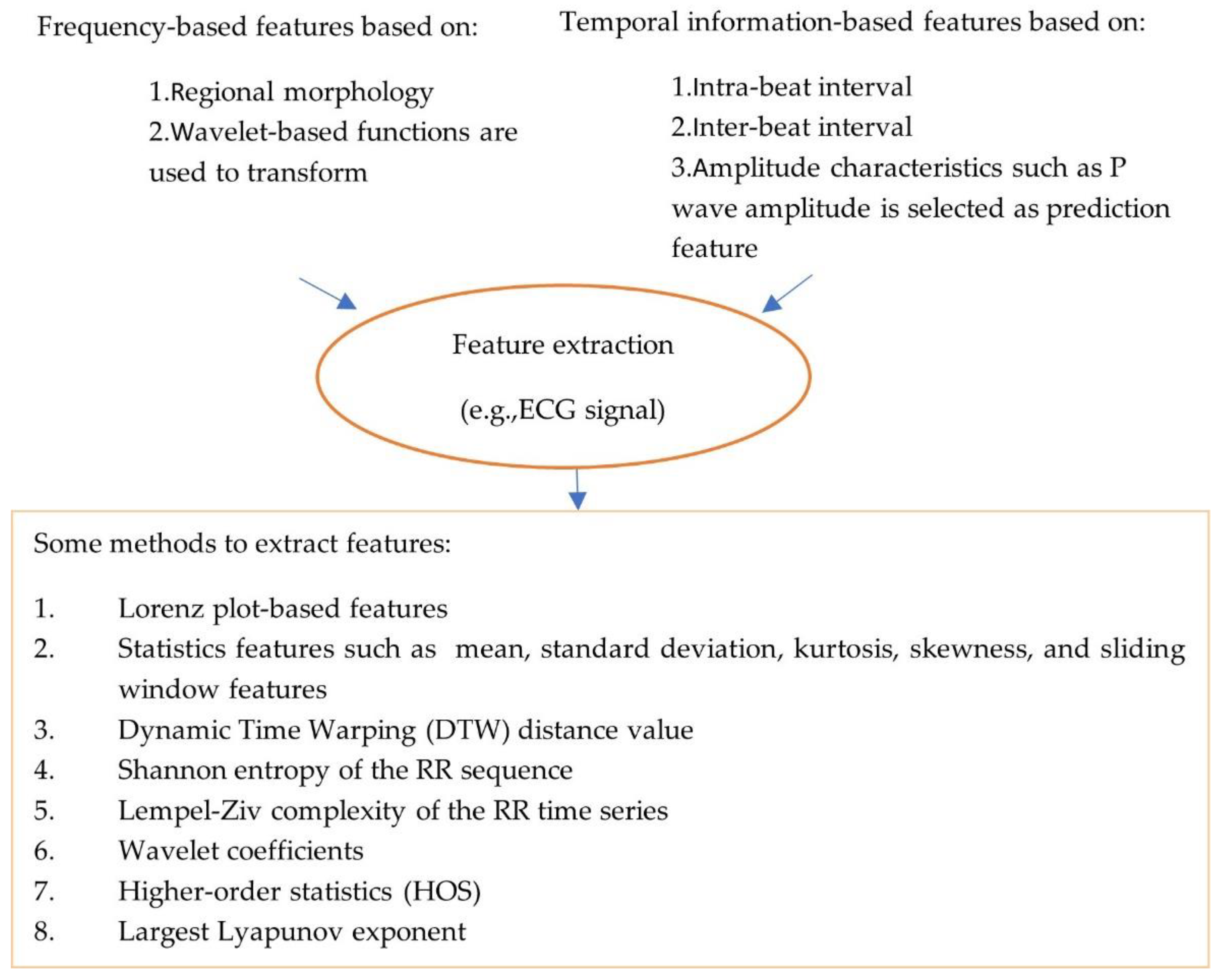
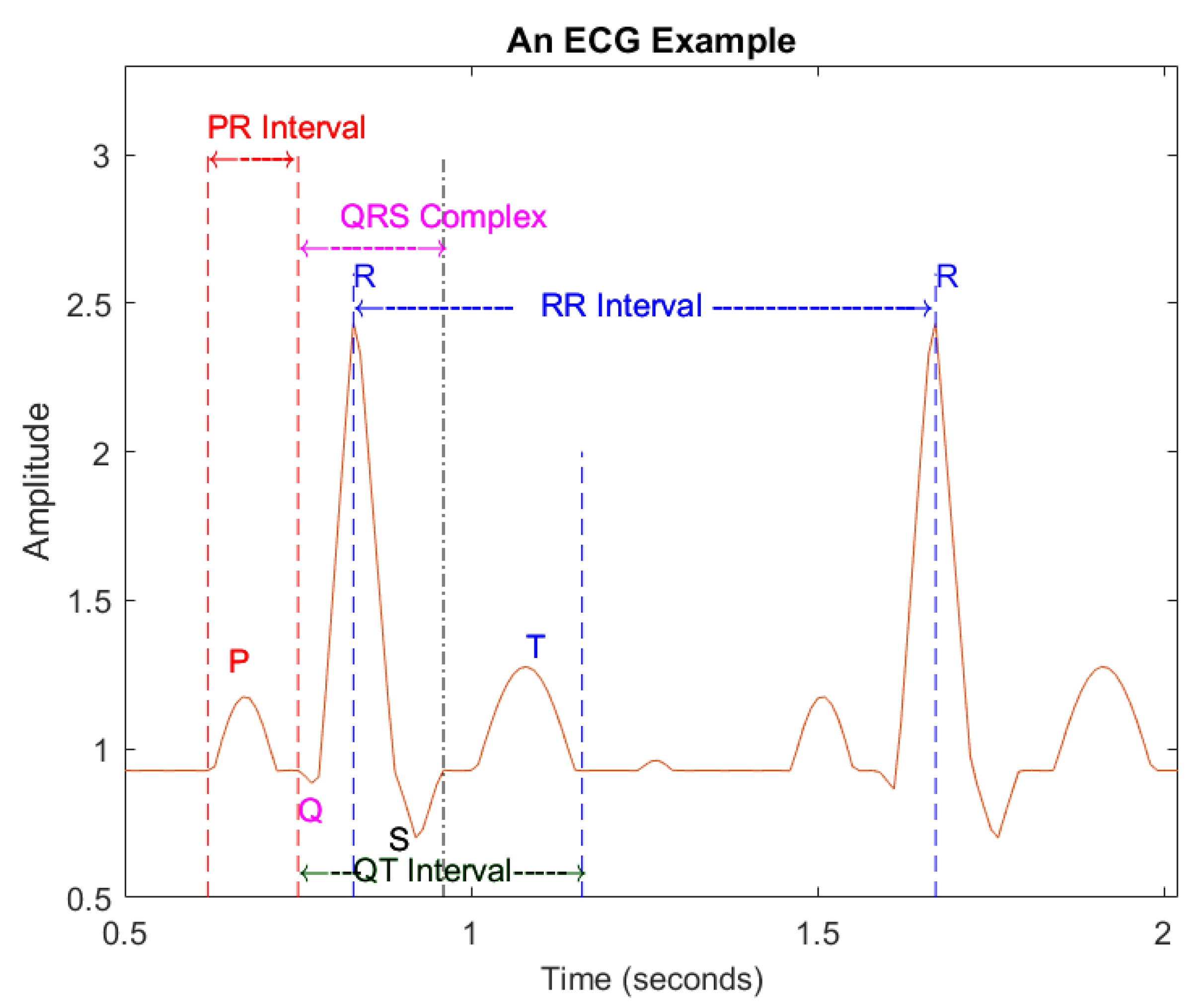
- Feature extraction:
- (3)
- Artificial intelligence, machine learning and deep learning
- Classical machine learning algorithms
- b.
