Hyperspectral Image Data and Waveband Indexing Methods to Estimate Nutrient Concentration on Lettuce (Lactuca sativa L.) Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Influence of N Concentration on Lettuce Growth Dynamics
2.2. HSI Capture
2.3. HSI Indices
2.4. Feature Extraction Models
2.5. PLS-VIP Method
2.6. Waveband Selection Methods
3. Results and Discussion
3.1. Hydroponic System
3.2. NFT System
3.3. HSI Capture
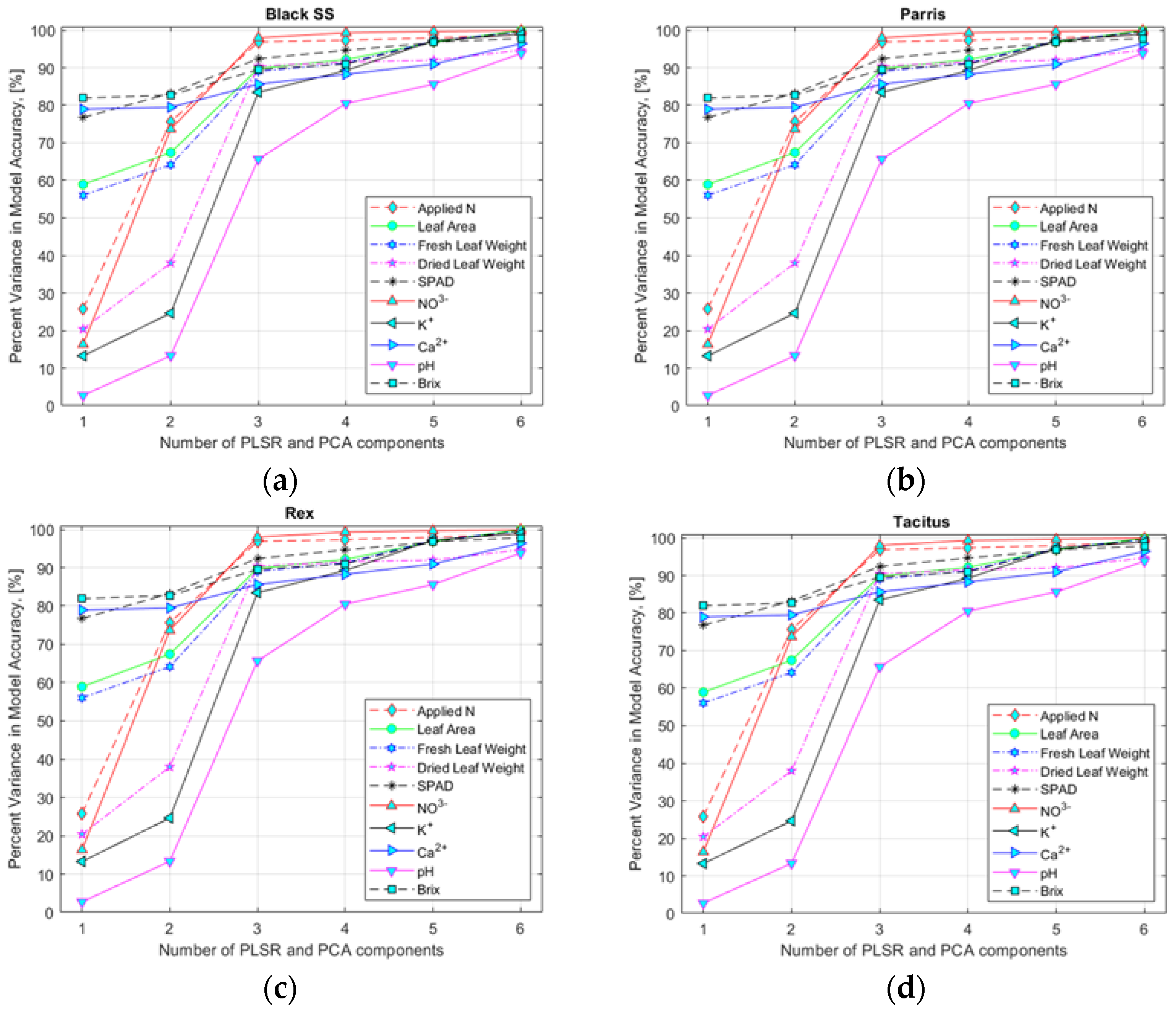
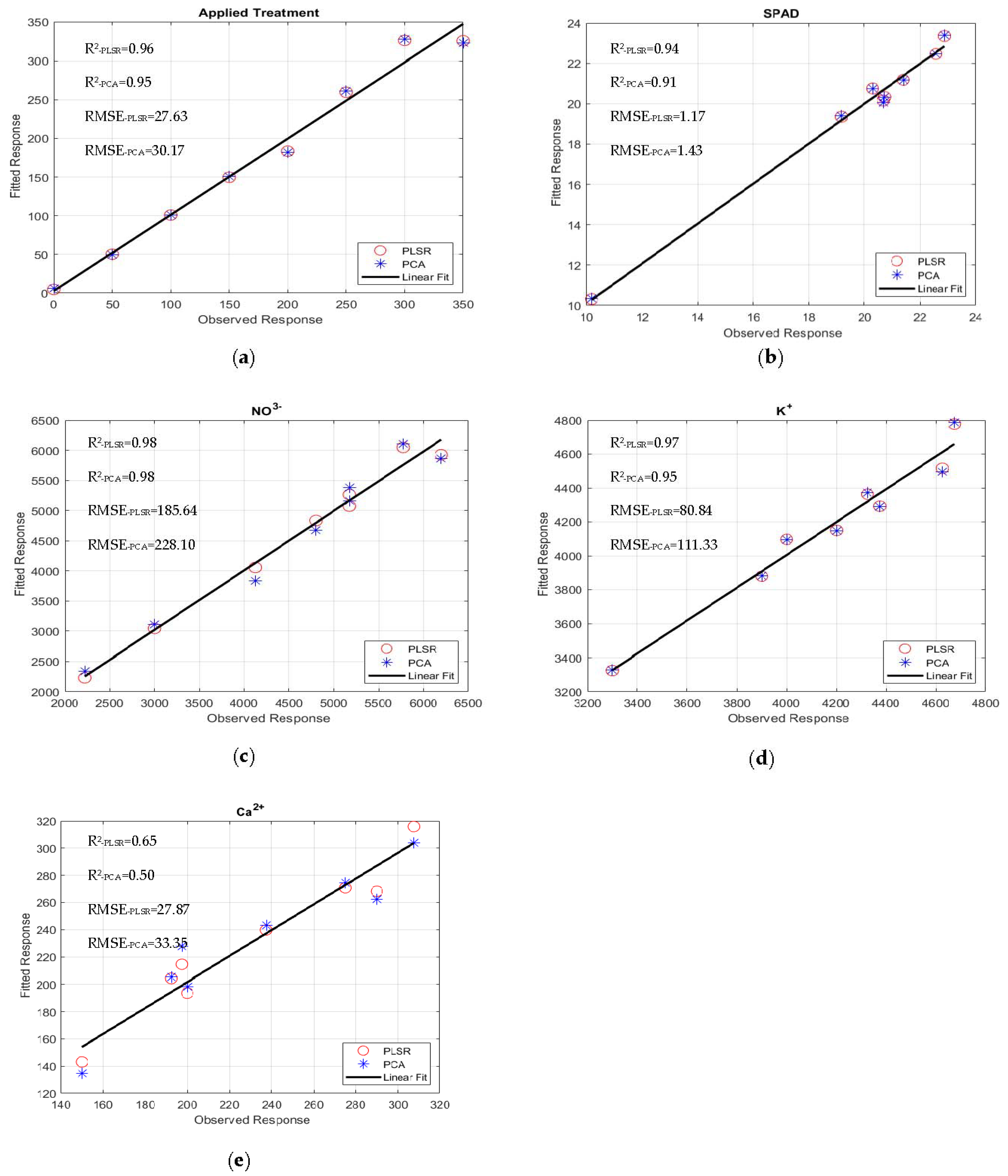
3.4. Comparison of Performance of FDR, PLSR/PCA, and VIP-Score Approach for Estimating Nutrient Content in Lettuce Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kimura, M.; Rodriguez-Amaya, D.B. Carotenoid composition of hydroponic leafy vegetables. J. Agric. Food Chem. 2003, 51, 2603–2607. [Google Scholar] [CrossRef] [PubMed]
- Pilbeam, D.J.; Barker, A.V. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Reddy, V. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Gianquinto, G.; Orsini, F.; Sambo, P.; D’Urzo, M.P. The use of diagnostic optical tools to assess nitrogen status and to guide the fertilization of vegetables. HortTechnology 2011, 21, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Odabas, M.S.; Simsek, H.; Lee, C.W.; İseri, İ. Multilayer perceptron neural network approach to estimate chlorophyll concentration index of lettuce (Lactuca sativa L.). Commun. Soil Sci. Plant Anal. 2017, 48, 162–169. [Google Scholar] [CrossRef]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of nutrient deficiency and abiotic environmental stresses on yield, phenolic compounds and antiradical activity in lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- Mahlangu, R.; Maboko, M.; Sivakumar, D.; Soundy, P.; Jifon, J. Lettuce (Lactuca sativa L.) growth, yield and quality response to nitrogen fertilization in a non-circulating hydroponic system. J. Plant Nutr. 2016, 39, 1766–1775. [Google Scholar] [CrossRef]
- Boussadia, O.; Steppe, K.; Zgallai, H.; El Hadj, S.B.; Braham, M.; Lemeur, R.; Van Labeke, M.-C. Effects of nitrogen deficiency on leaf photosynthesis, carbohydrate status, and biomass production in two olive cultivars’ Meski’and ‘Koroneiki’. Sci. Hortic. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Kamnev, A.; Sadovnikova, Y.N.; Antonyuk, L. Effects of nitrogen deficiency and wheat lectin on the composition and structure of some biopolymers of Azospirillum brasilense Sp245. Microbiology 2008, 77, 240–242. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Fei, P.; Song, J.; Li, D.; Ge, C.; Chen, W. Responses of rice leaf thickness, SPAD readings and chlorophyll a/b ratios to different nitrogen supply rates in paddy field. Field Crops Res. 2009, 114, 426–432. [Google Scholar]
- Shi, J.-Y.; Zou, X.-B.; Zhao, J.-W.; Wang, K.-L.; Chen, Z.-W.; Huang, X.-W.; Zhang, D.-T.; Mel, H. Non-destructive diagnostics of nitrogen deficiency by cucumber leaf chlorophyll distribution map based on near infrared hyperspectral imaging. Sci. Hortic. 2012, 138, 190–197. [Google Scholar]
