Molecularly Imprinted Polymer-Modified Microneedle Sensor for the Detection of Imidacloprid Pesticides in Food Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
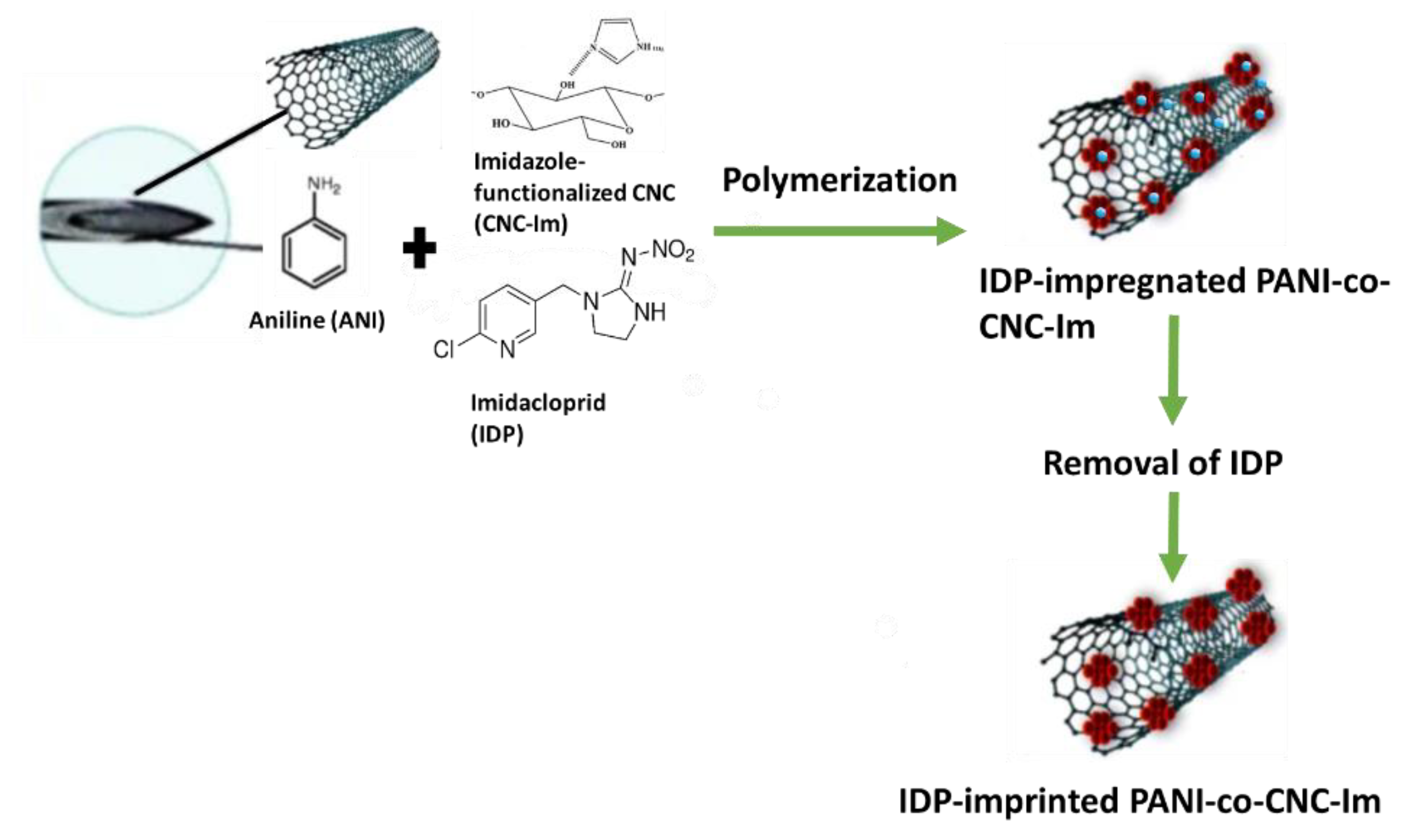
2.2. Fabrication of the IDP-Imprinted PANI-co-CNC-Im@CNT/CNC MN Sensors
2.3. Instrumentation
2.4. Electrochemical Measurements and Data Analysis