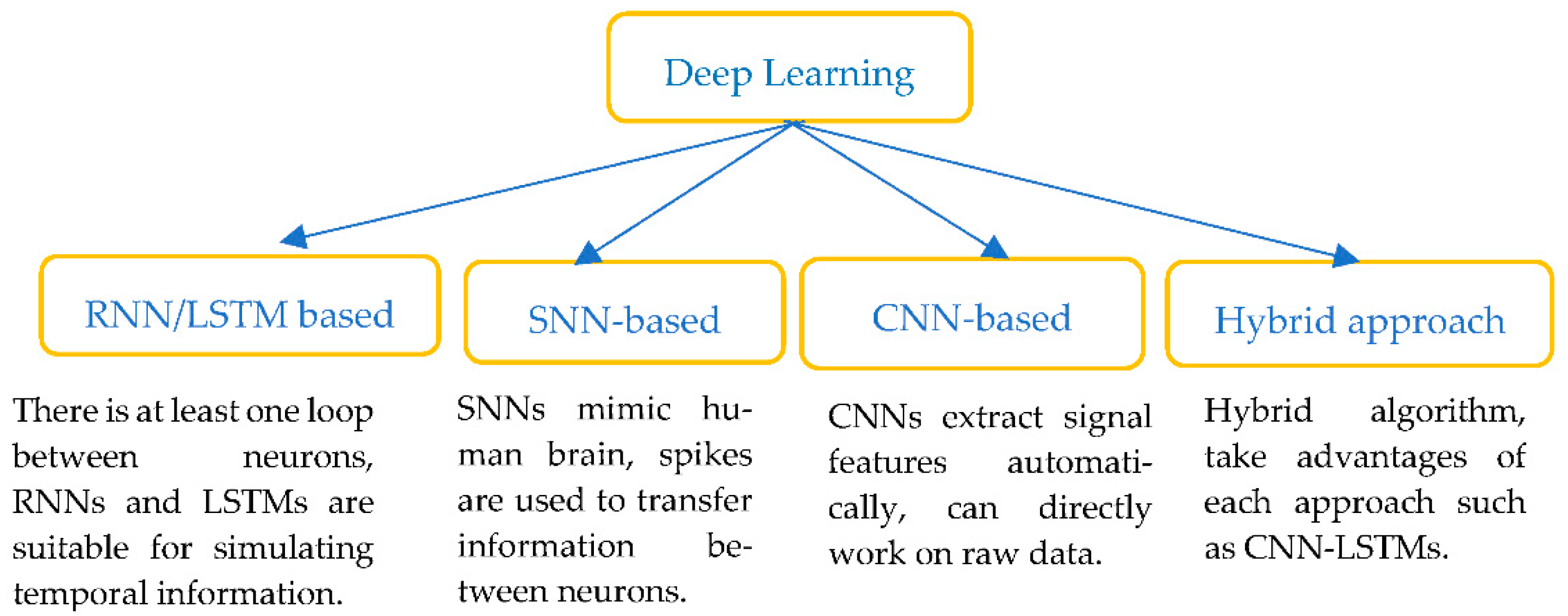
- Deep learning (DL)
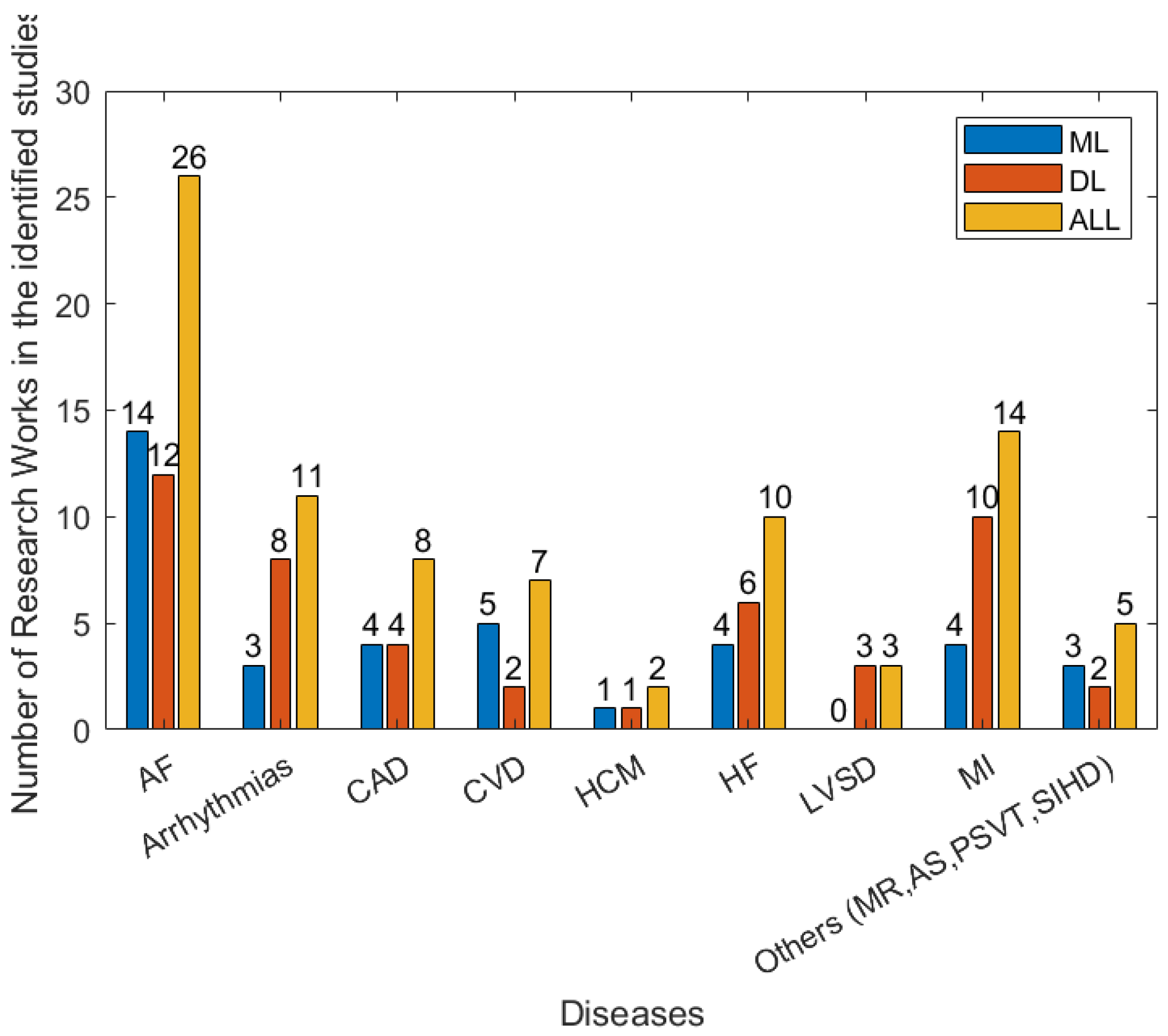
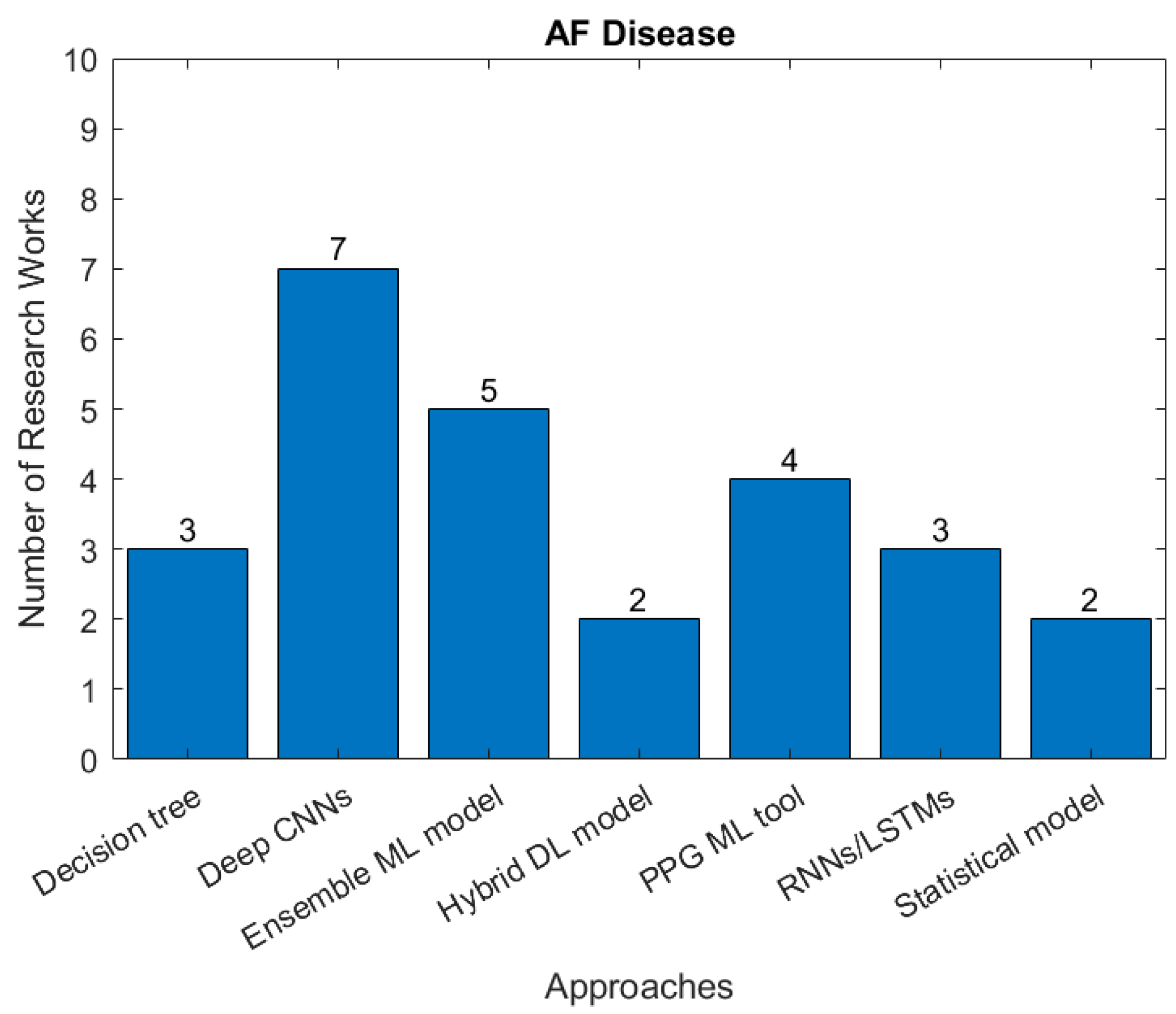
3.3. Types of Cardiovascular Disease and AI Methods
4. Discussion
4.1. AI Algorithms and Models
4.2. Functioning of Wearable Devices
4.3. Challenges and Opportunities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badnjević, A.; Pokvić, L.G.; Spahić, L. Cardiovascular techniques and technology. In Clinical Engineering Handbook; Academic Press: Cambridge, MA, USA, 2020; pp. 484–490. [Google Scholar]
- Benjamin, J.; Bllah, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; Deo, R.; et al. Heart disease and stroke statistics—2017 update: A report from the American heart association. Circulation 2017, 135, 146–160. [Google Scholar] [CrossRef] [PubMed]
- European Society of Cardiology. ESC Guidelines. 2019. Available online: www.escardio.org/Guidelines/Clinical-Practice-Guidelines (accessed on 15 May 2022).
