Acquisition and Analysis of Microcirculation Image in Septic Model Rats
Abstract
:1. Introduction
- To investigate the two indices of microcirculation under sepsis and therapy, namely, the velocity and vessel diameter of blood, two corresponding image-based estimation approaches have been proposed.
- By observing the temporal changes in blood velocity and vessel diameter, the effects of disease and TM alfa-induced therapy on sepsis have been confirmed using multiple indices.
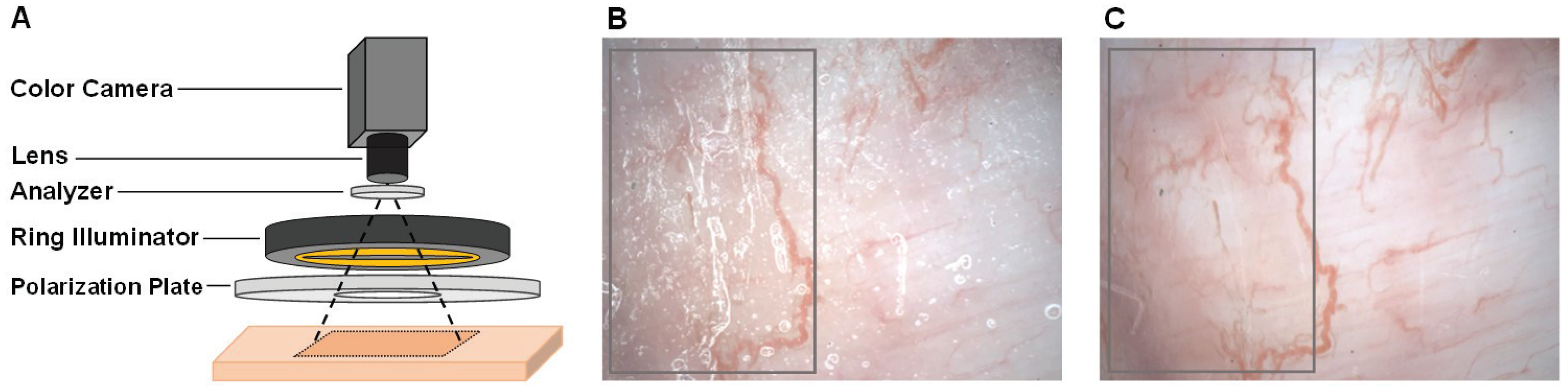
2. Non-Contact Imaging Setup
3. Proposed Image Analysis Method
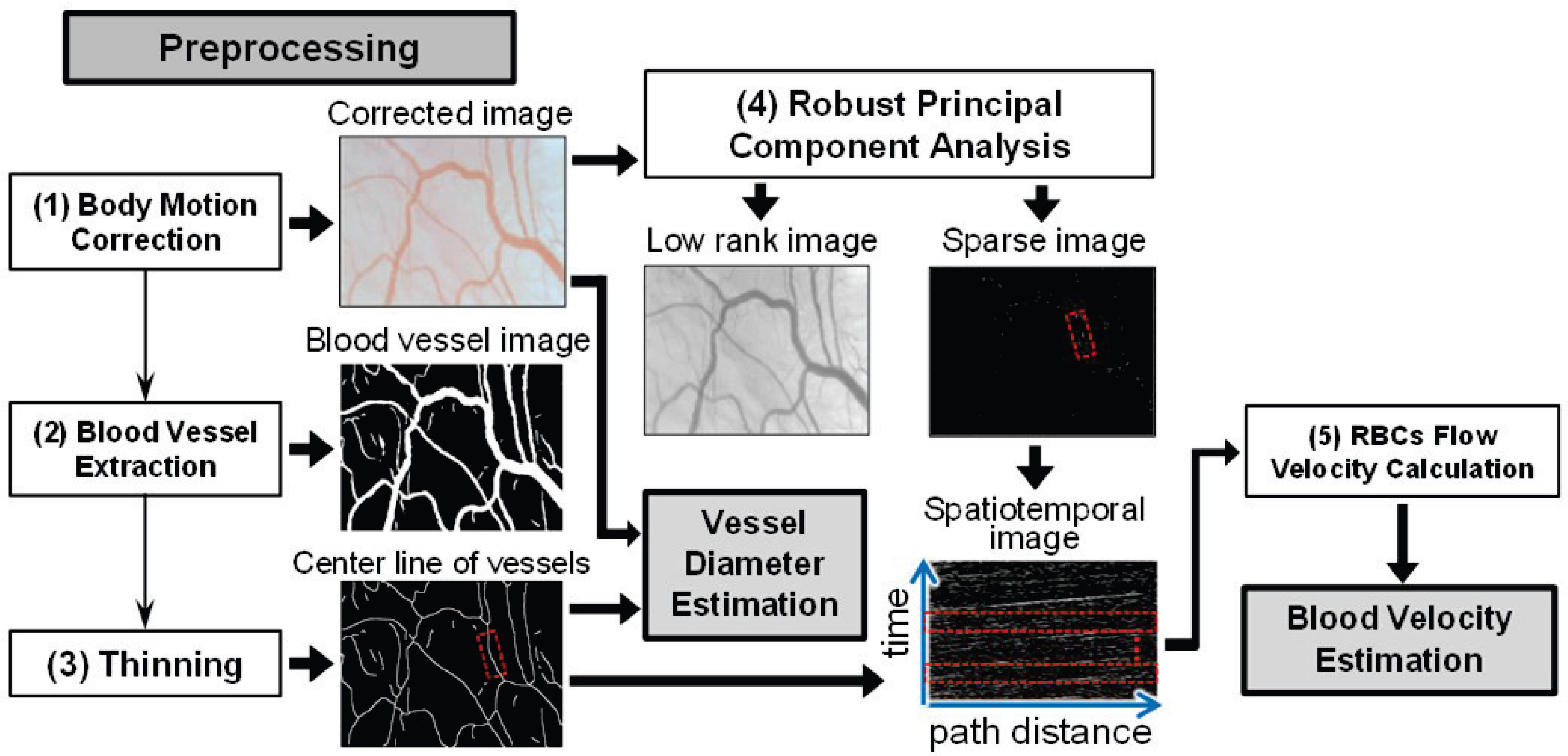
3.1. Preprocessing
- (1)
- Body Motion Correction: A motion correction is first executed on all the acquired images to obtain the corrected images due to the body motion-induced image blurring in the motion pictures. More concretely, the first image is defined as the target image, and each of the following images is registered to the target image. A common template-matching technique was used for this purpose.
- (2)
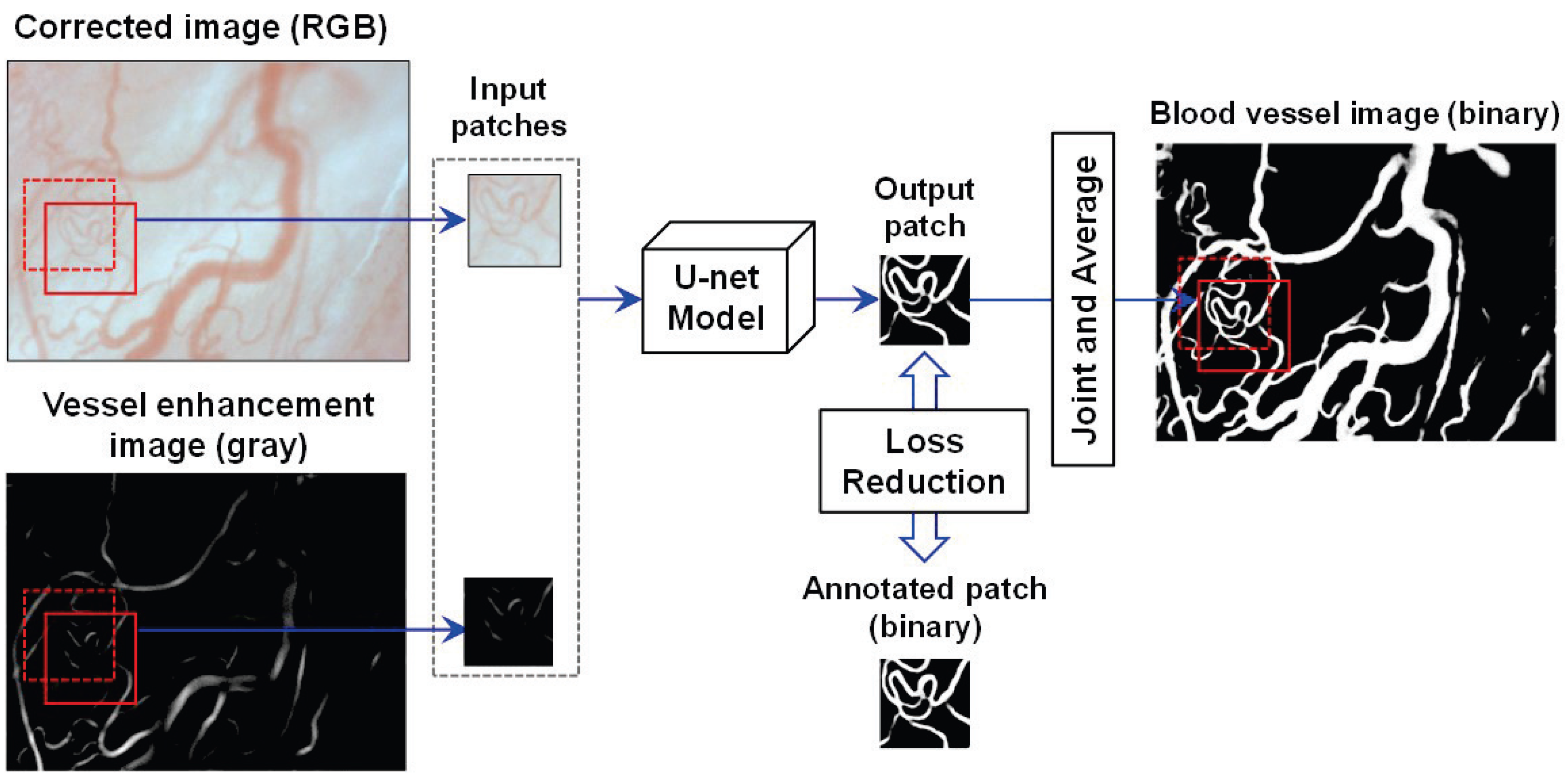