2.5. Honey Sample Electrochemical Analysis
2.6. Honey Sample Preparation for HPLC Analysis and Settings
3. Results and Discussion
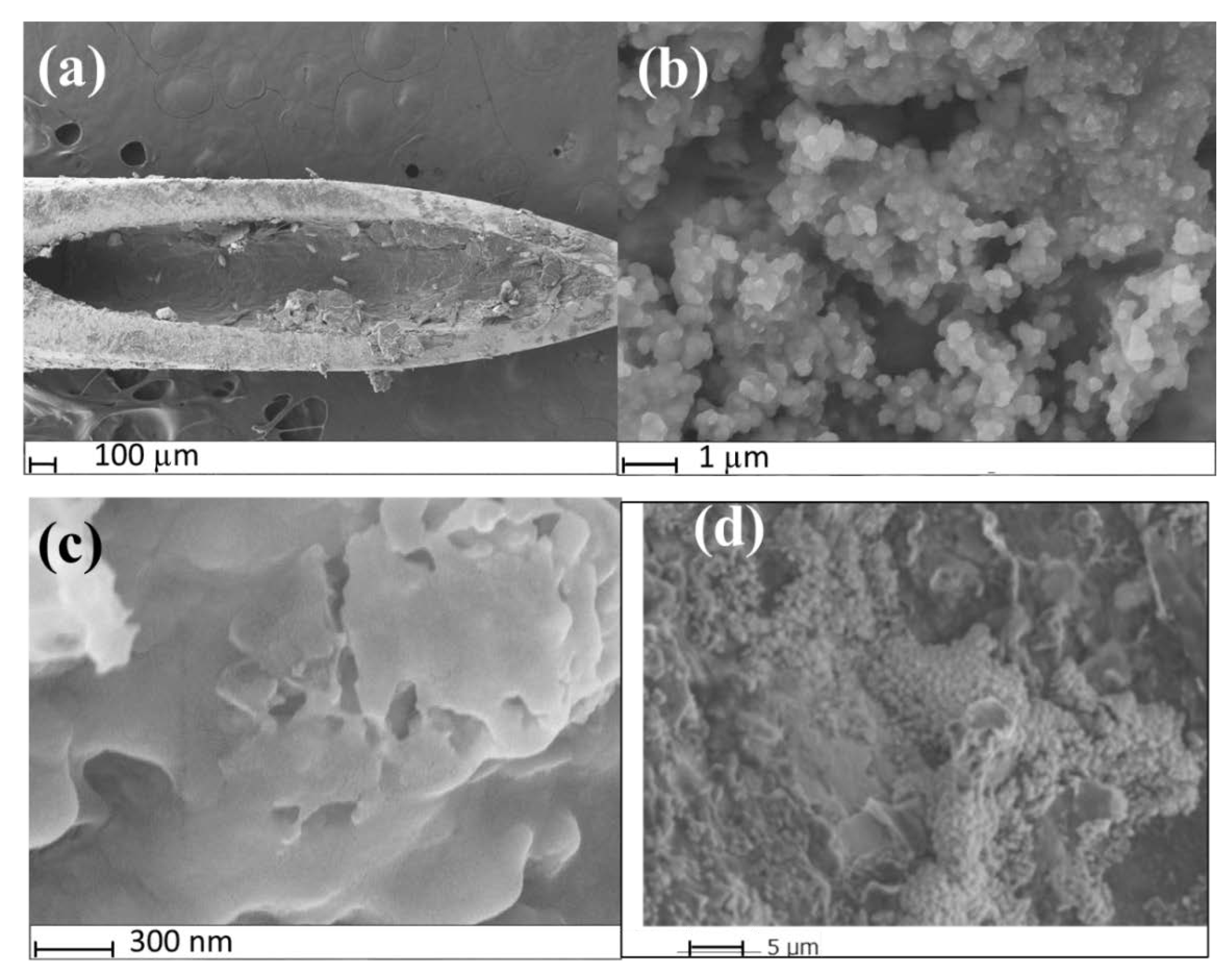
3.1. Surface Morphology