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; de Smedt, D.; Gale, C.P.; Maggioni, A.P.; Huculeci, R.; Kazakiewicz, D.; et al. Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Frederix, I.; Caiani, E.G.; Dendale, P.; Anker, S.; Bax, J.; Böhm, A.; Cowie, M.; Crawford, J.; de Groot, N.; Dilaveris, P.; et al. ESC e-Cardiology Working Group Position Paper: Overcoming challenges in digital health implementation in cardiovascular medicine. Eur. J. Prev. Cardiol. 2019, 26, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Turakhia, M.P.; Desai, S.A.; Harrington, R.A. The outlook of digital health for cardiovascular medicine: Challenges but also extraordinary opportunities. JAMA Cardiol. 2016, 1, 743–744. [Google Scholar] [CrossRef] [PubMed]
- Mesko, B.; Drobni, Z.; Benyei, E.; Gergely, B.; Gyorffy, Z. Digital health is a cultural transformation of traditional healthcare. Mhealth 2017, 3, 3–38. [Google Scholar] [CrossRef]
- Solam, L.; Chu, Y.; Ryu, J.; Park, Y.J.; Yang, S.; Koh, S.B. Artificial Intelligence for Detection of Cardiovascular-Related Diseases from Wearable Devices: A Systematic Review and Meta-Analysis. Yonsei Med. J. 2022, 63, S93. [Google Scholar]
- Acosta, J.N.; Falcone, G.J.; Rajpurkar, P.; Topol, E.J. Multimodal biomedical AI. Nat. Med. 2022, 28, 1773–1784. [Google Scholar] [CrossRef]
- David, D.; Ding, W.Y.; Etheridge, S.; Noseworthy, P.A.; Veltmann, C.; Yao, X.; Bunch, T.J.; Gupta, D. Smart wearables for cardiac monitoring—Real-world use beyond atrial fibrillation. Sensors 2021, 21, 2539. [Google Scholar]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; Martin, S.S.; Muse, E.D.; Turakhia, M.P.; Tarakji, K.G.; et al. Smart wearable devices in cardiovascular care: Where we are and how to move forward. Nat. Rev. Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef]
- Bouzid, Z.; Al-Zaiti, S.S.; Bond, R.; Sejdić, E. Remote and wearable ECG devices with diagnostic abilities in adults: A state-of-the-science scoping review. Heart Rhythm. 2022, 19, 1192–1201. [Google Scholar] [CrossRef]
- Marcelina, O.; Kapoor, A.; Kumar, P.S.; Rangasamy, G.; Ponnuchamy, M.; Rajagopal, M.; Banerje, P.N.E. Modeling of sugarcane bagasse conversion to levulinic acid using response surface methodology (RSM), artificial neural networks (ANN), and fuzzy inference system (FIS): A comparative evaluation. Fuel 2022, 329, 125409. [Google Scholar]
- Raj, M.M.; Riyaz, N.U.S.; Reddy, M.; Yalcin, H.C.; Ouakad, H.M.; Bahadur, I.; Al-Maadeed, S.; Sadasivuni, K.K. A review of smart sensors coupled with Internet of Things and Artificial Intelligence approach for heart failure monitoring. Med. Biol. Eng. Comput. 2021, 59, 2185–2203. [Google Scholar]
- Siontis, C.K.; Noseworthy, P.A.; Attia, Z.I.; Friedman, P.A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 2021, 18, 465–478. [Google Scholar] [CrossRef]
- Pevnick, M.J.; Birkeland, K.; Zimmer, R.; Elad, Y.; Kedan, I. Wearable technology for cardiology: An update and framework for the future. Trends Cardiovasc. Med. 2018, 28, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Anushree, T.; Dhir, A.; Islam, A.K.M.N.; Mäntymäki, M. Blockchain in healthcare: A systematic literature review, synthesizing framework and future research agenda. Comput. Ind. 2022, 122, 103290. [Google Scholar]
- Elassad, A.; Elamrani, Z.; Mousannif, H.; al Moatassime, H.; Karkouch, A. The application of machine learning techniques for driving behavior analysis: A conceptual framework and a systematic literature review. Eng. Appl. Artif. Intell. 2020, 87, 103312. [Google Scholar] [CrossRef]
- Regona, M.; Yigitcanlar, T.; Xia, B.; Li, R.Y.M. Opportunities and adoption challenges of AI in the construction industry: A PRISMA review. J. Open Innov. Technol. Mark. Complex. 2022, 8, 45. [Google Scholar] [CrossRef]
- Shao, M.; Bin, G.; Wu, S.; Bin, G.; Huang, J.; Zhou, Z. Detection of atrial fibrillation from ECG recordings using decision tree ensemble with multi-level features. Physiol. Meas. 2018, 39, 094008. [Google Scholar] [CrossRef]
- Fallet, S.; Lemay, M.; Renevey, P.; Leupi, C.; Pruvot, E.; Vesin, J.-M. Can one detect atrial fibrillation using a wrist-type photoplethysmographic device? Med. Biol. Eng. Comput. 2019, 57, 477–487. [Google Scholar] [CrossRef]
- Ghiasi, M.M.; Zendehboudi, S.; Mohsenipour, A.A. Decision tree-based diagnosis of coronary artery disease: CART model. Comput. Methods Programs Biomed. 2020, 192, 105400. [Google Scholar] [CrossRef]
- Tozlu, B.H.; Şimşek, C.; Aydemir, O.; Karavelioglu, Y. A High performance electronic nose system for the recognition of myocardial infarction and coronary artery diseases. Biomed. Signal Process. Control 2021, 64, 102247. [Google Scholar] [CrossRef]
- Qureshi, K.N.; Din, S.; Jeon, G.; Piccialli, F. An accurate and dynamic predictive model for a smart M-Health system using machine learning. Inf. Sci. 2020, 538, 486–502. [Google Scholar] [CrossRef]
- Mei, Z.; Gu, X.; Chen, H.; Chen, W. Automatic atrial fibrillation detection based on heart rate variability and spectral features. IEEE Access 2018, 6, 53566–53575. [Google Scholar] [CrossRef]
- Zuhair, I.; Lahdenoja, O.; Tadi, M.J.; Hurnanen, T.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; Koivisto, T.; Pänkäälä, M. Multiclass classifier based cardiovascular condition detection using smartphone mechanocardiography. Sci. Rep. 2018, 8, 9344. [Google Scholar]
- Sengupta, P.P.; Kulkarni, H.; Narula, J. Prediction of abnormal myocardial relaxation from signal processed surface ECG. J. Am. Coll. Cardiol. 2018, 71, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Sopic, D.; Aminifar, A.; Atienza, D. Real-time event-driven classification technique for early detection and prevention of myocardial infarction on wearable systems. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 982–992. [Google Scholar] [CrossRef]
- Meng, Y.; Speier, W.; Shufelt, C.; Joung, S.; Van Eyk, J.E.; Merz, C.N.B.; Lopez, M.; Spiegel, B.; Arnold, C.W. A machine learning approach to classifying self-reported health status in a cohort of patients with heart disease using activity tracker data. IEEE J. Biomed. Health Inform. 2019, 24, 878–884. [Google Scholar] [CrossRef]
- Akbulut, F.P.; Akan, A. A smart wearable system for short-term cardiovascular risk assessment with emotional dynamics. Measurement 2018, 128, 237–246. [Google Scholar] [CrossRef]
- Dunn, J.; Kidzinski, L.; Runge, R.; Witt, D.; Hicks, J.L.; Schüssler-Fiorenza Rose, S.M.; Li, X.; Bahmani, A.; Delp, S.L.; Hastie, T.; et al. Wearable sensors enable personalized predictions of clinical laboratory measurements. Nat. Med. 2021, 27, 1105–1112. [Google Scholar] [CrossRef]
- Lichy, H.; Askari, M.; Altman, R.B.; Schmitt, S.K.; Fan, J.; Bentley, J.P.; Narayan, S.M.; Turakhia, M.P. Atrial fibrillation burden signature and near-term prediction of stroke: A machine learning analysis. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005595. [Google Scholar]
- Hill, N.R.; Ayoubkhani, D.; McEwan, P.; Sugrue, D.M.; Farooqui, U.; Lister, S.; Lumley, M.; Bakhai, A.; Cohen, A.T.; O’Neill, M.; et al. Predicting atrial fibrillation in primary care using machine learning. PLoS ONE 2019, 14, e0224582. [Google Scholar] [CrossRef]
- Jabeen, F.; Maqsood, M.; Ghazanfar, M.A.; Aadil, F.; Khan, S.; Khan, M.F.; Mehmood, I. An IoT based efficient hybrid recommender system for cardiovascular disease. Peer Peer Netw. Appl. 2019, 12, 1263–1276. [Google Scholar] [CrossRef]
- Kańtoch, E. Recognition of sedentary behavior by machine learning analysis of wearable sensors during activities of daily living for telemedical assessment of cardiovascular risk. Sensors 2018, 18, 3219. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.C.; Yuan, N.; Ouyang, D. Predicting the Future with Wearable Technology. JACC Asia 2021, 1, 409–410. [Google Scholar] [CrossRef]