- Yu, K.-Q.; Zhao, Y.-R.; Li, X.-L.; Shao, Y.-N.; Liu, F.; He, Y. Hyperspectral imaging for mapping of total nitrogen spatial distribution in pepper plant. PLoS ONE 2014, 9, e116205. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci. Rep. 2015, 5, 13389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsan, M.; Eshkabilov, S.; Cemek, B.; Küçüktopcu, E.; Lee, C.W.; Simsek, H. Deep Learning Models to Determine Nutrient Concentration in Hydroponically Grown Lettuce Cultivars (Lactuca sativa L.). Sustainability 2021, 14, 416. [Google Scholar] [CrossRef]
- Eshkabilov, S.; Lee, A.; Sun, X.; Lee, C.W.; Simsek, H. Hyperspectral imaging techniques for rapid detection of nutrient content of hydroponically grown lettuce cultivars. Comput. Electron. Agric. 2021, 181, 105968. [Google Scholar] [CrossRef]
- Sun, J.; Yang, W.; Zhang, M.; Feng, M.; Xiao, L.; Ding, G. Estimation of water content in corn leaves using hyperspectral data based on fractional order Savitzky-Golay derivation coupled with wavelength selection. Comput. Electron. Agric. 2021, 182, 105989. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Jin, G.; Li, L.-Q.; Liu, Y.; Kalkhajeh, Y.K.; Ning, J.-M.; Zhang, Z.-Z. NIR hyperspectral imaging coupled with chemometrics for non-destructive assessment of phosphorus and potassium contents in tea leaves. Infrared Phys. Technol. 2020, 108, 103365. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, T.H.; Jin, G.; Wei, Y.M.; Li, L.Q.; Kalkhajeh, Y.K.; Ning, J.M.; Zhang, Z.Z. Qualitative and quantitative diagnosis of nitrogen nutrition of tea plants under field condition using hyperspectral imaging coupled with chemometrics. J. Sci. Food Agric. 2020, 100, 161–167. [Google Scholar] [CrossRef]
- Sabzi, S.; Pourdarbani, R.; Rohban, M.H.; García-Mateos, G.; Arribas, J.I. Estimation of nitrogen content in cucumber plant (Cucumis sativus L.) leaves using hyperspectral imaging data with neural network and partial least squares regressions. Chemom. Intell. Lab. Syst. 2021, 217, 104404. [Google Scholar] [CrossRef]
- Lopes, D.D.C.; Moura, L.D.O.; Steidle Neto, A.J.; Ferraz, L.D.C.L.; Carlos, L.D.A.; Martins, L.M. Spectral indices for non-destructive determination of lettuce pigments. Food Anal. Methods 2017, 10, 2807–2814. [Google Scholar] [CrossRef]
- Ghosh, D.; Kaabouch, N. A survey on image mosaicing techniques. J. Vis. Commun. Image Represent. 2016, 34, 1–11. [Google Scholar] [CrossRef]
- Ren, Z.-Q.; Rao, Z.-H.; Ji, H.-Y. Identification of different concentrations pesticide residues of dimethoate on spinach leaves by hyperspectral image technology. IFAC-Pap. 2018, 51, 758–763. [Google Scholar]
- Steidle Neto, A.J.; Moura, L.D.O.; Lopes, D.D.C.; Carlos, L.D.A.; Martins, L.M.; Ferraz, L.D.C.L. Non-destructive prediction of pigment content in lettuce based on visible–NIR spectroscopy. J. Sci. Food Agric. 2017, 97, 2015–2022. [Google Scholar] [CrossRef]