- Vessel Extraction: The blood vessels are segmented by U-net [23] based on fully convolutional networks (FCN) [25], relying on the corrected image. The architecture of the U-net is composed of a contracted path for data information capture and a symmetric expanding path for accurate localization. In [23], U-net was proven to realize the precise and efficient segmentation of biomedical images by classifying each pixel. In the training phase, the pixel values of the vessels in the corrected images are enhanced to obtain vessel enhancement images using the enhancement filtering technique in [26]. Both the corrected images and the obtained vessel enhancement images were used to acquire a learning model that can generate images of blood vessels.Figure 3 illustrates the U-net model training and prediction for blood vessel extraction. To better segment the vessel fields, both the corrected image and its vessel enhancement image are used as input images. Here, the corrected image is an RGB one with three channels, and the enhancement image is a gray one with one channel. To simplify the segmentation task, the patches in the same field of the corrected and enhancement images are input simultaneously into the U-net model with overlaps to other patches for seamless segmentation, as in [23]. A binary patch from the annotated image is used to calculate and reduce the loss with the updating output patch for training the model. Using the trained U-net model, the binary vessel patches can be predicted, which are finally joined into an entire vessel image by averaging overlaps.
- (3)
- Thinning: After vessel extraction based on the U-net, the thinning technique presented in [27] narrows down the regions of the vessel in the blood vessel image to obtain the center line of the vessels.
3.2. Estimation Approach of Blood Velocity
- (1)
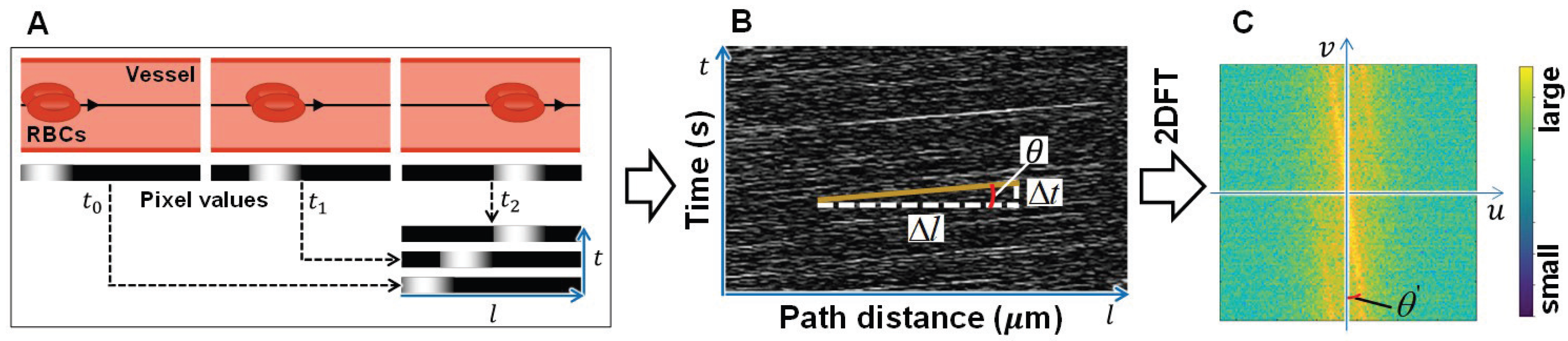
- RPCA: Robust principal component analysis (RPCA) can perform singular value decomposition (SVD)-based dimensionality reduction, similar to standard PCA, and extract the sparse component using the introduced sparse penalty [21,22]. Thus, RPCA was adopted on the corrected image to extract the RBCs existing in the blood vessels. By RPCA, the obtained low-rank component of tissue and vessels contains elements with less temporal changes, whereas the extracted sparse component of RBCs and residual body motion noise contain elements with obvious temporal changes. The resultant image composed of the sparse component is called the sparse image in this study and is used for the calculation of the RBCs flow velocity, that is, blood velocity.