- Li, B.; Ding, S.; Song, G.; Li, J.; Zhang, Q. Computer-aided diagnosis and clinical trials of cardiovascular diseases based on artificial intelligence technologies for risk-early warning model. J. Med. Syst. 2019, 43, 1–10. [Google Scholar] [CrossRef]
- Yang, W.; Si, Y.; Wang, D.; Guo, B. Automatic recognition of arrhythmia based on principal component analysis network and linear support vector machine. Comput. Biol. Med. 2018, 101, 22–32. [Google Scholar] [CrossRef]
- Yang, C.; Aranoff, N.D.; Green, P.; Tavassolian, N. Classification of aortic stenosis using time–frequency features from chest cardio-mechanical signals. IEEE Trans. Biomed. Eng. 2019, 67, 1672–1683. [Google Scholar] [CrossRef]
- Hui, Y.; Wei, Z. Arrhythmia recognition and classification using combined parametric and visual pattern features of ECG morphology. IEEE Access 2020, 8, 47103–47117. [Google Scholar]
- Bumgarner, J.M.; Lambert, C.T.; Hussein, A.A.; Cantillon, D.J.; Baranowski, B.; Wolski, K.; Lindsay, B.D.; Wazni, O.M.; Tarakji, K.G. Smartwatch algorithm for automated detection of atrial fibrillation. J. Am. Coll. Cardiol. 2018, 71, 2381–2388. [Google Scholar] [CrossRef]
- Dörr, M.; Nohturfft, V.; Brasier, N.; Bosshard, E.; Djurdjevic, A.; Gross, S.; Raichle, C.J.; Rhinisperger, M.; Stöckli, R.; Eckstein, J. The WATCH AF trial: SmartWATCHes for detection of atrial fibrillation. JACC Clin. Electrophysiol. 2019, 5, 199–208. [Google Scholar] [CrossRef]
- Yong-Yan, F.; Li, Y.; Li, J.; Cheng, W.; Shan, Z.; Wang, Y.; Guo, Y. Diagnostic performance of a smart device with photoplethysmography technology for atrial fibrillation detection: Pilot study (Pre-mAFA II registry). JMIR mHealth uHealth 2019, 7, e11437. [Google Scholar]
- Green, E.M.; van Mourik, R.; Wolfus, C.; Heitner, S.B.; Dur, O.; Semigran, M.J. Machine learning detection of obstructive hypertrophic cardiomyopathy using a wearable biosensor. NPJ Digit. Med. 2019, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, H.; Zhang, H.; Liu, T.; Liang, Z.; Xia, Y.; Yan, L.; Xing, Y.; Shi, H.; Li, S.; et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J. Am. Coll. Cardiol. 2019, 74, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Karwath, A.; Bunting, K.V.; Gill, S.K.; Tica, O.; Pendleton, S.; Aziz, F.; Barsky, A.D.; Chernbumroong, S.; Duan, J.; Mobley, A.R.; et al. Redefining β-blocker response in heart failure patients with sinus rhythm and atrial fibrillation: A machine learning cluster analysis. Lancet 2021, 398, 1427–1435. [Google Scholar] [CrossRef]
- Khan, M.A.; Algarni, F. A healthcare monitoring system for the diagnosis of heart disease in the IoMT cloud environment using MSSO-ANFIS. IEEE Access 2020, 8, 122259–122269. [Google Scholar] [CrossRef]
- Zeng, W.; Yuan, J.; Yuan, C.; Wang, Q.; Liu, F.; Wang, Y. Classification of myocardial infarction based on hybrid feature extraction and artificial intelligence tools by adopting tunable-Q wavelet transform (TQWT), variational mode decomposition (VMD) and neural networks. Artif. Intell. Med. 2020, 106, 101848. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- Shao, M.; Zhou, Z.; Bin, G.; Bai, Y.; Wu, S. A wearable electrocardiogram telemonitoring system for atrial fibrillation detection. Sensors 2020, 20, 606. [Google Scholar] [CrossRef]
- Spaccarotella, C.A.M.; Polimeni, A.; Migliarino, S.; Principe, E.; Curcio, A.; Mongiardo, A.; Sorrentino, S.; De Rosa, S.; Indolfi, C. Multichannel electrocardiograms obtained by a smartwatch for the diagnosis of ST-segment changes. JAMA Cardiol. 2020, 5, 1176–1180. [Google Scholar] [CrossRef]
- Stehlik, J.; Schmalfuss, C.; Bozkurt, B.; Nativi-Nicolau, J.; Wohlfahrt, P.; Wegerich, S.; Rose, K.; Ray, R.; Schofield, R.; Deswal, A.; et al. Continuous wearable monitoring analytics predict heart failure hospitalization: The LINK-HF multicenter study. Circ. Heart Fail. 2020, 13, e006513. [Google Scholar] [CrossRef]
- Steinhubl, S.R.; Waalen, J.; Edwards, A.M.; Ariniello, L.M.; Mehta, R.R.; Ebner, G.S.; Carter, C.; Baca-Motes, K.; Felicione, E.; Sarich, T.; et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: The mSToPS randomized clinical trial. JAMA 2018, 320, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Samuel, O.W.; Yang, B.; Geng, Y.; Asogbon, M.G.; Pirbhulal, S.; Mzurikwao, D.; Idowu, O.P.; Ogundele, T.J.; Li, X.; Chen, S.; et al. A new technique for the prediction of heart failure risk driven by hierarchical neighborhood component-based learning and adaptive multi-layer networks. Future Gener. Comput. Syst. 2020, 110, 781–794. [Google Scholar] [CrossRef]
- Mohammad, A.M.; Olesen, K.K.W.; Koul, S.; Gale, C.P.; Rylance, R.; Jernberg, T.; Baron, T.; Spaak, J.; James, S.; Lindahl, B.; et al. Development and validation of an artificial neural network algorithm to predict mortality and admission to hospital for heart failure after myocardial infarction: A nationwide population-based study. Lancet Digit. Health 2022, 4, e37–e45. [Google Scholar] [CrossRef]
- Kwon, J.-M.; Kim, K.-H.; Eisen, H.J.; Cho, Y.; Jeon, K.-H.; Lee, S.Y.; Park, J.; Oh, B.-H. Artificial intelligence assessment for early detection of heart failure with preserved ejection fraction based on electrocardiographic features. Eur. Heart J. Digit. Health 2021, 2, 106–116. [Google Scholar] [CrossRef]
- Chocron, A.; Oster, J.; Biton, S.; Mandel, F.; Elbaz, M.; Zeevi, Y.Y.; Behar, J.A. Remote atrial fibrillation burden estimation using deep recurrent neural network. IEEE Trans. Biomed. Eng. 2020, 68, 2447–2455. [Google Scholar] [CrossRef]
- Feng, K.; Pi, X.; Liu, H.; Sun, K. Myocardial infarction classification based on convolutional neural network and recurrent neural network. Appl. Sci. 2019, 9, 1879. [Google Scholar] [CrossRef]
- Saadatnejad, S.; Oveisi, M.; Hashemi, M. LSTM-based ECG classification for continuous monitoring on personal wearable devices. IEEE J. Biomed. Health Inform. 2019, 24, 515–523. [Google Scholar] [CrossRef]
- Chang, K.-C.; Hsieh, P.-H.; Wu, M.-Y.; Wang, Y.-C.; Wei, J.-T.; Shih, E.S.C.; Hwang, M.-J.; Lin, W.-Y.; Lee, K.-J.; Wang, T.-H. Usefulness of multi-labelling artificial intelligence in detecting rhythm disorders and acute ST-elevation myocardial infarction on 12-lead electrocardiogram. Eur. Heart J. Digit. Health 2021, 2, 299–310. [Google Scholar] [CrossRef]
- Dami, S.; Yahaghizadeh, M. Predicting cardiovascular events with deep learning approach in the context of the internet of things. Neural Comput. Appl. 2021, 33, 7979–7996. [Google Scholar] [CrossRef]
- Faust, O.; Shenfield, A.; Kareem, M.; San, T.R.; Fujita, H.; Acharya, U.R. Automated detection of atrial fibrillation using long short-term memory network with RR interval signals. Comput. Biol. Med. 2018, 102, 327–335. [Google Scholar] [CrossRef]
- Tadesse, G.A.; Javed, H.; Weldemariam, K.; Liu, Y.; Liu, J.; Chen, J.; Zhu, T. DeepMI: Deep multi-lead ECG fusion for identifying myocardial infarction and its occurrence-time. Artif. Intell. Med. 2021, 121, 102192. [Google Scholar] [CrossRef] [PubMed]
- Lui, H.W.; Chow, K.L. Multiclass classification of myocardial infarction with convolutional and recurrent neural networks for portable ECG devices. Inform. Med. Unlocked 2018, 13, 26–33. [Google Scholar] [CrossRef]
- Tan, J.H.; Hagiwara, Y.; Pang, W.; Lim, I.; Oh, S.L.; Adam, M.; Tan, R.S.; Chen, M.; Acharya, U.R. Application of stacked convolutional and long short-term memory network for accurate identification of CAD ECG signals. Comput. Biol. Med. 2018, 94, 19–26. [Google Scholar] [PubMed]
- Alireza, A.; Hashemi, M. ECG classification algorithm based on STDP and R-STDP neural networks for real-time monitoring on ultra low-power personal wearable devices. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1483–1493. [Google Scholar]
- Yan, Z.; Zhou, J.; Wong, W.-F. Energy efficient ECG classification with spiking neural network. Biomed. Signal Process. Control 2021, 63, 102170. [Google Scholar] [CrossRef]