- Asante, E.A.; Du, Z.; Lu, Y.; Hu, Y. Detection and assessment of nitrogen effect on cold tolerance for tea by hyperspectral reflectance with PLSR, PCR, and LM models. Inf. Process. Agric. 2021, 8, 96–104. [Google Scholar] [CrossRef]
- Jin, H.; Li, L.; Cheng, J. Rapid and non-destructive determination of moisture content of peanut kernels using hyperspectral imaging technique. Food Anal. Methods 2015, 8, 2524–2532. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Liu, P.; Choo, K.-K.R.; Huang, F. Spectral–spatial multi-feature-based deep learning for hyperspectral remote sensing image classification. Soft Comput. 2017, 21, 213–221. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [Green Version]
- Höskuldsson, A. PLS regression methods. J. Chemom. 1988, 2, 211–228. [Google Scholar] [CrossRef]
- Qin, S.J. Survey on data-driven industrial process monitoring and diagnosis. Annu. Rev. Control. 2012, 36, 220–234. [Google Scholar] [CrossRef]
- Kiers, H.A.; Smilde, A.K. A comparison of various methods for multivariate regression with highly collinear variables. Stat. Methods Appl. 2007, 16, 193–228. [Google Scholar] [CrossRef]
- Gabrielsson, J.; Lindberg, N.O.; Lundstedt, T. Multivariate methods in pharmaceutical applications. J. Chemom. A J. Chemom. Soc. 2002, 16, 141–160. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Yue, H.H.; Qin, S.J. Reconstruction-based fault identification using a combined index. Ind. Eng. Chem. Res. 2001, 40, 4403–4414. [Google Scholar] [CrossRef]
- Rangkuti, M.Y.; Saputro, A.H.; Imawan, C. Prediction of soluble solid contents mapping on Averrhoa carambola using hyperspectral imaging. In Proceedings of the 2017 International Conference on Sustainable Information Engineering and Technology (SIET), Batu City, Indonesia, 24–25 November 2017; pp. 414–419. [Google Scholar]
- Mukherjee, R.; Sengupta, D.; Sikdar, S.K. Selection of Sustainable Processes Using Sustainability Footprint Method: A Case Study of Methanol Production from Carbon Dioxide. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2015; Volume 36, pp. 311–329. [Google Scholar]
- Sapkota, S.; Sapkota, S.; Liu, Z. Effects of nutrient composition and lettuce cultivar on crop production in hydroponic culture. Horticulturae 2019, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Miyasaka, S.C.; Hamasaki, R.T.; De la Pena, R.S. Nutrient Deficiencies and Excesses in Taro; Soil and Crop Management; SCM-4; University of Hawaii: Honolulu, HI, USA, 2002; 14p. [Google Scholar]
- Sahin, S.; Dincer Seckin, S. Effects of different LED light and nitrogen application on growth of lettuce plants and leaf nitrate content. J. Plant Nutr. 2022, 45, 1–11. [Google Scholar] [CrossRef]