- (2)
- RBCs Flow Velocity Calculation: Finally, the spatiotemporal image is constructed by the pixel values of the sequential sparse component (in the sparse images) along the center line of the vessel, and the slope of the obvious moving tracks of RBCs on the spatiotemporal images is calculated as the blood velocity.
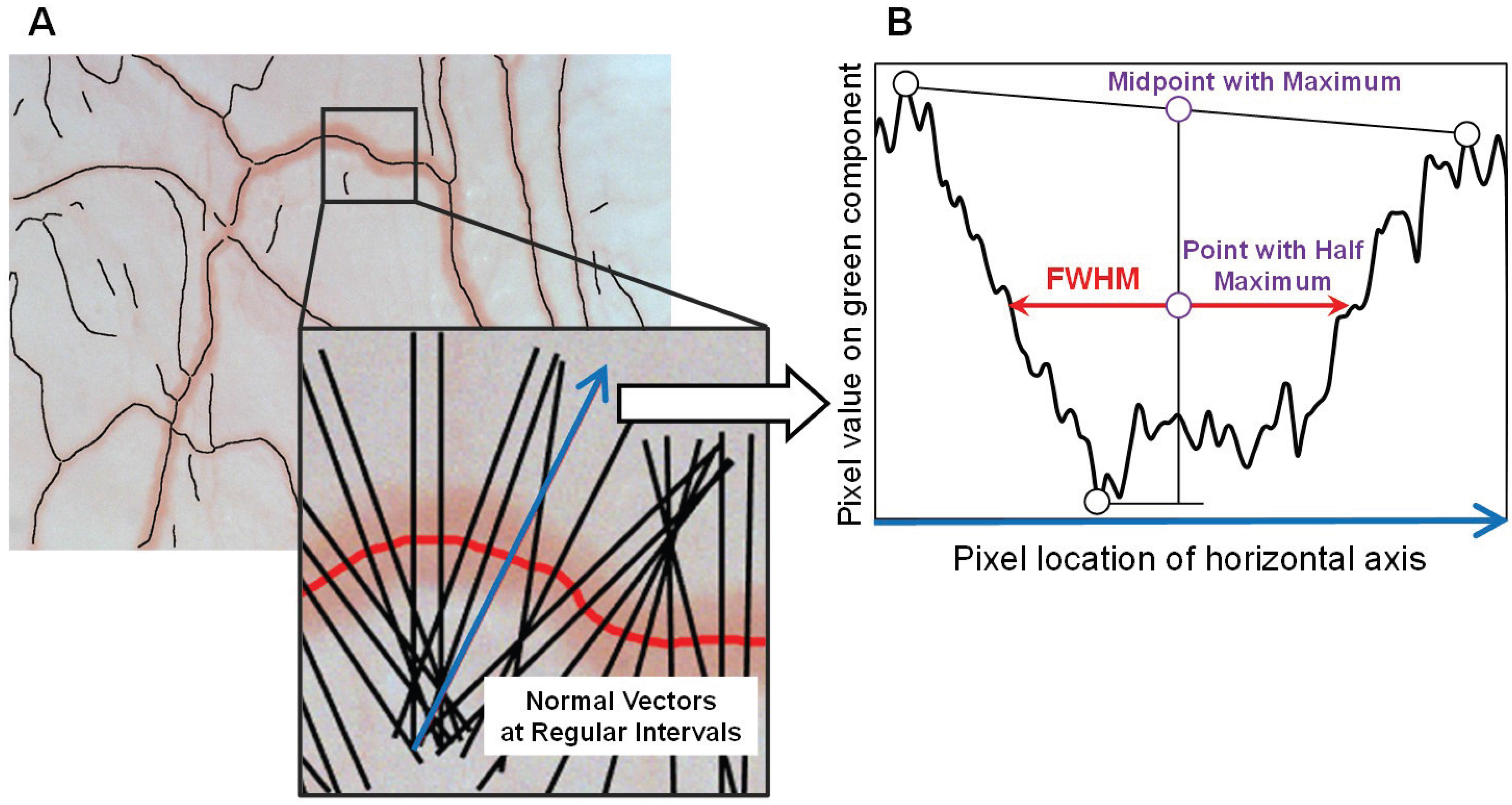
3.3. Estimation Approach of Blood Vessel Diameter
4. Experiments
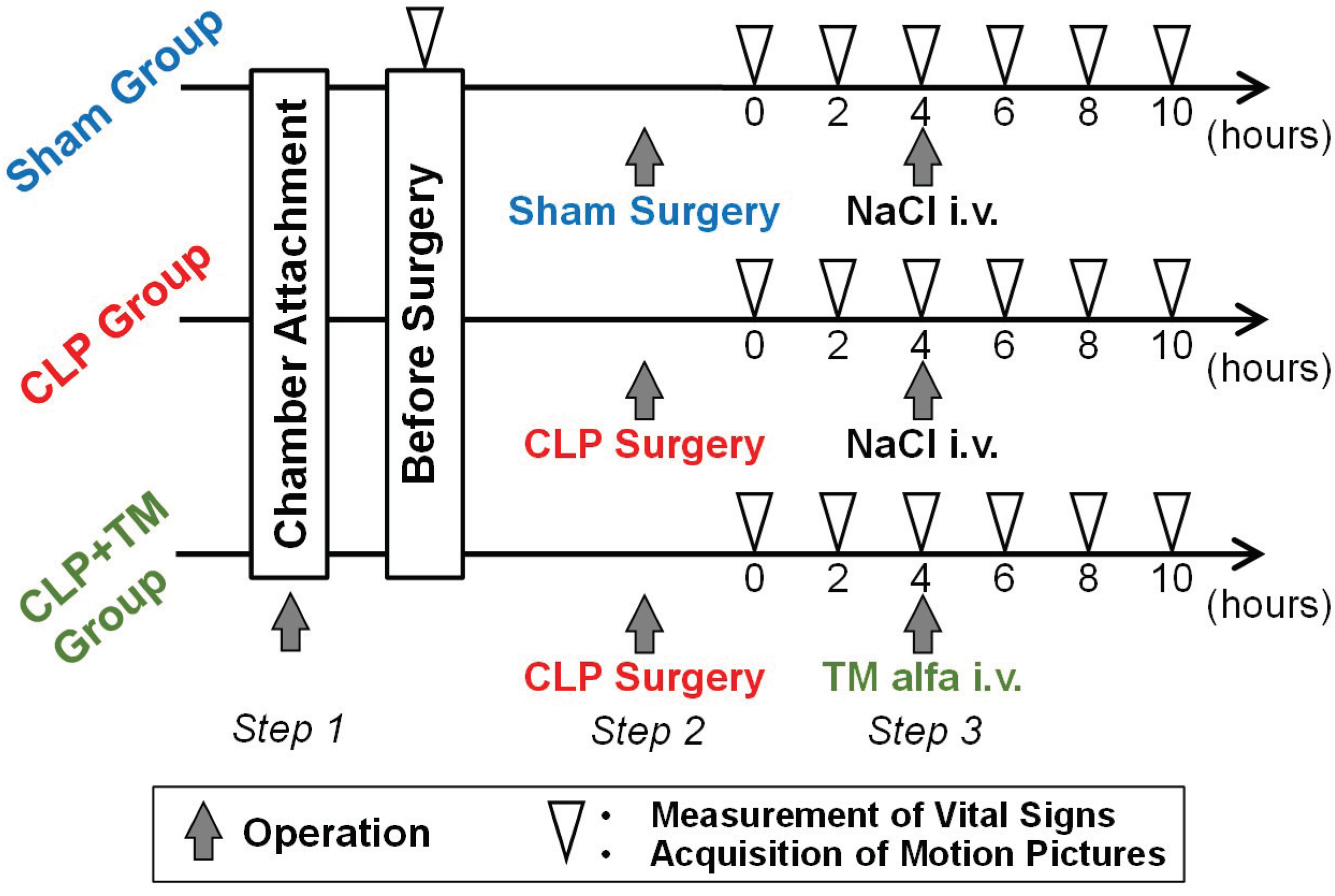
4.1. Dataset and Condition
4.2. Blood Vessel Extraction by U-net
4.2.1. Parameter Setting
4.2.2. Performance Evaluation
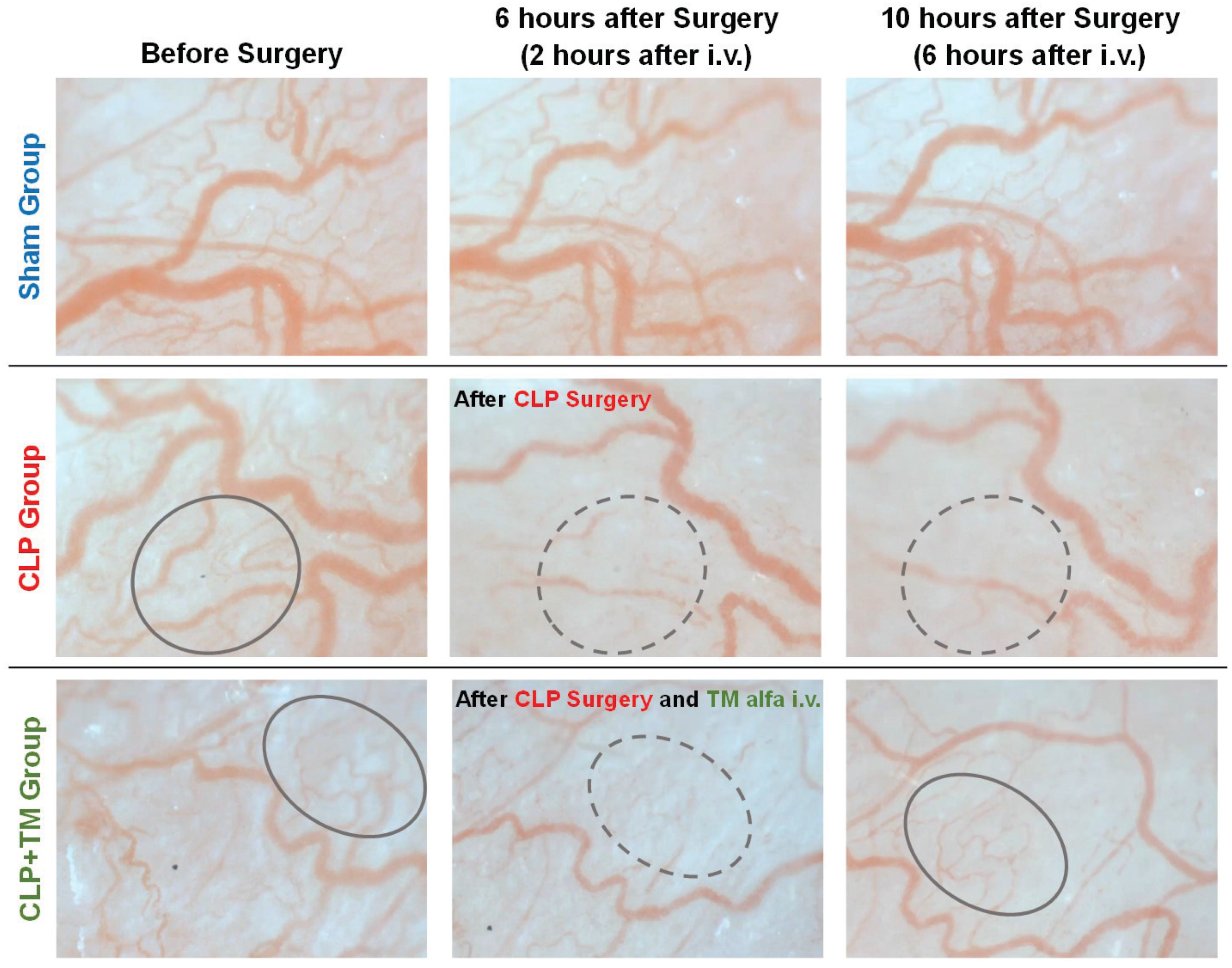
4.3. Results
4.3.1. Vital Signs Measurements on Temporal Changes
4.3.2. Estimations on Temporal Changes of Blood Velocity and Vessel Diameter
5. Discussion
5.1. Normalization with Centralization
5.2. Significant Difference Test
5.3. Typical Individual Rats
5.4. Limitations
6. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Francois, B.; Zabolotskikh, I.; Daga, M.K.; Lascarrou, J.-B.; Kirov, M.Y.; Pettilä, V.; Wittebole, X.; Meziani, F.; Mercier, E.; et al. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy. JAMA 2019, 321, 1993–2002. [Google Scholar] [CrossRef] [Green Version]
- Arioka, T.; Tahara, S.; Nakatsugawa, M.; Sakai, T. Effects of thrombomodulin alfa on disseminated intravascular coagulation and organ injury in cecal ligation and puncture-induced sepsis in rats. Jpn. Pharmacol. Ther. 2014, 42, 477–483. (In Japanese) [Google Scholar]
- Helms, J.; Clere-Jehl, R.; Bianchini, E.; Borgne, P.L.; Burban, M.; Zobairi, F.; Diehl, J.