- Attia, Z.I.; Kapa, S.; Lopez-Jimenez, F.; McKie, P.M.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Enriquez-Sarano, M.; Noseworthy, P.A.; Munger, T.M.; et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat. Med. 2019, 25, 70–74. [Google Scholar] [CrossRef]
- Attia, Z.I.; Kapa, S.; Yao, X.; Lopez-Jimenez, F.; Mohan, T.L.; Pellikka, P.A.; Carter, R.E.; Shah, N.D.; Friedman, P.A.; Noseworthy, P.A. Noseworthy. Prospective validation of a deep learning electrocardiogram algorithm for the detection of left ventricular systolic dysfunction. J. Cardiovasc. Electrophysiol. 2019, 30, 668–674. [Google Scholar] [CrossRef]
- Attia, I.Z.; Tseng, A.S.; Benavente, E.D.; Medina-Inojosa, J.R.; Clark, T.G.; Malyutina, S.; Kapa, S.; Schirmer, H.; Kudryavtsev, A.V.; Noseworthy, P.A.; et al. External validation of a deep learning electrocardiogram algorithm to detect ventricular dysfunction. Int. J. Cardiol. 2021, 329, 130–135. [Google Scholar]
- Bachtiger, P.; Petri, C.F.; Scott, F.E.; Park, S.R.; Kelshiker, M.A.; Sahemey, H.K.; Dumea, B.; Alquero, R.; Padam, P.S.; Hatrick, I.R.; et al. Point-of-care screening for heart failure with reduced ejection fraction using artificial intelligence during ECG-enabled stethoscope examination in London, UK: A prospective, observational, multicentre study. Lancet Digit. Health 2022, 4, e117–e125. [Google Scholar] [CrossRef]
- Betancur, J.; Commandeur, F.; Motlagh, M.; Sharir, T.; Einstein, A.J.; Bokhari, S.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.; Sinusas, A.J.; et al. Deep learning for prediction of obstructive disease from fast myocardial perfusion SPECT: A multicenter study. JACC: Cardiovasc. Imaging 2018, 11, 1654–1663. [Google Scholar]
- Wenjie, C.; Hu, D. QRS complex detection using novel deep learning neural networks. IEEE Access 2020, 8, 97082–97089. [Google Scholar]
- Jinwoo, C.; Lee, B.; Kwon, J.; Lee, Y.; Park, H.; Oh, B.; Jeon, K.; Park, J.; Kim, K. Artificial intelligence algorithm for screening heart failure with reduced ejection fraction using electrocardiography. ASAIO J. 2021, 67, 314–321. [Google Scholar]
- Christopoulos, G.; Graff-Radford, J.; Lopez, C.L.; Yao, X.; Attia, Z.I.; Rabinstein, A.A.; Petersen, R.C.; Knopman, D.S.; Mielke, M.M.; Kremers, W.; et al. Artificial intelligence–electrocardiography to predict incident atrial fibrillation: A population-based study. Circ. Arrhythmia Electrophysiol. 2020, 13, e009355. [Google Scholar] [CrossRef]
- Han, C.; Song, Y.; Lim, H.-S.; Tae, Y.; Jang, J.-H.; Lee, B.T.; Lee, Y.; Bae, W.; Yoon, D. Automated Detection of Acute Myocardial Infarction Using Asynchronous Electrocardiogram Signals-Preview of Implementing Artificial Intelligence with Multichannel Electrocardiographs Obtained from Smartwatches: Retrospective Study. J. Med. Internet Res. 2021, 23, e31129. [Google Scholar] [CrossRef] [PubMed]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Jo, Y.-Y.; Kwon, J.-M.; Jeon, K.-H.; Cho, Y.-H.; Shin, J.-H.; Lee, Y.-J.; Jung, M.-S.; Ban, J.-H.; Kim, K.-H.; Lee, S.Y.; et al. Artificial intelligence to diagnose paroxysmal supraventricular tachycardia using electrocardiography during normal sinus rhythm. Eur. Heart J. Digit. Health 2021, 2, 290–298. [Google Scholar] [CrossRef]
- Kiyasseh, D.; Zhu, T.; Clifton, D. A clinical deep learning framework for continually learning from cardiac signals across diseases, time, modalities, and institutions. Nat. Commun. 2021, 12, 4221. [Google Scholar] [CrossRef]
- Ko, W.-Y.; Siontis, K.C.; Attia, Z.I.; Carter, R.E.; Kapa, S.; Ommen, S.R.; Demuth, S.J.; Ackerman, M.J.; Gersh, B.J.; Arruda-Olson, A.M.; et al. Detection of hypertrophic cardiomyopathy using a convolutional neural network-enabled electrocardiogram. J. Am. Coll. Cardiol. 2020, 75, 722–733. [Google Scholar] [CrossRef]
- Kwon, J.-M.; Kim, K.-H.; Akkus, Z.; Jeon, K.-H.; Park, J.; Oh, B.-H. Artificial intelligence for detecting mitral regurgitation using electrocardiography. J. Electrocardiol. 2020, 59, 151–157. [Google Scholar] [CrossRef]
- Lai, D.; Bu, Y.; Su, Y.; Zhang, X.; Ma, C.-S. Non-standardized patch-based ECG lead together with deep learning based algorithm for automatic screening of atrial fibrillation. IEEE J. Biomed. Health Inform. 2020, 24, 1569–1578. [Google Scholar] [CrossRef]
- Li, G.; Watanabe, K.; Anzai, H.; Song, X.; Qiao, A.; Ohta, M. Pulse-wave-pattern classification with a convolutional neural network. Sci. Rep. 2019, 9, 14930. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, E.B.; Paglinawan, A.C.; Chung, W.Y.; Paa, G.L.S. ECG diagnostic support system (EDSS): A deep learning neural network based classification system for detecting ECG abnormal rhythms from a low-powered wearable biosensors. Sens. Bio-Sens. Res. 2021, 31, 100398. [Google Scholar] [CrossRef]
- Ribeiro, A.L.P.; Ribeiro, M.H.; Paixão, G.M.M.; Oliveira, D.M.; Gomes, P.R.; Canazart, J.A.; Ferreira, M.P.S.; Andersson, C.R.; Macfarlane, P.W.; Meira, W.; et al. Automatic diagnosis of the 12-lead ECG using a deep neural network. Nat. Commun. 2020, 11, 1760. [Google Scholar] [CrossRef] [PubMed]
- Tison, G.H.; Sanchez, J.M.; Ballinger, B.; Singh, A.; Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Lee, E.S.; Fan, S.M.; Gladstone, R.A.; et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 2018, 3, 409–416. [Google Scholar] [CrossRef]
- Torres-Soto, J.; Ashley, E.A. Multi-task deep learning for cardiac rhythm detection in wearable devices. NPJ Digit. Med. 2020, 3, 116. [Google Scholar] [CrossRef]
- Wasserlauf, J.; You, C.; Patel, R.; Valys, A.; Albert, D.; Passman, R. Smartwatch performance for the detection and quantification of atrial fibrillation. Circ. Arrhythmia Electrophysiol. 2019, 12, e006834. [Google Scholar] [CrossRef]
- Yao, X.; McCoy, R.G.; Friedman, P.A.; Shah, N.D.; Barry, B.A.; Behnken, E.M.; Inselman, J.W.; Attia, Z.I.; Noseworthy, P.A. ECG AI-Guided Screening for Low Ejection Fraction (EAGLE): Rationale and design of a pragmatic cluster randomized trial. Am. Heart J. 2020, 219, 31–36. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, J.; Hou, Y.; Zhu, M.; Lu, Y.; Xu, Y.; Teliewubai, J.; Liu, W.; Xu, X.; Li, X.; et al. Early detection of ST-segment elevated myocardial infarction by artificial intelligence with 12-lead electrocardiogram. Int. J. Cardiol. 2020, 317, 223–230. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, C.; Yin, H.; Li, X.; Zuo, P.; Ding, J.; Lin, F.; Wang, J.; Zhou, B.; Li, Y.; et al. Automatic multilabel electrocardiogram diagnosis of heart rhythm or conduction abnormalities with deep learning: A cohort study. Lancet Digit. Health 2020, 2, e348–e357. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, Z.; Wang, S.; Lu, G.; Xv, G.; Liu, Q.; Zhu, X. Atrial fibrillation detection based on multi-feature extraction and convolutional neural network for processing ECG signals. Comput. Methods Programs Biomed. 2021, 202, 106009. [Google Scholar] [CrossRef]
- Younghoon, C.; Kwon, J.; Kim, K.; Medina-Inojosa, J.R.; Jeon, K.; Cho, S.; Lee, S.Y.; Park, J.; Oh, B.-H. Artificial intelligence algorithm for detecting myocardial infarction using six-lead electrocardiography. Sci. Rep. 2020, 10, 20495. [Google Scholar]
- Jahmunah, V.; Ng, E.; San, T.R.; Acharya, U.R. Automated detection of coronary artery disease, myocardial infarction and congestive heart failure using GaborCNN model with ECG signals. Comput. Biol. Med. 2021, 134, 104457. [Google Scholar] [PubMed]
- Shu, L.O.; Jahmunah, V.; San, T.R.; Ciaccio, E.J.; Yamakawa, T.; Tanabe, M.; Kobayashi, M.; Faust, O.; Acharya, U.R. Comprehensive electrocardiographic diagnosis based on deep learning. Artif. Intell. Med. 2020, 103, 101789. [Google Scholar]
- Caiyun, M.; Wei, S.; Chen, T.; Zhong, J.; Liu, Z.; Liu, C. Integration of results from convolutional neural network in a support vector machine for the detection of atrial fibrillation. IEEE Trans. Instrum. Meas. 2020, 70, 1–10. [Google Scholar]