3.2. Electrochemical Characterization
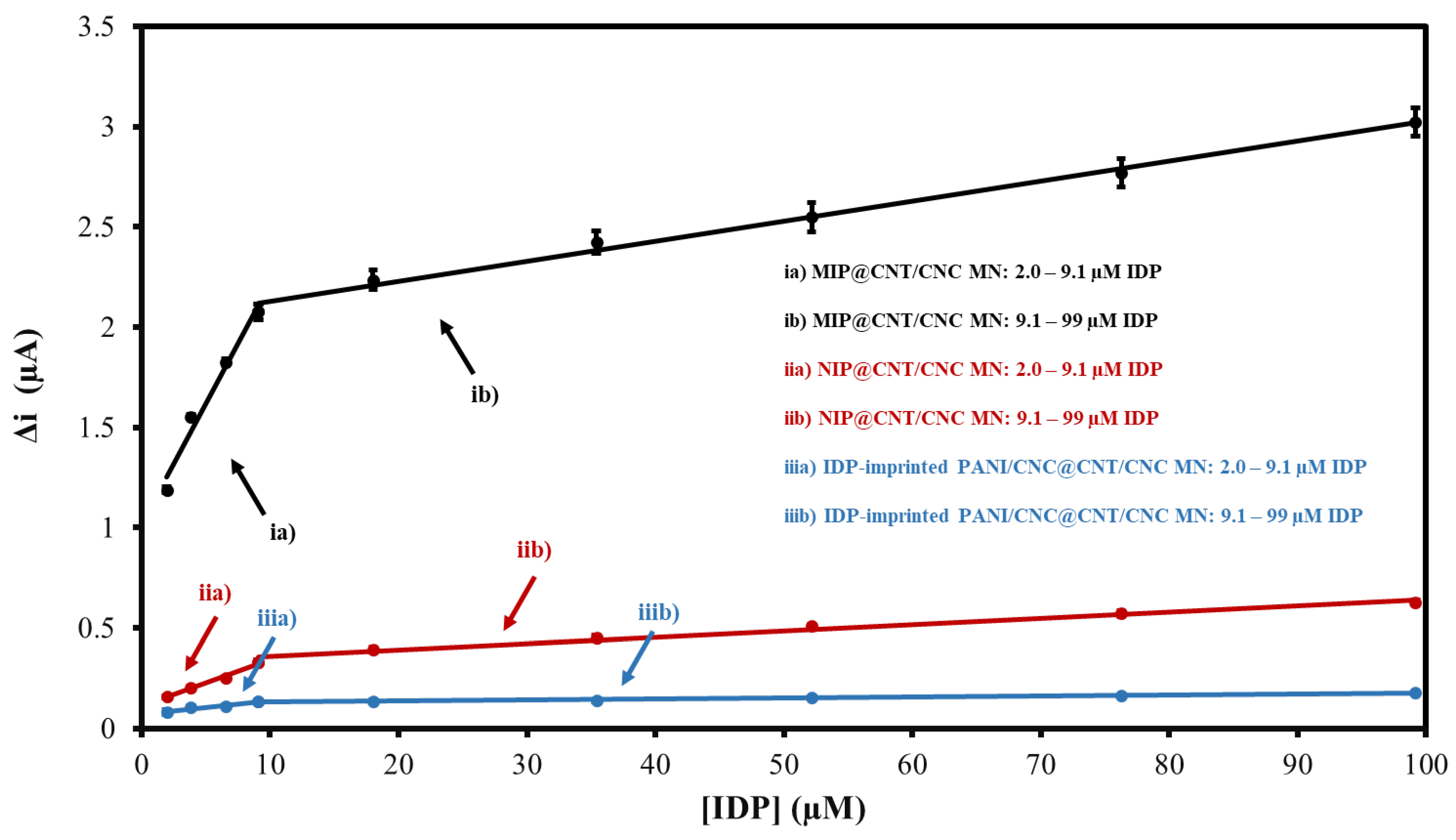
3.3. CV Performance Evaluation
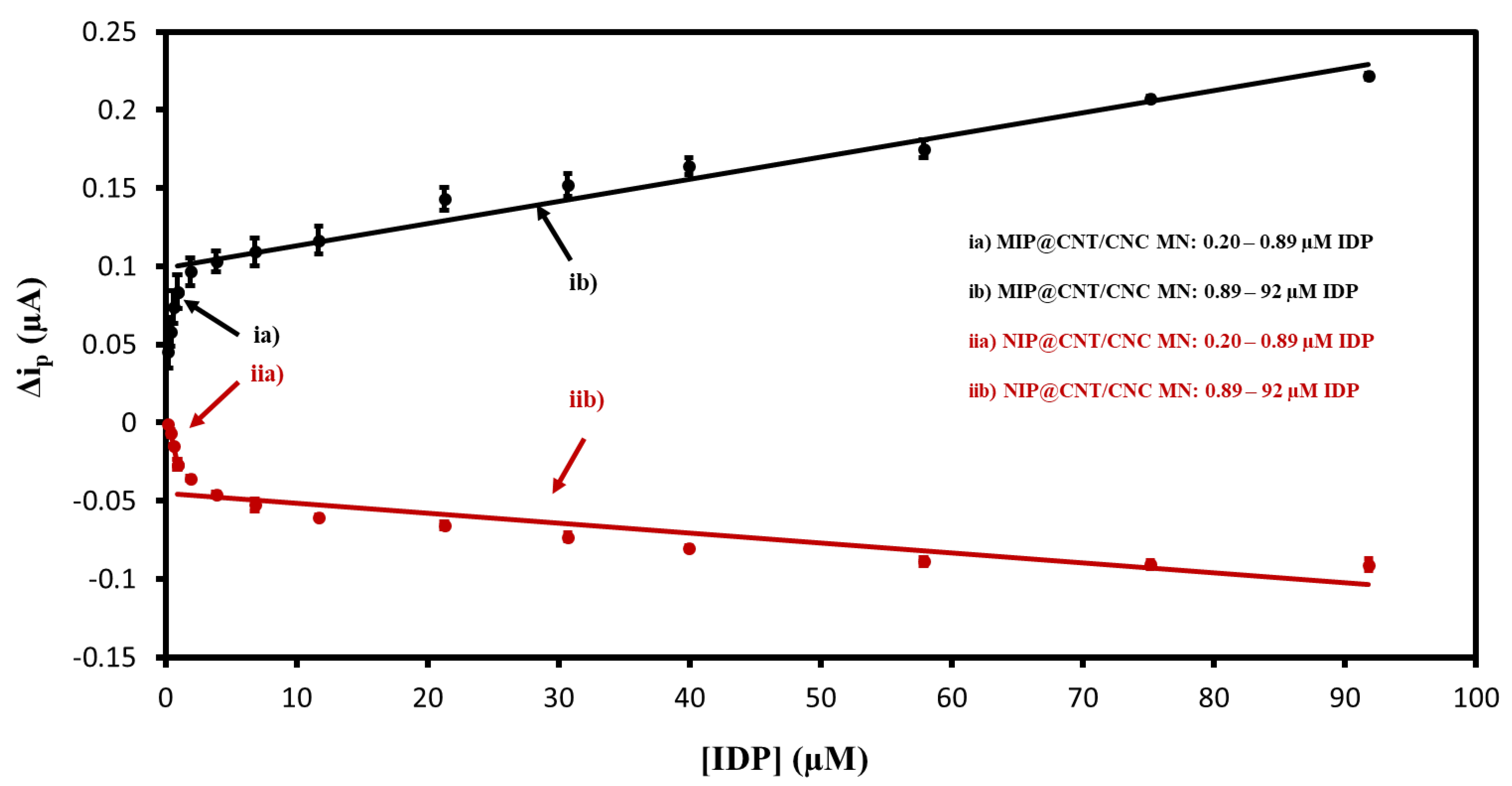