-L.; Grunebaum, L.; Toti, F.; Meziani, F.; et al. Thrombomodulin favors leukocyte microvesicle fibrinolytic activity, reduces NETosis and prevents septic shock-induced coagulopathy in rats. Ann. Intensive Care 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eg, M.; Ogura, H.; Yatabe, T.; Atagi, K.; Inoue, S.; Iba, T.; Kakihana, Y.; Kawasaki, T.; Kushimoto, S.; Kuroda, Y.; et al. The Japanese clinical practice guidelines for management of sepsis and septic shock 2020 (J-SSCG 2020). Acute Med. Surg. 2021, 8, e659. [Google Scholar]
- Ocak, I.; Kara, A.; Ince, C. Monitoring microcirculation. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 407–418. [Google Scholar] [CrossRef]
- Groner, W.; Winkelman, J.W.; Harris, A.G.; Ince, C.; Bouma, G.J.; Messmer, K.; Nadeau, R.G. Orthogonal polarization spectral imaging: A new method for study of the microcirculation. Nat. Med. 1999, 5, 1209–1212. [Google Scholar] [CrossRef]
- Ince, C. Sidestream dark field imaging: An improved technique to observe sublingual microcirculation. Crit. Care. 2005, 9, 72. [Google Scholar] [CrossRef]
- Erdem, O.; Ince, C.; Tibboel, D.; Kuiper, J.W. Assessing the microcirculation with handheld vital microscopy in critically ill neonates and children: Evolution of the technique and its potential for critical care. Front. Pediatr. 2019, 7, 273. [Google Scholar] [CrossRef]
- Machikhin, A.S.; Volkov, M.V.; Khokhlov, D.D.; Lovchikova, E.D.; Potemkin, A.V.; Danilycheva, I.V.; Dorofeeva, I.V.; Shulzhenko, A.E. Exoscope-based videocapillaroscopy system for in vivo skin microcirculation imaging of various body areas. Biomed. Opt. Express 2021, 12, 4627–4636. [Google Scholar] [CrossRef]
- Lal, C.; Leahy, M.J. An updated review of methods and advancements in microvascular blood flow imaging. Microcirculation 2016, 23, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Spronk, P.E.; Ince, C.; Gardien, M.J.; Mathura, K.R.; Straaten, H.M.O.; Zandstra, D.F. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 2002, 360, 1395–1396. [Google Scholar] [CrossRef]