- Sajad, M.; Fotoohinasab, A.; Afghah, F. Single-modal and multi-modal false arrhythmia alarm reduction using attention-based convolutional and recurrent neural networks. PLoS ONE 2020, 15, e0226990. [Google Scholar]
- Zhao, Z.; Liu, C.; Li, Y.; Li, Y.; Wang, J.; Lin, B.; Li, J. Noise rejection for wearable ECGs using modified frequency slice wavelet transform and convolutional neural networks. IEEE Access 2019, 7, 34060–34067. [Google Scholar] [CrossRef]
- Saritha, C.; Sukanya, V.; Murthy, Y.N. Ecg signal analysis using wavelet transforms. Bulg. J. Phys. 2008, 35, 155–163. [Google Scholar]
- Tinati, A.M.; Mozaffary, B. A wavelet packets approach to electrocardiograph baseline drift cancellation. Int. J. Biomed. Imaging 2006, 2006, 97157. [Google Scholar] [CrossRef]
- Wang, R.; Fan, J.; Li, Y. Deep multi-scale fusion neural network for multi-class Arrhythmia detection. IEEE J. Biomed. Health Inform. 2020, 24, 2461–2472. [Google Scholar] [CrossRef]
- Watson, H.A.; Tribe, R.M.; Shennan, A.H. The role of medical smartphone apps in clinical decision-support: A literature review. Artif. Intell. Med. 2019, 100, 101707. [Google Scholar] [CrossRef]
- Foster, K.R.; Torous, J. The opportunity and obstacles for smartwatches and wearable sensors. IEEE Pulse 2019, 10, 22–25. [Google Scholar] [CrossRef]
- Sajeev, J.K.; Koshy, A.N.; Teh, A.W. Wearable devices for cardiac arrhythmia detection: A new contender? Intern. Med. J. 2019, 49, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.T.; Treskes, R.W.; Caiani, E.G.; Casado-Arroyo, R.; Cowie, M.R.; Dilaveris, P.; Duncker, D.; Di Rienzo, M.; Frederix, I.; De Groot, N.; et al. ESC working group on e-cardiology position paper: Use of commercially available wearable technology for heart rate and activity tracking in primary and secondary cardiovascular prevention—In collaboration with the European Heart Rhythm Association, European Association of Preventive Cardiology, Association of Cardiovascular Nursing and Allied Professionals, Patient Forum, and the Digital Health Committee. Eur. Heart J. Digit. Health 2021, 2, 49–59. [Google Scholar]
- Oliver, F.; Ciaccio, E.J.; Acharya, U.R. A review of atrial fibrillation detection methods as a service. Int. J. Environ. Res. Public Health 2020, 17, 3093. [Google Scholar]
- Witt, D.R.; Kellogg, R.A.; Snyder, M.P.; Dunn, J. Windows into human health through wearables data analytics. Curr. Opin. Biomed. Eng. 2019, 9, 28–46. [Google Scholar] [CrossRef]
- Giorgio, Q.; Arnaout, R.; Henne, M.; Arnaout, R. Machine learning and the future of cardiovascular care: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 77, 300–313. [Google Scholar]
- David, T.; Patterson, C. From machine learning to artificial intelligence applications in cardiac care: Real-world examples in improving imaging and patient access. Circulation 2018, 138, 2569–2575. [Google Scholar]
- Friedrich, S.; Groß, S.; König, I.R.; Engelhardt, S.; Bahls, M.; Heinz, J.; Huber, C.; Kaderali, L.; Kelm, M.; Leha, A.; et al. Applications of artificial intelligence/machine learning approaches in cardiovascular medicine: A systematic review with recommendations. Eur. Heart J. Digit. Health 2021, 2, 424–436. [Google Scholar] [CrossRef]
- Chayakrit, K.; Johnson, K.W.; Rosenson, R.S.; Wang, Z.; Aydar, M.; Baber, U.; Min, J.K.; Tang, W.H.W.; Halperin, J.L.; Narayan, S.M. Deep learning for cardiovascular medicine: A practical primer. Eur. Heart J. 2019, 40, 2058–2073. [Google Scholar]
- Yan, Y.; Zhang, J.; Zang, G.; Pu, J. The primary use of artificial intelligence in cardiovascular diseases: What kind of potential role does artificial intelligence play in future medicine? J. Geriatr. Cardiol. 2019, 16, 585. [Google Scholar]
- Wongvibulsin, S.; Martin, S.S.; Steinhubl, S.R.; Muse, E.D. Connected health technology for cardiovascular disease prevention and management. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.D.; Lee, S.; Robertus, J.; Nienaber, C.A.; Trayanova, N.A.; Ernst, S. Artificial intelligence in the diagnosis and management of arrhythmias. Eur. Heart J. 2021, 42, 3904–3916. [Google Scholar] [CrossRef] [PubMed]
- Manjurul, A.M.; Siddique, Z. Machine learning-based heart disease diagnosis: A systematic literature review. Artif. Intell. Med. 2022, 128, 102289. [Google Scholar]
- Attia, I.Z.; Harmon, D.M.; Behr, E.R.; Friedman, P.A. Application of artificial intelligence to the electrocardiogram. Eur. Heart J. 2021, 42, 4717–4730. [Google Scholar] [CrossRef]
- Hinai, G.A.; Jammoul, S.; Vajihi, Z.; Afilalo, J. Deep learning analysis of resting electrocardiograms for the detection of myocardial dysfunction, hypertrophy, and ischaemia: A systematic review. Eur. Heart J. Digit. Health 2021, 2, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Krichen, M. Anomalies detection through smartphone sensors: A review. IEEE Sens. J. 2021, 21, 7207–7217. [Google Scholar] [CrossRef]
- Aizatul, F.; Mohd, S.; Thevarajah, T.M.; Khor, S.M.; Chang, S. A review of risk prediction models in cardiovascular disease: Conventional approach vs. artificial intelligent approach. Comput. Methods Programs Biomed. 2021, 207, 106190. [Google Scholar]
- Guo, Y. A New Paradigm of Real-Time Stroke Risk Prediction and Integrated Care Management in the Digital Health Era: Innovations Using Machine Learning and Artificial Intelligence Approaches. Thromb. Haemost. 2022, 122, 5–7. [Google Scholar] [CrossRef]
- Graves, A.; Fernandez, F.; Liwicki, M.; Bunke, H.; Schmidhuber, J. Unconstrained Online Handwriting Recognition with Recurrent Neural Networks. Adv. Neural Inf. Process. Syst. 2007, p. 20. Available online: https://proceedings.neurips.cc/paper/2007/file/4b0250793549726d5c1ea3906726ebfe-Paper.pdf (accessed on 1 September 2022).
- Malhotra, P.; Vig, L.; Shroff, G.; Agarwal, P. Long short-term memory networks for anomaly detection in time series. In Proceedings of the 23rd European Symposium on Artificial Neural Networks, Computational Intelligence and Machine Learning, Bruges, Belgium, 22–24 April 2015; pp. 89–94. [Google Scholar]
- Lin, C.C.; Yang, C.M. Heartbeat classification using normalized RR intervals and morphological features. Math. Probl. Eng. 2014, 2014, 712474. [Google Scholar]
- Osowski, S.; Linh, T.H. ECG Beat recognition using fuzzy hybrid neural network. IEEE Trans. Biomed. Eng. 2001, 48, 1265–1271. [Google Scholar]
- Mar, T.; Zaunseder, S.; Martínez, J.P.; Llamedo, M.; Poll, R. Optimization of ECG classification by means of feature selection. IEEE Trans. Biomed. Eng. 2011, 58, 2168–2177. [Google Scholar]
- Tutuko, B.; Rachmatullah, M.N.; Darmawahyuni, A.; Nurmaini, S.; Tondas, A.E.; Passarella, R.; Partan, R.U.; Rifai, A.; Sapitri, A.I.; Firdaus, F. Short Single-Lead ECG Signal Delineation-Based Deep Learning: Implementation in Automatic Atrial Fibrillation Identification. Sensors 2022, 22, 2329. [Google Scholar] [CrossRef]
- Guo, A.; Pasque, M.; Loh, F.; Mann, D.L.; Payne, P.R.O. Heart failure diagnosis, readmission, and mortality prediction using machine learning and artificial intelligence models. Curr. Epidemiol. Rep. 2020, 7, 212–219. [Google Scholar] [CrossRef]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation 2019, 139, 5. [Google Scholar] [CrossRef]
- Dagher, L.; Shi, H.; Zhao, Y.; Marrouche, N.F. Wearables in cardiology: Here to stay. Heart Rhythm 2020, 17, 889–895. [Google Scholar] [CrossRef]
- Jahfari, A.N.; Tax, D.; Reinders, M.; van der Bilt, I. Machine Learning for Cardiovascular Outcomes from Wearable Data: Systematic Review from a Technology Readiness Level Point of View. JMIR Med. Inform. 2022, 10, e29434. [Google Scholar] [CrossRef]
- Amanda, C.; Cadaret, L.M.; Liu, K. Machine learning in electrocardiography and echocardiography: Technological advances in clinical cardiology. Curr. Cardiol. Rep. 2020, 22, 161. [Google Scholar]
- Brijesh, P.; Sengupta, P. Machine learning for predicting cardiac events: What does the future hold? Expert Rev. Cardiovasc. Ther. 2020, 18, 77–84. [Google Scholar]