- Stefanelli, D.; Brady, S.; Winkler, S.; Jones, R.; Tomkins, B. Lettuce (Lactuca sativa L.) growth and quality response to applied nitrogen under hydroponic conditions. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 927, Lisbon, Portugal, 22–27 August 2010; pp. 353–359. [Google Scholar]
- Mendoza-Tafolla, R.O.; Juarez-Lopez, P.; Ontiveros-Capurata, R.-E.; Sandoval-Villa, M.; Iran, A.-T.; Alejo-Santiago, G. Estimating nitrogen and chlorophyll status of romaine lettuce using SPAD and at LEAF readings. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 751–756. [Google Scholar] [CrossRef] [Green Version]
- Westerveld, S.M.; McKeown, A.W.; Mcdonald, M.R.; Scott-Dupree, C.D. Assessment of Chlorophyll and Nitrate Meters as Field Tissue Nitrogen Tests for Cabbage, Onions, and Carrots. Horttechnology 2004, 14, 179–188. [Google Scholar] [CrossRef]
- Rhezali, A.; Aissaoui, A.E. Feasibility Study of Using Absolute SPAD Values for Standardized Evaluation of Corn Nitrogen Status. Nitrogen 2021, 2, 20. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Davis, S.M.; Landgrebe, D.A.; Phillips, T.L.; Swain, P.H.; Hoffer, R.M.; Lindenlaub, J.C.; Silva, L.F. Remote Sensing: The Quantitative Approach; McGraw-Hill International Book Co.: New York, NY, USA, 1978. [Google Scholar]
- Nguyen, H.D.; Nansen, C. Hyperspectral remote sensing to detect leafminer-induced stress in bok choy and spinach according to fertilizer regime and timing. Pest Manag. Sci. 2020, 76, 2208–2216. [Google Scholar] [CrossRef] [Green Version]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Methods 2017, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Xu, J.-H. Hyperspectral image information fusion-based detection of soluble solids content in red globe grapes. Comput. Electron. Agric. 2022, 196, 106822. [Google Scholar] [CrossRef]
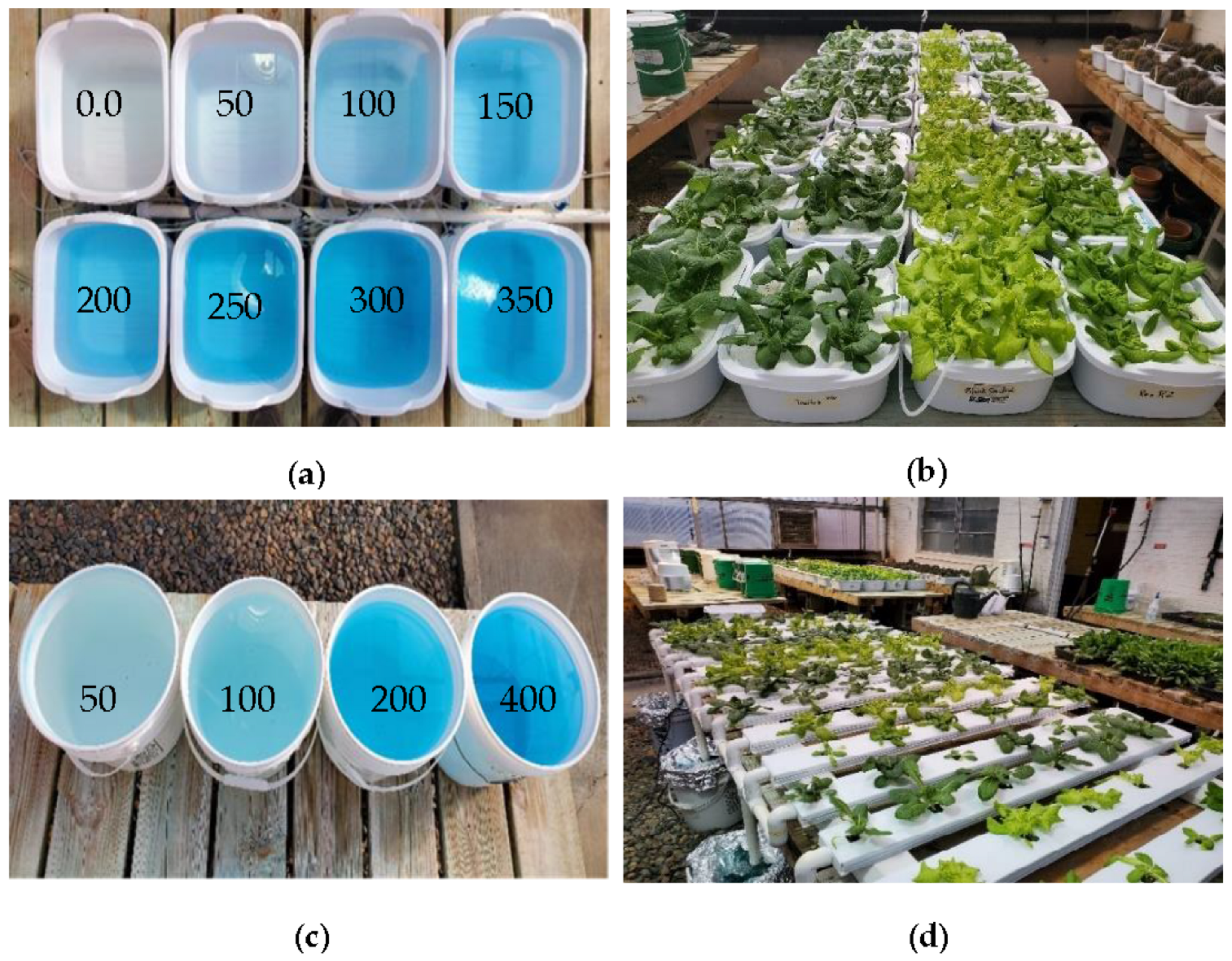
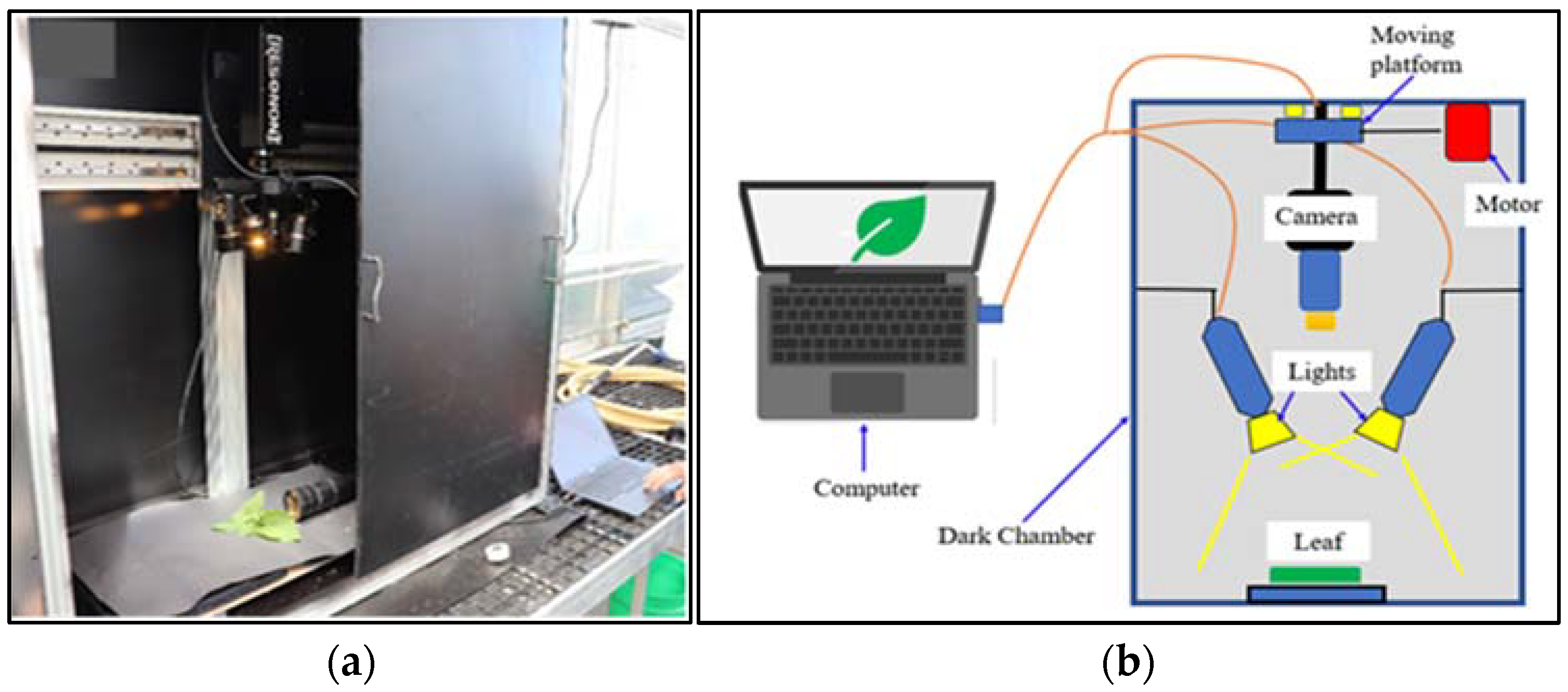
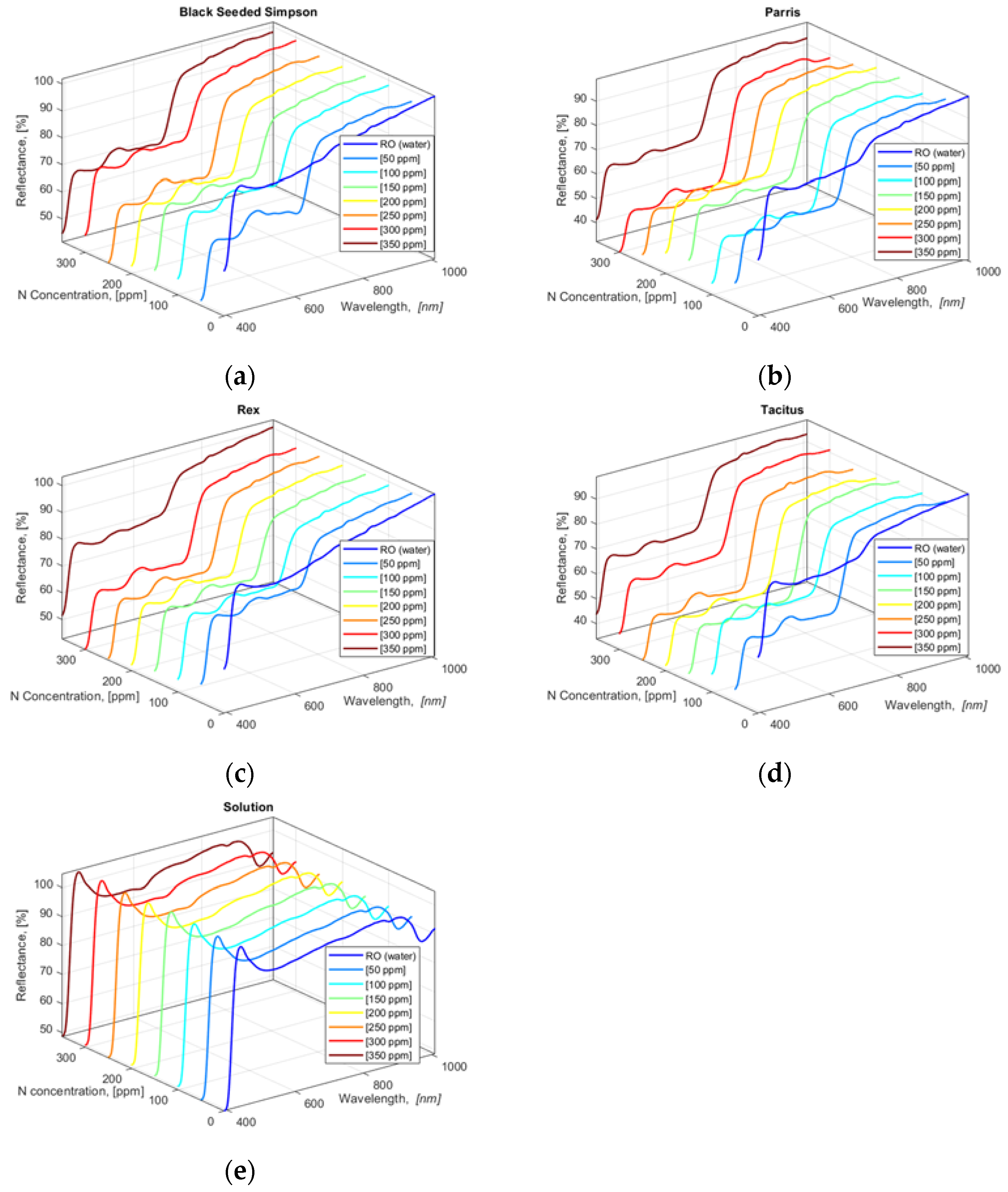
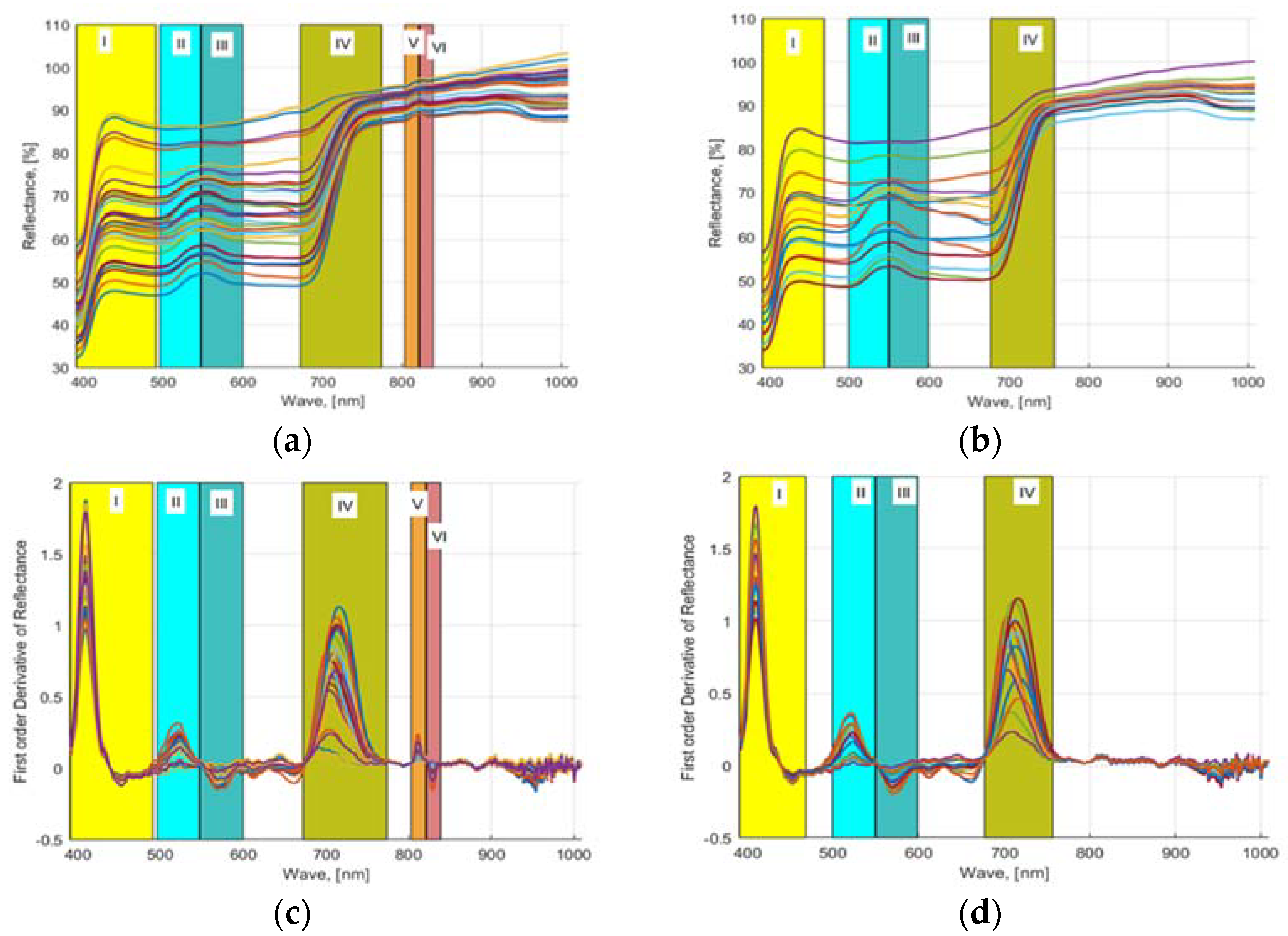



| Treatment (ppm N) | Fresh Weight (g/Plant) | Dry Weight (g/Plant) | Visual Quality * | Leaf-Edge Burns ** | SPAD |
|---|---|---|---|---|---|
| Black Seeded Simpson | |||||
| 0 | 4.8 ± 1.0 | - | 2.0 | 5.0 | 10.2 ± 1.5 |
| 50 | 69.0 ± 16.7 | 3.2 ± 1.2 | 5.0 | 5.0 | 19.2 ± 2.2 |
| 100 | 80.8 ± 34.9 | 3.6 ± 2.3 | 5.0 | 5.0 | 20.7 ± 2.2 |
| 150 | 59.0 ± 23.2 | 2.6 ± 0.9 | 5.0 | 5.0 | 22.6 ± 6.1 |
| 200 | 72.5 ± 18.3 | 2.9 ± 0.8 | 5.0 | 5.0 | 20.3 ± 3.5 |
| 250 | 104.8 ± 21.7 | 5.1 ± 1.0 | 5.0 | 5.0 | 20.7 ± 3.5 |
| 300 | 78.0 ± 36.2 | 4.1 ± 2.7 | 5.0 | 4.5 | 21.4 ± 1.5 |
| 350 | 68.0 ± 5.3 | 3.5 ± 0.2 | 5.0 | 4.5 | 22.9 ± 4.9 |
| Parris Island | |||||
| 0 | 5.5 ± 3.1 | - | 2.0 | 5.0 | 30.3 ± 6.2 |
| 50 | 59.3 ± 18.8 | 8.1 ± 3.2 | 5.0 | 5.0 | 39.6 ± 2.7 |
| 100 | 79.8 ± 31.0 | 10.9 ± 5.3 | 5.0 | 5.0 | 42.6 ± 2.7 |
| 150 | 116.8 ± 21.9 | 4.7 ± 1.2 | 5.0 | 5.0 | 39.6 ± 3.5 |
| 200 | 111.5 ± 30.9 | 5.0 ± 2.2 | 5.0 | 5.0 | 40.4 ± 2.7 |
| 250 | 121.0 ± 51.0 | 4.3 ± 2.2 | 5.0 | 5.0 | 42.5 ± 1.4 |