- Ince, C. The microcirculation is the motor of sepsis. Crit. Care 2005, 9, S13–S19. [Google Scholar] [CrossRef] [Green Version]
- Backer, D.D.; Creteur, J.; Preiser, J.C.; Dubois, M.J.; Vincent, J.L. Microvascular blood flow is altered in patients with sepsis. Am. Respir. Crit. Care Med. 2002, 166, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Backer, D.D.; Hollenberg, S.; Boerma, C.; Goedhart, P.; Buchele, G.; Tascon, G.O.; Dobbe, I.; Ince, C. How to evaluate the microcirculation: Report of a round table conference. Crit. Care 2007, 11, R101. [Google Scholar] [CrossRef] [Green Version]
- Dobbe, J.G.G.; Streekstra, G.J.; Atasever, B.; Zijderveld, R.V.; Ince, C. Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med. Biol. Eng. Comput. 2008, 46, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, R.; Kurata, T.; Sekine, M.; Nakano, K.; Ohnishi, T.; Haneishi, H. Two-wavelength oximetry of tissue microcirculation based on sidestream dark-field imaging. J. Biomed. Optics 2008, 24, 031013. [Google Scholar] [CrossRef]
- Takahashi, M.; Kurata, T.; Ohnishi, T.; Haneishi, H. Quantitative evaluation of blood flow obstruction in microcirculation with sidestream dark-field images. In Proceedings of the SPIE Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues XV, San Francisco, CA, USA, 16 February 2017; p. 100680A. [Google Scholar]
- Hassanein, A.S.; Mohammad, S.; Sameer, M.; Ragab, M.E. A survey on Hough transform, theory, techniques and applications. arXiv 2015, arXiv:1502.02160. [Google Scholar]
- Wichterman, K.A.; Baue, A.E.; Chaudry, I.H. Sepsis and septic shock–A review of laboratory models and a proposal. J. Surg. Res. 1980, 29, 189–201. [Google Scholar] [CrossRef]