| Author(s)/Database | Types of Diseases/Data | CML-Algorithms | Application | Evaluation |
|---|---|---|---|---|
| Shao et al. (2018) [20]/ 2017 PhysioNet CinC Challenge (CinC: Computing in Cardiology) | AF/ECG | DT, AdaBoosted DT ensemble | Classification (4 classes) | F1-score: 0.82 |
| Fallet et al. (2019) [21]/ 17 patients (catheter ablation of cardiac arrhythmia) | AF and Ventricular arrhythmia/PPG, ECG, ACC-signals (ACC signals: three-axis accelerometer signals) | DT | Classification (2 classes) | ACC: 95.0% SPE: 92.8% SEN: 96.2% |
| Ghiasi et al. (2020) [22]/ Z-Alizadeh Sani CAD dataset: 303 patients | CAD/Databank: 55 independent parameters | DT-based CART (classification and regression tree) | Classification (2 classes) | ACC: 92.41%, TNR: 77.01%, TPR: 98.61% |
| Tozlu et al. (2021) [23]/ 33 MI patients, 22 CAD patients, 26 normal. | MI and CAD/ Electronic noses (19 gas sensors) | SVM | Classification (2 classes) | ACC: MI: 97.19%, CAD: 81.48% |
| Qureshi et al. (2020) [24]/ ~250 patients, Extracted CVD dataset | CVD/Physiological signals and clinical data | SVM and DT | Classification (2 categories) | ACC: 86.72%, SEN: 67.0%, SPE: 89.0% |
| Mei et al. (2018) [25]/ CinC 2017, (MIT-BIH AF) database | AF/ECG | SVM and Bagging trees | Classification (2 classes, 3 classes) | ACC: 92.0%-96.6% (Varies noise levels), 82.0% (3 classes) |
| Iftikhar et al. (2018) [26]/ 23 healthy people, 40 AF, 21 CAD, 21 MI patients | AF/SCG and GCG (seismo- and gyro- cardiogram-signals) | RF and SVM | Multiclass model (SR, AF, CAD, STEMI) | ACC: 75.24% F1: 74% (RF) |
| Sengupta et al. (2018) [27]/ 188 subjects | Abnormal Myocardial Relaxation (AMR)/spECG (spECG: Signal Processed Surface ECG) | RF/Monte Carlo cross-validation | Prediction | AUC: 91%, SEN: 80%, SPE: 84% |
| Sopic et al. (2018) [28]/ Physionet (PTB Diagnostic ECG database) | MI/ECG | RF | Classification and prediction | ACC: 83.26%, SEN: 87.95%, SPE: 78.82% |
| Meng et al. (2019) [29]/ Activity tracker data | SIHD/Tracker data | HMM | Output health status over time | AUC: 0.79 |
| Akbulut & Akan (2018) [30]/ 30 participants | CVD/ECG | Decision Forest (DF), Logistic Regression (LR), NNs | Risk assessment | ACC: 96.0% |
| Dunn et al. (2021) [31]/ 54 integrative personal omics profiling (iPOP) participants | CVD/PPG, wVS HR, Electrodermal activity (EDA), physical activities | RF and Lasso models, canonical correlation analysis (CCA) | Prediction | wVS (wearable vital sigh) models outperform cVS (clinical vital sigh) models |
| Han et al. (2019) [32]/ 9530 controls, 306 cases | AF/AF burden signatures | Convolutional NN (CNN), RF and L1 regularized LR (LASSO) | Prediction of short-term stroke in 30-day window | AUC: RF: 0.662, Ensemble: 0.634 |
| Hill et al. (2019) [33]/ CPRD (CPRD: UK Clinical Practice Research Datalink) 2,994,837 individuals (3.2% AF) | AF/ECG | Statistical/Models (NNs, LASSO, RF, SVM and Cox Regression) | Prediction | AUROC: 0.827 SEN: 75% |
| Jabeen et al. (2019) [34]/ UCI repository, 100 cardiac patients | CVD/Medical records | SVM, Naïve Bayes (NB), RF, Multilayer Perceptron (MLP) | Classification (8 classes) | ACC: 98% for Community-based heuristic approach |
| Kantoch E. (2018) [35]/ 5 participants, SPPB (SPPB: Short Physical Performance Battery task) test task | Sedentary Behavior (CVD risk)/Ambulatory and Daily activities | Linear Discriminant Analysis (LDA), DT, KNN, SVM, NB, Artificial NNs (ANNs) | Classification (6 activities) | ACC: 95.00% ± 2.11% |
| Kwan et al. (2021) [36]/ 50 participants | AF/PPG | XGBoost, RF, SVM and Gradient Boosting DT | Prediction | AF predicted 4 h in advance |
| Li, B. et al. (2019) [37]/ Hypertension patients, 3 datasets (stroke, HF, renal failure) | CVD/Medical records | Spark MLlib library (LR, SVM, NB) | A risk early warning model | LR(HF): AUC: 0.9269, ACC:0.8529, F1: 0.8456 |
| Yang et al. (2018) [38]/ MIT-BIH arrhythmia Database | Arrhythmia/ECG | PCANet andand L-SVM, Back Propagation (BP)-NN, KNN | Identification (5 types) | ACC: 97.77% (skewed) 97.08% (noised) |
| Yang et al. (2020) [39]/ 20 AS patients, 20 health persons | AS/SCG and GCG | DT, RF and ANNs | Classification (2-classes, multi-classes) | ACC: (2/multi-classes): RF 97.43%/92.99% |
| Yang and Wei, (2020) [40]/ MIT-BIH AF database | Cardiac Arrhythmias/ECG | KNN, SVM and NNs | Classification (6 main types) | Best ACC: 97.70% (KNN) |
| Bumgarner et al. (2018) [41]/ 100 patients | AF/ECG | Kardia Band (KB) algorithm supported by Physician | Classification (2 classes) | SEN: 99%, SPE: 83%, K coefficient: 0.83 |
| Dörr et al. (2019) [42]/ 672 participants | AF/PPG, iECG | Heartbeats PPG algorithm | Classification (2 classes) | ACC: 96.1%, SEN: 93.7%, SPE: 98.2% |
| Fan et al. (2019) [43]/ 112 participants | AF/Waveform recording from PPG | PRO AF PPG algorithm | Classification (2 classes) | Smart bands: ACC: 97.72%, SEN: 95.36%, SPE: 99.70% |
| Green et al. (2019) [44]/ 19 patients and 64 healthy volunteers | oHCM (with left ventricular outflow tract obstruction)/PPG | Multiple-instance ML model | Classification (2 classes) | SEN: 95%, SPE: 98%, C-statistic: 0.99 |
| Guo et al. (2019) [45]/ 187,912 used smart devices | AF/PPG | Discrimination rule PPG algorithm | Prediction | Positive predictive value: 91.6% (95% CI: 91.5% to91.8%) |
| Karwath et al. (2021) [46]/ 18,637 patients (LVEF < 50) | HFrEF/ECG | Hierarchical clustering | Statistical analysis | Mean Jaccard score: 0·571 (SD 0·073; p < 0·0001) |
| Khan and Algarni, (2020) [47]/ UCI dataset https://www.kaggle.com/datasets, accessed on 15 April 2020. | Heart disease/LoMT (LoMT: Internet of Medical Things) Sensor data and medical records | MSSO-ANFIS | Prediction | ACC: 99.45%, PRE: 96.54% |
| Zeng et al. (2020) [48]/ PTB database:290 subjects, in which 148 patients with MI and 52 controls | MI/ECG | TQWT-VMD- Radial Basis Function (RBF) | Classification (2 classes) | ACC:97.98% |
| Perez et al. (2019) [49]/ 419,297 participants | AF/PPG, ECG patch | Irregular pulse notification algorithm | Identification | Positive predictive value: 84% (95% CI, 76 to 92) |
| Shao et al. (2020) [50]/ AFDB-2017, MIT-BIH AF (MITBIH-AFDB) | AF/ECG patch | CatBoost-based method | Classification (4 classes) | F1: 0.92 |
| Spaccarotella et al. (2020) [51]/ 100 participants, 54 STEMI, 27 non-STEMI, 19 normal | Acute coronary syndromes/ECG | Cohen κ coefficient and Bland–Altman analysis | Earlier diagnosis | For STEMI: SEN: 93%, SPE: 95% |
| Stehlik et al. (2020) [52]/ 100 subjects | HF/PPG | Similarity-based | Prediction | SEN: 88%, SPE: 85% |
| Steinhubl et al. (2018) [53]/ 2659 participants | AF/ECG | Statistical analysis | Assessment | 3.0% difference (immediate vs. delayed monitoring) |
| Samuel et al. (2020) [54]/ UCI repository Cleveland HF disease dataset: 303 patients | HF/Medical records | HNCL (HNCL: Hierarchical Neighborhood Component-based-Learning)/adaptive multi-layer networks (AMLN) | Prediction | ACC: 97.8%, SEN: 95.45%, SPE: 100% |
| Author(s)/Database | Types of Diseases/Data | DL-Algorithms | Application | Evaluation |
|---|---|---|---|---|
| Mohammad et al. (2022) [55]/ 139,288 patients | MI/Medical records | ANN | Prediction 1-year-all-cause-mortality after MI | AUC: 0.87, ACC: 77.1%, SPE: 76.3%, SEN: 84.6% |
| Kwon et al. (2021) [56]/ 32,671 ECGs of 20,169 patients | HFpEF/12-lead ECG | Ensemble NN | Detect HFpEF | AUC: [0.866 0.869] |
| Chocron et al. (2020) [57]/ 2891 patients, PhysioNet LTAF test database. | AF/ECG | ArNet (a deep RNNs) | Estimation of the AF burden (AFB) | Estimation error: EAF: 1.2% (0.1–6.7)% |
| Feng et al. (2019) [58]/ Physikalisch–Technische Bundesanstalt (PTB) database | MI/I-lead ECG | CNNs/RNN | Classification (2 classes) | ACC: 95.4%, SEN: 98.2%, SPE: 86.5%, |
| Saadatnejad et al. (2019) [59]/ MIT-BIH arrhythmia database | AF/ECG | Wavelet transform (WT)/ LSTM-RNNs | Classification (7 types) | ACC: 99.2%, SEN: 93.0%, SPE: 99.8% |