| 300 | 98.3 ± 13.8 | 4.4 ± 0.9 | 5.0 | 5.0 | 43.6 ± 6.5 |
| 350 | 117.3 ± 29.7 | 5.7 ± 1.9 | 5.0 | 5.0 | 45.8 ± 3.6 |
| Rex RZ | |||||
| 0 | 5.5 ± 3.7 | - | 2.5 | 5.0 | 23.0 ± 2.2 |
| 50 | 54.0 ± 3.7 | 2.3 ± 0.3 | 5.0 | 5.0 | 30.9 ± 2.7 |
| 100 | 76.0 ± 20.3 | 3.8 ± 1.7 | 5.0 | 5.0 | 29.5 ± 1.4 |
| 150 | 69.0 ± 13.6 | 2.8 ± 0.8 | 5.0 | 5.0 | 31.3 ± 2.9 |
| 200 | 69.0 ± 13.1 | 3.5 ± 2.0 | 5.0 | 5.0 | 30.6 ± 1.1 |
| 250 | 70.3 ± 5.7 | 2.8 ± 0.3 | 5.0 | 5.0 | 30.4 ± 4.5 |
| 300 | 62.5 ± 24.4 | 2.5 ± 1.1 | 5.0 | 5.0 | 32.2 ± 3.5 |
| 350 | 72.5 ± 36.5 | 3.9 ± 2.4 | 5.0 | 4.5 | 28.7 ± 1.7 |
| Tacitus | |||||
| 0 | 5.0 ± 2.2 | - | 2.5 | 5.0 | 36.6 ± 2.1 |
| 50 | 59.8 ± 14.3 | 2.8 ± 0.9 | 5.0 | 5.0 | 44.3 ± 1.6 |
| 100 | 73.8 ± 36.3 | 3.0 ± 2.1 | 5.0 | 5.0 | 45.3 ± 2.7 |
| 150 | 97.3 ± 18.3 | 4.3 ± 2.4 | 5.0 | 5.0 | 44.7 ± 2.3 |
| 200 | 105.8 ± 26.3 | 4.0 ± 1.2 | 5.0 | 5.0 | 46.5 ± 1.7 |
| 250 | 84.0 ± 29.1 | 3.2 ± 1.8 | 5.0 | 5.0 | 45.9 ± 3.1 |
| 300 | 102.3 ± 35.5 | 4.6 ± 1.9 | 5.0 | 5.0 | 45.4 ± 3.5 |
| 350 | 94.8 ± 32.9 | 5.1 ± 3.8 | 5.0 | 4.0 | 47.7 ± 2.6 |
| Treatment (ppm N) | Tissue NO3− (ppm) | Tissue K+ (ppm) | Tissue Ca2+ (ppm) | Tissue pH | Brix (%) |
|---|---|---|---|---|---|
| Black Seeded Simpson | |||||
| 0 | 3000 ± 1074 | 3300 ± 1046 | 308 ± 113 | 6.2 ± 0.7 | 12.5 ± 0.5 |
| 50 | 2225 ± 763 | 4325 ± 585 | 333 ± 48 | 5.9 ± 0.1 | 7.4 ± 0.3 |
| 100 | 4125 ± 690 | 4625 ± 171 | 223 ± 95 | 5.9 ± 0.1 | 6.9 ± 1.3 |
| 150 | 5175 ± 1315 | 3900 ± 726 | 243 ± 40 | 5.9 ± 0.2 | 6.5 ± 1.7 |
| 200 | 4800 ± 663 | 4000 ± 1078 | 265 ± 26 | 5.9 ± 0.3 | 6.7 ± 0.9 |
| 250 | 5175 ± 1209 | 4375 ± 624 | 240 ± 16 | 6.0 ± 0.1 | 7.8 ± 0.7 |
| 300 | 6200 ± 683 | 4200 ± 825 | 248 ± 25 | 6.0 ± 0.3 | 6.0 ± 0.7 |
| 350 | 5775 ± 785 | 6025 ± 606 | 308 ± 115 | 6.1 ± 0.3 | 7.8 ± 1.5 |
| Parris Island | |||||
| 0 | 1650 ± 173 | 5300 ± 956 | 150 ± 25 | 6.1 ± 0.3 | 15.3 ± 4.9 |
| 50 | 2050 ± 412 | 5250 ± 834 | 238 ± 47 | 6.2 ± 0.2 | 7.8 ± 1.2 |
| 100 | 4450 ± 1034 | 5750 ± 1622 | 193 ± 13 | 6.0 ± 0.1 | 7.3 ± 0.8 |
| 150 | 5025 ± 299 | 4500 ± 510 | 275 ± 29 | 5.9 ± 0.1 | 6.3 ± 0.9 |
| 200 | 5975 ± 866 | 4050 ± 433 | 200 ± 29 | 6.0 ± 0.1 | 5.9 ± 0.9 |
| 250 | 5875 ± 1028 | 4800 ± 993 | 308 ± 39 | 5.8 ± 0.2 | 6.7 ± 1.0 |
| 300 | 5333 ± 306 | 4200 ± 346 | 290 ± 26 | 5.9 ± 0.1 | 7.3 ± 1.1 |
| 350 | 6675 ± 834 | 5475 ± 525 | 198 ± 35 | 6.1 ± 0.1 | 8.2 ± 0.2 |
| Rex RZ | |||||
| 0 | 3325 ± 754 | 4175 ± 1021 | 318 ± 99 | 7.2 ± 1.0 | 18.7 ± 2.5 |
| 50 | 2138 ± 221 | 5350 ± 1047 | 335 ± 34 | 6.3 ± 0.3 | 7.6 ± 1.2 |
| 100 | 4825 ± 465 | 5050 ± 755 | 298 ± 34 | 5.9 ± 0.1 | 7.2 ± 1.0 |
| 150 | 5775 ± 556 | 4925 ± 499 | 233 ± 40 | 5.9 ± 0.1 | 6.9 ± 0.9 |
| 200 | 6825 ± 704 | 5500 ± 990 | 310 ± 83 | 6.0 ± 0.1 | 6.7 ± 0.7 |
| 250 | 7850 ± 387 | 5425 ± 1034 | 278 ± 82 | 5.9 ± 0.1 | 6.5 ± 0.7 |
| 300 | 7375 ± 574 | 4975 ± 465 | 255 ± 53 | 6.0 ± 0.4 | 6.7 ± 1.1 |
| 350 | 7450 ± 1162 | 4725 ± 411 | 213 ± 38 | 6.0 ± 0.1 | 7.0 ± 0.8 |