- Candes, E.J.; Li, X.; Ma, Y.; Wright, J. Robust principal component analysis? J. ACM 2011, 58, 1–37. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, R.; Su, Z. Linearized alternating direction method with adaptive penalty for low rank representation. In Proceedings of the International Conference on Neural Information Processing Systems, Granada, Spain, 12–17 December 2011; pp. 612–620. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015, Munich, Germany, 5–9 October 2015; pp. 234–241. [Google Scholar]
- Kawasaki, M.; Nakano, K.; Ohnishi, T.; Sekine, M.; Watanabe, E.; Oda, S.; Nakada, T.; Haneishi, H. Investigation into effect of thrombomodulin alfa for septic model rats based on microcirculation image analysis. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) IEEE, Montreal, ON, Canada, 20–24 July 2020. [Google Scholar]
- Long, J.; Shelhamer, E.; Darrell, T. Fully convolutional networks for semantic segmentation. In Proceedings of the 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015. [Google Scholar]
- Frangi, A.F.; Niessen, W.J.; Vincken, K.L.; Viergever, M.A. Multiscale vessel enhancement filtering. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI’98, Cambridge, MA, USA, 11–13 October 1998; pp. 130–137. [Google Scholar]
- Zhang, T.Y.; Suen, C.Y. A fast parallel algorithm for thinning digital patterns. Commu. ACM 1984, 27, 236–239. [Google Scholar] [CrossRef]
- Faber, D.J.; Aalders, M.C.G.; Mik, E.G.; Hooper, B.A.; van Gemert, M.J.C.; van Leeuwen, T.G. Oxygen saturation-dependent absorption and scattering of blood. Phys. Rev. Lett. 2004, 93, 028102-1–028102-4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, T.; Wu, X.; Wang, W.; Li, H.; Bradley, J.; Peberdy, M.A.; Ornato, J.P.; Tang, W. Micro- and macrocirculatory changes during sepsis and septic shock in a rat model. Shock 2018, 49, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Wellek, S. Testing Statistical Hypotheses of Equivalence and Noninferiority, 2nd ed.; Chapman and Hall/CRC: London, UK, 2010. [Google Scholar]