| Chang et al. (2021) [60]/ 35,981 patients | 12 heart rhythms and STEMI/ECG | Deep BiLSTM | STEMI detection | ACC: 98.7%, AUC: 0.997, F1: 0.909 |
| Dami and Yahaghizadeh (2021) [61]/ Four publicly available datasets | (Super)-ventricular ectopic beats/ECG | Deep Belief Network (DBN)/LSTM | Prediction in advance of a few weeks or months | ACC: DB1:88.74%, DB2: 93.24%, DB3-4: 80.41% |
| Faust et al. (2018) [62]/ MIT-BIH AF database | AF/ECG, HR signals | Deep LSTMs | Classification (2 classes) | ACC: 98.51%, SEN: 98.32%, SPE: 98.67% |
| Tadesse et al. (2021) [63]/ 17,000 patients Evaluation: PTB | MI/ECG | End-to-end deep learning (Dense-LSTM) | Classification (4 classes) | AUC: 94.0% |
| Lui et al. (2018) [64]/ Physionet PTB dataset, AF-Challenge 2017 | MI/I-lead ECG | CNN/LSTM stacking | Classification (MI, healthy, other CVD, noisy) | SEN: 92.4%, SPE: 97.7%, PPV: 97.2% |
| Tan et al. (2018) [65]/ PhysioNet, 7 CAD and 40 normal subjects | CAD/ECG | CNN/LSTM | Classification (2 classes) | ACC: 99.85% PRE: 0.9985 F1: 0.9952 |
| Amirshahi and Hashemi (2019) [66]/ MIT-BIH arrhythmia database | Arrhythmia Patterns/ECG | Deep SNNs | Classification (4 types) | ACC: 97.9%, SEN: 80.2%, SPE: 99.8% |
| Yan et al. (2021) [67]/ MIT-BIH arrhythmia database | Arrhythmia Patterns/ECG | CNNS/SNNs | Classification (2 to 4 classes) | ACC: 90.0% |
| Attia et al. (2019) [68]/ 44,959 subjects, tested on 52,870 patients | ALVD/Paired 12-lead ECG and transthoracic echocardiogram (TTE) | AI-ECG algorithm (CNNs-based) | Prediction (EF ≤ 35%) | AUC: 0.93, SEN: 86.3%, SPE: 85.7%, ACC: 85.7% |
| Attia et al. (2019) [69]/ 16,056 patients | LVSD/12-lead ECG | Deep-CNNs | Prediction (EF ≤ 35%) | AUC: 0.918, SEN: 82.5%, SPE: 86.8%, ACC: 86.5% |
| Attia et al. (2021) [70]/ 4277 subjects | LVSD/12-lead ECG | AI-ECG algorithm (CNNs-based) | Validation in an external population Prediction (EF ≤ 35%) | AUC: 0.82, SEN: 96.9%, SPE: 97.4%, ACC:97.0% |
| Bachtiger et al. (2022) [71]/ 1050 patients | HF (LVEF)/ 1-lead ECG | AI-ECG algorithm (CNNs-based) | Prediction | AUC:0.91 SEN: 91.9% SPE: 80.2% |
| Betancur et al. (2018) [72]/ 1638 patients | Obstructive CAD/MPI | DNN | Prediction | AUC: 0.80/0.76 SPE: 82.3/69.8 per patient/vessel |
| Cai and Hu (2020) [73]/ Four open-access ECG databases | AF/ECG | SENet, CRNN | Predict QRS locations | ACC: 99.0% F1: 99.0% |
| Cho et al. (2021) [74]/ Internal validation (IV): 2908 patients. External (EV): 4176 patients | HFrEF (EF < 40%)/12-lead ECG | CNNs | Detection | AUC: (IV/EV) 0.913/0.961 ACC: 77.5%/91.1% |
| Christopoulos et al. (2020) [75]/ 1936 participants | AF/ECG | CNNs/Statistical analysis | prediction | C statistics: 0.69 (95% CI, 0.66–0.72) |
| Han et al. (2021) [76]/ 97,742 patients | MI/ECG | Residual networks | Detection cardiac disorders | AUC: 12-lead: 0.880 1-lead: 0.768 |
| Hannun et al. (2019) [77]/ 91,232 single-lead ECGs from 53,549 patients | Arrhythmias/ECG | End-to-end DNNs | Classification rhythm diagnoses | ROC: 0.97, F1:0.837 > 0.780 (Cardiologists) |
| Jo et al. (2021) [78]/12,955 patients with normal sinus rhythm | PSVT/ECG | Deep residual NNs | Identify patients with PSVT | ACC: 97%, SEN: 86.8%, SPE: 97.2% |
| Kiyasseh et al. (2021) [79]/ Four publicly available datasets | Cardiac Arrhythmias/ 1-lead ECG | CLOPS (Deep CNNs) | Diagnose in various continual learning (CL) scenarios | AUC: 0.796 (SD 0.013) |
| Ko et al. (2020) [80]/Train/Test HCM: 2,448/612 Control: 51,153/12,788 | HCM/ 12-lead ECG | Deep CNNs | Classification (2 classes: HCM and control) | AUC: 0.96 SEN: 87% SPE: 90% |
| Kwon et al. (2020) [81]/ 38,393 patients | MR/ECG | Deep CNNs | Prediction | AUC: 0.816 (Internal) 0.877 (External) |
| Lai et al. (2020) [82]/ 55 consecutive AF patients | AF/ECG | CNNs | Classification (2 classes) | ACC: 93.1%, SEN: 93.1%, SPE: 93.4% |
| Li, G.Y. et al. (2019) [83]/412 Subjects Data1: medical records Data2: physiological parameters | CVD/ Medical records, disease’s metrics | Deep CNNs | Pulse-wave Classification (5 diseases) | ACC: Data1: 95.0% Data2: 88.0% |
| Panganiban et al. (2021) [84]/ 4 datasets from PhysioNet | Arrhythmia/ECG | Spectrograms Image 2D-CNNs | Classification (2, 5 classes) | ACC: 98.73% binary & 97.33% for quinary |
| Ribeiro et al. (2020) [85]/ 2,322,513 ECG records from 1,676,384 patients | AF/ECG | DNN Residual network | Classification (6-abnormality) | SPE: 1.000, SEN: 0.769, F1: 0.870 |
| Tison et al. (2018) [86]/ 9750 (347) participants (with AF) | AF/HR (PPG) | DNN | Classification (2 classes) | C statistic: 0.97 (95% CI, 0.94–1.00; p < 0.001) |
| Torres-Soto and Ashley (2020) [87]/ Tr:(Synapse ID: syn21985690), Ambulatory dataset | AF/PPG | DeepBeat (transfer learning with autoencoder) | Classification (2 classes: AF and Sinus Rhythm) | SEN: 98.0%, SPE:99.0% F1 score: 0.93 |
| Wasserlauf et al. (2019) [88]/ 7500 AliveCor users, 26 patients for validation | AF/ECG, HR, activity | DCNN | Classification (2 classes: AF and Sinus Rhythm) | SEN: 74.8%, SPE: 90.0% |
| Yao et al. (2020) [89]/ ~400 clinicians and 20,000 patients | Low ejection fraction (EF)/12-lead ECG from EHR | DL | Statistical analysis | To prospectively evaluate a novel AI screening tool |
| Zhao, Y. et al. (2020) [90]/ 667 STEMI ECGs, 7571 control ECGs | STEMI/ECG | Deep AI CNNs | Classification (2 classes) | AUC: 0.9954, SEN: 96.75%, SPE: 99.20%, ACC: 99.01% |
| Zhu et al. (2020) [91]/ 70,692 patients (aged ≥18 years) | AF/ECG | Deep CNN | Real-time analysis | F1 score: 0·887 AUC: 0·983, SEN: 0·867, SPE: 0·995 |
| Chen et al. (2021) [92]/ MIT-BIH database | AF/ECG | multi-feature extraction/ CNNs | Classification (2 classes) | ACC: 98.92%, SPE: 97.04%, SEN: 97.19% |
| Cho et al. (2020) [93]/ 9536, 1301, 1768 ECGs of adult patients | MI/ECG (6, 12-lead) | DL/variational autoencoder (VAE) | Detection | AUROC: 0.880 (internal) 0.854 (external) |
| Jahmunah et al. (2021) [94]/ 92 healthy controls, 7 CAD, 148 MI and 15 CHF patients | CAD, MI, C-HF/ECG | CNN and GaborCNN | Classification (4 classes) | ACC: 98.5% |
| Lih et al. (2020) [95]/ 92 normal, 7 CAD, 148 MI, and 15 C-HF patients | CAD, MI, C-HF/ECG | Deep CNNs/LSTMs | Classification (4 classes) | ACC: 98.5% |
| Ma et al. (2020) [96]/ MIT-BIH AF (train), PhysioNet/CinC 2017, CPSC 2018 databases | AF/ECG | CNNs/SVM | Classification (2 classes) | (ACC for 30s ECG episodes) 98.48%/99.21% |
| Mousavi et al. (2020) [97]/ PhysioNet/CinC 2015 | Arrhythmia/ABP, PPG, ECG | DL (CNNs+RNNs) | Suppress the false alarm, classification | SEN: 93.88%, SPE: 92.05% |
| Zhao, Z. et al. (2019) [98]/ Collected 1000 10s ECG segments | CVDs/ Smart ECG vest | MFSWT (MFSWT: Modified frequency slice wavelet transforms)/Deep-CNNs | Identify the noisy ECG segments (3 classes) | ACC: 86.3%, Kappa coefficient: [0.61 0.80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-D.; Wang, J.; Ramsey, E.; Leavey, G.; Chico, T.J.A.; Condell, J. Applying Artificial Intelligence to Wearable Sensor Data to Diagnose and Predict Cardiovascular Disease: A Review. Sensors 2022, 22, 8002. https://doi.org/10.3390/s22208002
Huang J-D, Wang J, Ramsey E, Leavey G, Chico TJA, Condell J. Applying Artificial Intelligence to Wearable Sensor Data to Diagnose and Predict Cardiovascular Disease: A Review. Sensors. 2022; 22(20):8002. https://doi.org/10.3390/s22208002
Chicago/Turabian StyleHuang, Jian-Dong, Jinling Wang, Elaine Ramsey, Gerard Leavey, Timothy J. A. Chico, and Joan Condell. 2022. "Applying Artificial Intelligence to Wearable Sensor Data to Diagnose and Predict Cardiovascular Disease: A Review" Sensors 22, no. 20: 8002. https://doi.org/10.3390/s22208002
APA StyleHuang, J.-D., Wang, J., Ramsey, E., Leavey, G., Chico, T. J. A., & Condell, J. (2022). Applying Artificial Intelligence to Wearable Sensor Data to Diagnose and Predict Cardiovascular Disease: A Review. Sensors, 22(20), 8002. https://doi.org/10.3390/s22208002