| Tacitus | |||||
| 0 | 2425 ± 435 | 4850 ± 881 | 185 ± 37 | 6.1 ± 0.5 | 19.3 ± 1.4 |
| 50 | 1625 ± 435 | 5250 ± 881 | 318 ± 66 | 6.2 ± 0.4 | 8.5 ± 0.8 |
| 100 | 3375 ± 403 | 4700 ± 707 | 283 ± 59 | 6.0 ± 0.2 | 7.5 ± 1.6 |
| 150 | 4800 ± 1197 | 4250 ± 676 | 248 ± 64 | 5.9 ± 0.1 | 7.0 ± 1.3 |
| 200 | 4575 ± 465 | 4450 ± 526 | 323 ± 114 | 5.9 ± 0.1 | 6.9 ± 1.0 |
| 250 | 5475 ± 525 | 4600 ± 497 | 298 ± 69 | 6.2 ± 0.2 | 6.8 ± 1.0 |
| 300 | 5600 ± 852 | 4150 ± 480 | 295 ± 72 | 6.2 ± 0.1 | 7.6 ± 0.6 |
| 350 | 6650 ± 575 | 4600 ± 825 | 298 ± 56 | 6.1 ± 0.3 | 9.2 ± 0.3 |
| Treatment (ppm N) | Fresh Weight (g/Plant) | Dry Weight (g/Plant) | Visual Quality * | Leaf Edge Burns ** | SPAD | Tissue NO3− (ppm) | Tissue K+ (ppm) | Tissue Ca2+ (ppm) | Tissue pH | Brix (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Black Seeded Simpson | ||||||||||
| 50 | 82.5 ± 35.1 | 4.4 ± 2.3 | 4.0 | 5.0 | 20.1 ± 4.2 | 2925 ± 670 | 3325 ± 613 | 218 ± 30 | 5.9 ± 0.1 | 6.6 ± 0.9 |
| 100 | 198.5 ± 54.6 | 15.0 ± 10.0 | 4.5 | 4.5 | 21.4 ± 2.0 | 4850 ± 252 | 4025 ± 655 | 290 ± 20 | 5.9 ± 0.1 | 6.7 ± 0.8 |
| 200 | 254.0 ± 46.0 | 18.7 ± 9.5 | 5.0 | 4.0 | 24.7 ± 3.8 | 5925 ± 492 | 3975 ± 842 | 250 ± 26 | 5.9 ± 0.2 | 8.0 ± 0.7 |
| 400 | 65.0 ± 10.8 | 7.8 ± 1.4 | 2.0 | 3.0 | 32.2 ± 5.1 | 8300 ± 1214 | 5925 ± 896 | 147 ± 44 | 5.6 ± 0.2 | 14.1 ± 0.7 |
| Parris Island | ||||||||||
| 50 | 128.8 ± 22.7 | 2.8 ± 1.2 | 4.5 | 5.0 | 38.8 ± 3.6 | 2475 ± 826 | 4000 ± 1707 | 218 ± 46 | 6.3 ± 0.4 | 6.8 ± 1.0 |
| 100 | 170.0 ± 37.1 | 3.9 ± 1.7 | 5.0 | 5.0 | 40.1 ± 4.4 | 5550 ± 404 | 4550 ± 819 | 238 ± 38 | 6.0 ± 0.1 | 6.0 ± 1.2 |
| 200 | 141.0 ± 31.1 | 8.4 ± 4.7 | 4.3 | 4.8 | 45.7 ± 2.4 | 6675 ± 1053 | 4650 ± 574 | 208 ± 40 | 5.9 ± 0.1 | 6.8 ± 1.0 |
| 400 | 86.0 ± 11.9 | 7.3 ± 0.1 | 3.8 | 4.3 | 46.2 ± 4.6 | 5825 ± 685 | 8000 ± 735 | 325 ± 159 | 6.3 ± 0.4 | 11.7 ± 2.5 |
| Rex RZ | ||||||||||
| 50 | 110.3 ± 31.5 | 7.9 ± 3.2 | 4.5 | 5.0 | 29.2 ± 1.8 | 6725 ± 763 | 3700 ± 816 | 240 ± 25 | 5.9 ± 0.1 | 6.1 ± 0.8 |
| 100 | 145.8 ± 31.5 | 11.1 ± 6.5 | 5.0 | 5.0 | 28.9 ± 2.0 | 7025 ± 378 | 4575 ± 222 | 263 ± 19 | 5.8 ± 0.1 | 5.9 ± 0.6 |
| 200 | 125.8 ± 38.2 | 6.8 ± 4.1 | 4.8 | 4.8 | 29.0 ± 2.4 | 6100 ± 956 | 5125 ± 655 | 260 ± 22 | 5.9 ± 0.1 | 7.1 ± 2.7 |
| 400 | 92.0 ± 25.3 | 7.0 ± 3.1 | 4.0 | 4.0 | 35.7 ± 6.8 | 4750 ± 1147 | 4450 ± 2089 | 176 ± 36 | 5.8 ± 0.1 | 7.2 ± 0.4 |
| Tacitus | ||||||||||
| 50 | 88.3 ± 34.5 | 2.2 ± 3.8 | 4.8 | 5.0 | 42.6 ± 1.3 | 2875 ± 427 | 3425 ± 403 | 210 ± 8 | 6,1 ± 0.2 | 8.5 ± 0.3 |
| 100 | 123.5 ± 58.3 | 12.6 ± 9.9 | 5.0 | 5.0 | 41.4 ± 3.7 | 6750 ± 569 | 5450 ± 968 | 210 ± 55 | 5.9 ± 0.1 | 5.9 ± 0.8 |
| 200 | 158.5 ± 42.2 | 15.4 ± 9.2 | 4.3 | 4.5 | 42.8 ± 1.4 | 6800 ± 860 | 5025 ± 299 | 258 ± 77 | 5.8 ± 0.2 | 6.9 ± 1.2 |
| 400 | 74.5 ± 21.4 | 7.0 ± 2.3 | 3.0 | 3.8 | 48.9 ± 5.5 | 3550 ± 881 | 5400 ± 1520 | 195 ± 45 | 5.8 ± 0.0 | 22.6 ± 2.7 |
| Treatment (ppm N) | ||||
|---|---|---|---|---|