| Property | Specification |
|---|---|
| Breed | Wistar |
| Age | 12 weeks |
| Gender | Male |
| Body weight | 240–290 g |
| Amount of rats | 15 |
| Number of rats in each group | 5 |
| Group names | (1) Sham; |
| (2) CLP; | |
| (3) CLP+TM |
| Property | Specification |
|---|---|
| Image size (width×height) | 1384 × 1032 pixel |
| No. of images | 36:9:5 |
| (training:verif.:prediction) | |
| No. of channels in input image | 4 (RGB + gray) |
| No. of channels in output image | 1 (binary) |
| Patch size | 256 × 256 pixel |
| Batch size | 16 |
| Loss function | Cross-entropy+Dice |
| Optimizer | Adam |
| Learning rate | 0.001 |
| No. of iterations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Kawasaki, M.; Nakano, K.; Ohnishi, T.; Watanabe, E.; Oda, S.; Nakada, T.-A.; Haneishi, H. Acquisition and Analysis of Microcirculation Image in Septic Model Rats. Sensors 2022, 22, 8471. https://doi.org/10.3390/s22218471
Ye C, Kawasaki M, Nakano K, Ohnishi T, Watanabe E, Oda S, Nakada T-A, Haneishi H. Acquisition and Analysis of Microcirculation Image in Septic Model Rats. Sensors. 2022; 22(21):8471. https://doi.org/10.3390/s22218471
Chicago/Turabian StyleYe, Chen, Mami Kawasaki, Kazuya Nakano, Takashi Ohnishi, Eizo Watanabe, Shigeto Oda, Taka-Aki Nakada, and Hideaki Haneishi. 2022. "Acquisition and Analysis of Microcirculation Image in Septic Model Rats" Sensors 22, no. 21: 8471. https://doi.org/10.3390/s22218471
APA StyleYe, C., Kawasaki, M., Nakano, K., Ohnishi, T., Watanabe, E., Oda, S., Nakada, T.-A., & Haneishi, H. (2022). Acquisition and Analysis of Microcirculation Image in Septic Model Rats. Sensors, 22(21), 8471. https://doi.org/10.3390/s22218471