| Black Seeded Simpson | Parris | Rex RZ | Tacitus | |
| 0 | 0.979 | 0.975 | 0.981 | 0.979 |
| 50 | 0.821 | 0.908 | 0.920 | 0.873 |
| 100 | 0.893 | 0.855 | 0.904 | 0.925 |
| 150 | 0.912 | 0.919 | 0.876 | 0.873 |
| 200 | 0.879 | 0.888 | 0.861 | 0.885 |
| 250 | 0.851 | 0.872 | 0.879 | 0.843 |
| 300 | 0.918 | 0.844 | 0.864 | 0.925 |
| 350 | 0.881 | 0.936 | 0.952 | 0.941 |
| Type | FDR Index | PLSR/PCA Index | VIP-Score Index | |||
|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | |
| Black Seeded Simpson | ||||||
| Applied Treatment | 0.82 | 62.01 | 0.96 | 27.63 | 0.92 | 36.78 |
| Fresh Leaf Weight | 0.97 | 5.93 | 0.89 | 10.98 | 0.94 | 7.68 |
| Dried Leaf Weight | 0.91 | 0.33 | 0.82 | 0.47 | 0.86 | 0.38 |
| SPAD | 0.95 | 1.05 | 0.94 | 1.171 | 0.99 | 0.03 |
| NO3− | 0.93 | 437.19 | 0.98 | 185.63 | 0.99 | 111.51 |
| K+ | 0.97 | 83.69 | 0.97 | 80.84 | 0.99 | 30.02 |
| Ca2+ | 0.75 | 23.76 | 0.65 | 27.87 | 0.65 | 25.42 |
| pH | 0.95 | 0.03 | 0.98 | 0.02 | 0.98 | 0.016 |
| Brix | 0.98 | 0.36 | 0.99 | 0.21 | 0.99 | 0.03 |
| Parris | ||||||
| Applied Treatment | 0.97 | 22.43 | 0.99 | 11.91 | 0.99 | 0.73 |
| Fresh Leaf Weight | 0.92 | 13.05 | 0.88 | 16.08 | 0.99 | 0.76 |
| Dried Leaf Weight | 0.92 | 0.89 | 0.96 | 0.63 | 0.95 | 0.63 |
| SPAD | 0.99 | 0.44 | 0.98 | 0.731 | 0.99 | 0.26 |
| NO3− | 0.87 | 788.66 | 0.88 | 760.39 | 0.95 | 419.61 |
| K+ | 0.63 | 449.38 | 0.95 | 160.99 | 0.80 | 302.20 |
| Ca2+ | 0.99 | 5.51 | 0.94 | 15.57 | 0.94 | 14.35 |
| pH | 0.58 | 0.09 | 0.49 | 0.098 | 0.85 | 0.05 |
| Brix | 0.97 | 0.92 | 0.97 | 0.90 | 0.97 | 0.85 |
| Rex RZ | ||||||
| Applied Treatment | 0.99 | 8.04 | 0.99 | 12.437 | 0.87 | 47.79 |
| Fresh Leaf Weight | 0.87 | 9.91 | 0.99 | 0.840 | 0.99 | 0.27 |
| Dried Leaf Weight | 0.55 | 0.52 | 0.99 | 0.079 | 0.94 | 0.17 |
| SPAD | 0.98 | 0.41 | 0.99 | 0.061 | 0.94 | 0.72 |
| NO3− | 0.88 | 854.39 | 0.99 | 125.99 | 0.99 | 97.51 |
| K+ | 0.80 | 227.47 | 0.99 | 48.52 | 0.99 | 48.55 |
| Ca2+ | 0.35 | 41.35 | 0.99 | 3.196 | 0.95 | 10.79 |
| pH | 0.90 | 0.16 | 0.99 | 0.019 | 0.99 | 0.016 |
| Brix | 0.92 | 1.42 | 0.99 | 0.345 | 0.96 | 0.87 |
| Tacitus | ||||||
| Applied Treatment | 0.87 | 52.88 | 0.99 | 15.50 | 0.98 | 16.90 |
| Fresh Leaf Weight | 0.90 | 12.45 | 0.98 | 4.911 | 0.97 | 6.403 |
| Dried Leaf Weight | 0.73 | 0.63 | 0.95 | 0.264 | 0.91 | 0.33 |
| SPAD | 0.82 | 1.724 | 0.91 | 1.22 | 0.91 | 1.09 |
| NO3− | 0.96 | 361.98 | 0.97 | 311.36 | 0.99 | 152.50 |
| K+ | 0.97 | 75.04 | 0.96 | 85.37 | 0.96 | 78.75 |
| Ca2+ | 0.71 | 28.47 | 0.96 | 9.95 | 0.91 | 14.56 |
| pH | 0.83 | 0.057 | 0.74 | 0.07 | 0.74 | 0.06 |
| Brix | 0.97 | 0.76 | 0.98 | 0.61 | 0.99 | 0.20 |
| Type | FDR Index | PLSR/PCA Index | VIP-Score Index | |||
|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | |
| Black Seeded Simpson | ||||||
| Applied Treatment | 0.99 | 18.04 | 0.99 | 15.19 | 0.99 | 15.494 |
| Fresh Leaf Weight | 0.48 | 80.13 | 0.51 | 78.37 | 0.50 | 78.548 |
| Dried Leaf Weight | 0.51 | 5.63 | 0.54 | 5.44 | 0.54 | 5.45 |
| SPAD | 0.99 | 0.37 | 0.99 | 0.27 | 0.99 | 0.28 |
| NO3− | 0.97 | 425.41 | 0.98 | 363.325 | 0.98 | 369.58 |
| K+ | 0.97 | 227.76 | 0.96 | 253.99 | 0.96 | 251.38 |
| Ca2+ | 0.96 | 14.93 | 0.96 | 14.19 | 0.96 | 14.26 |
| pH | 0.98 | 0.02 | 0.98 | 0.02 | 0.98 | 0.02 |
| Brix | 0.99 | 0.47 | 0.98 | 0.52 | 0.98 | 0.51 |
| Parris | ||||||
| Applied Treatment | 0.99 | 20.56 | 0.98 | 22.86 | 0.98 | 24.71 |
| Fresh Leaf Weight | 0.69 | 23.71 | 0.66 | 24.80 | 0.66 | 24.77 |
| Dried Leaf Weight | 0.96 | 0.66 | 0.94 | 0.75 | 0.94 | 0.81 |
| SPAD | 0.96 | 0.95 | 0.94 | 1.06 | 0.94 | 1.13 |
| NO3− | 0.86 | 830.42 | 0.88 | 775.79 | 0.89 | 734.28 |
| K+ | 0.99 | 57.30 | 0.99 | 66.37 | 0.99 | 77.89 |
| Ca2+ | 0.99 | 6.43 | 0.99 | 6.66 | 0.99 | 6.59 |
| pH | 0.74 | 0.118 | 0.76 | 0.114 | 0.77 | 0.11 |
| Brix | 0.94 | 0.754 | 0.94 | 0.757 | 0.94 | 0.76 |
| Rex RZ | ||||||
| Applied Treatment | 0.98 | 23.34 | 0.98 | 25.45 | 0.98 | 25.88 |
| Fresh Leaf Weight | 0.97 | 4.37 | 0.998 | 1.1830 | 0.99 | 1.07 |
| Dried Leaf Weight | 0.75 | 1.23 | 0.63 | 1.49 | 0.57 | 1.59 |
| SPAD | 0.92 | 1.15 | 0.94 | 0.99 | 0.96 | 0.83 |
| NO3− | 0.99 | 128.67 | 0.97 | 186.55 | 0.97 | 218.64 |
| K+ | 0.45 | 526.96 | 0.62 | 456.71 | 0.72 | 388.02 |
| Ca2+ | 0.87 | 17.27 | 0.91 | 14.13 | 0.95 | 11.31 |
| pH | 0.93 | 0.007 | 0.88 | 0.01 | 0.86 | 0.011 |
| Brix | 0.69 | 0.46 | 0.64 | 0.49 | 0.65 | 0.49 |
| Tacitus | ||||||
| Applied Treatment | 0.99 | 18.67 | 0.98 | 25.99 | 0.97 | 29.16 |
| Fresh Leaf Weight | 0.96 | 9.60 | 0.98 | 5.71 | 0.99 | 4.25 |
| Dried Leaf Weight | 0.99 | 0.05 | 0.99 | 0.49 | 0.98 | 0.68 |
| SPAD | 0.99 | 0.16 | 0.99 | 0.17 | 0.99 | 0.18 |
| NO3− | 0.93 | 671.45 | 0.88 | 862.95 | 0.86 | 938.71 |
| K+ | 0.61 | 731.12 | 0.51 | 812.18 | 0.48 | 841.57 |
| Ca2+ | 0.67 | 19.12 | 0.74 | 16.97 | 0.77 | 15.91 |
| pH | 0.98 | 0.02 | 0.95 | 0.028 | 0.94 | 0.03 |
| Brix | 0.99 | 0.22 | 0.99 | 0.297 | 0.99 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eshkabilov, S.; Stenger, J.; Knutson, E.N.; Küçüktopcu, E.; Simsek, H.; Lee, C.W. Hyperspectral Image Data and Waveband Indexing Methods to Estimate Nutrient Concentration on Lettuce (Lactuca sativa L.) Cultivars. Sensors 2022, 22, 8158. https://doi.org/10.3390/s22218158
Eshkabilov S, Stenger J, Knutson EN, Küçüktopcu E, Simsek H, Lee CW. Hyperspectral Image Data and Waveband Indexing Methods to Estimate Nutrient Concentration on Lettuce (Lactuca sativa L.) Cultivars. Sensors. 2022; 22(21):8158. https://doi.org/10.3390/s22218158
Chicago/Turabian StyleEshkabilov, Sulaymon, John Stenger, Elizabeth N. Knutson, Erdem Küçüktopcu, Halis Simsek, and Chiwon W. Lee. 2022. "Hyperspectral Image Data and Waveband Indexing Methods to Estimate Nutrient Concentration on Lettuce (Lactuca sativa L.) Cultivars" Sensors 22, no. 21: 8158. https://doi.org/10.3390/s22218158