3.4. DPV Performance Evaluation and Comparison to Other Works
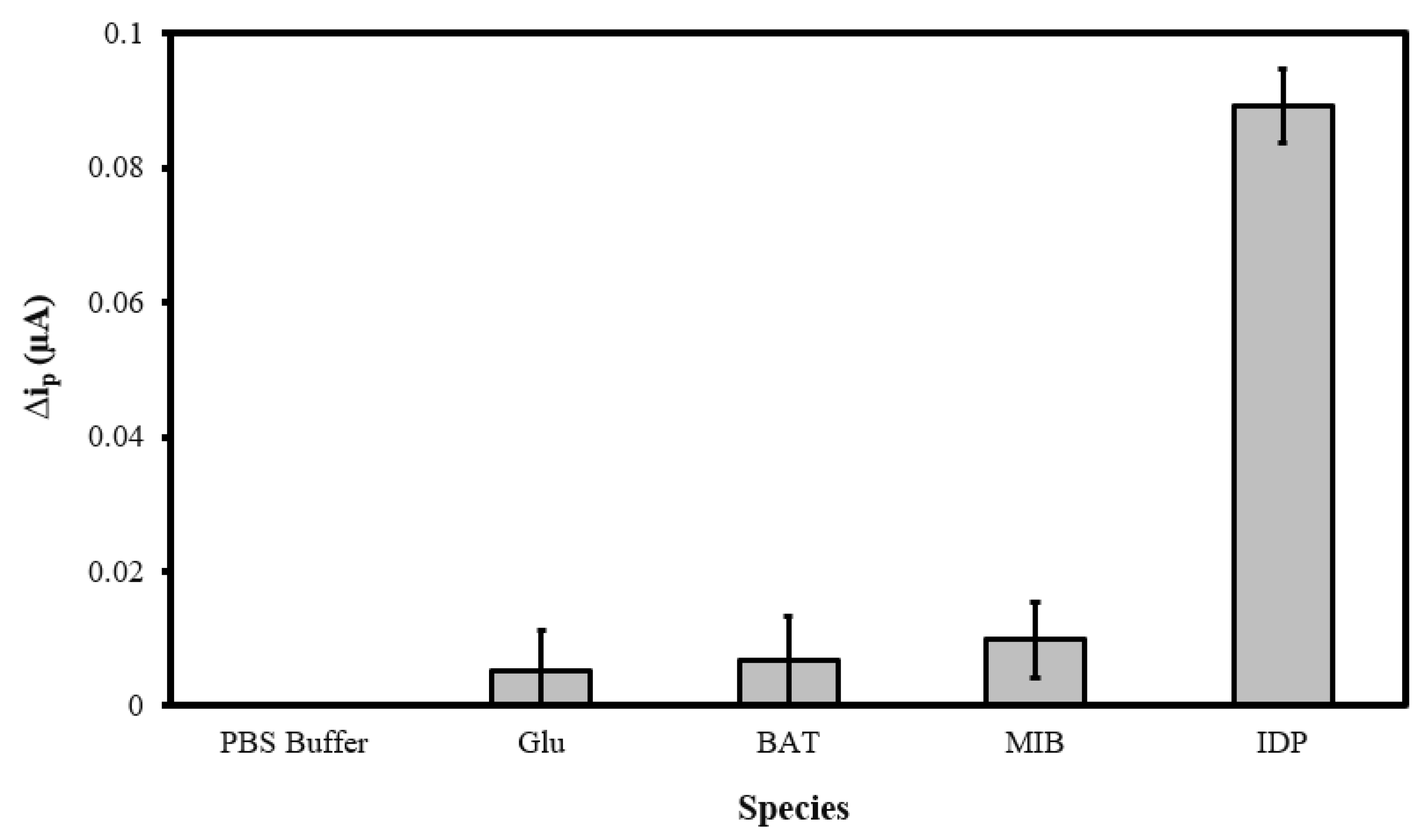
3.5. Sensor Selectivity Evaluation
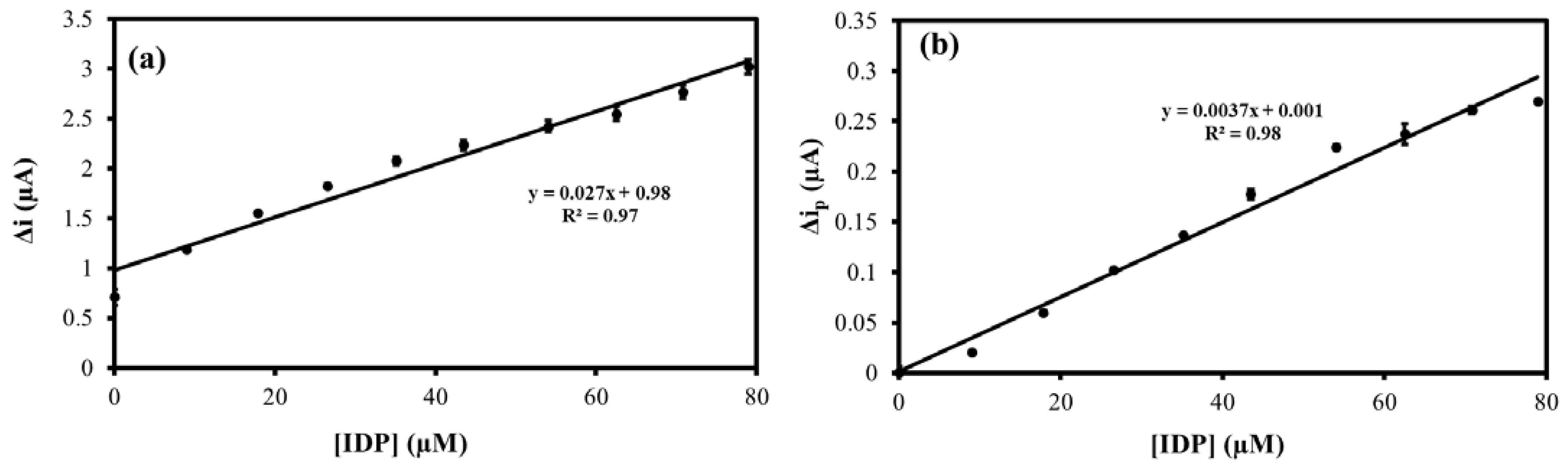
3.6. Honey Sample Analysis
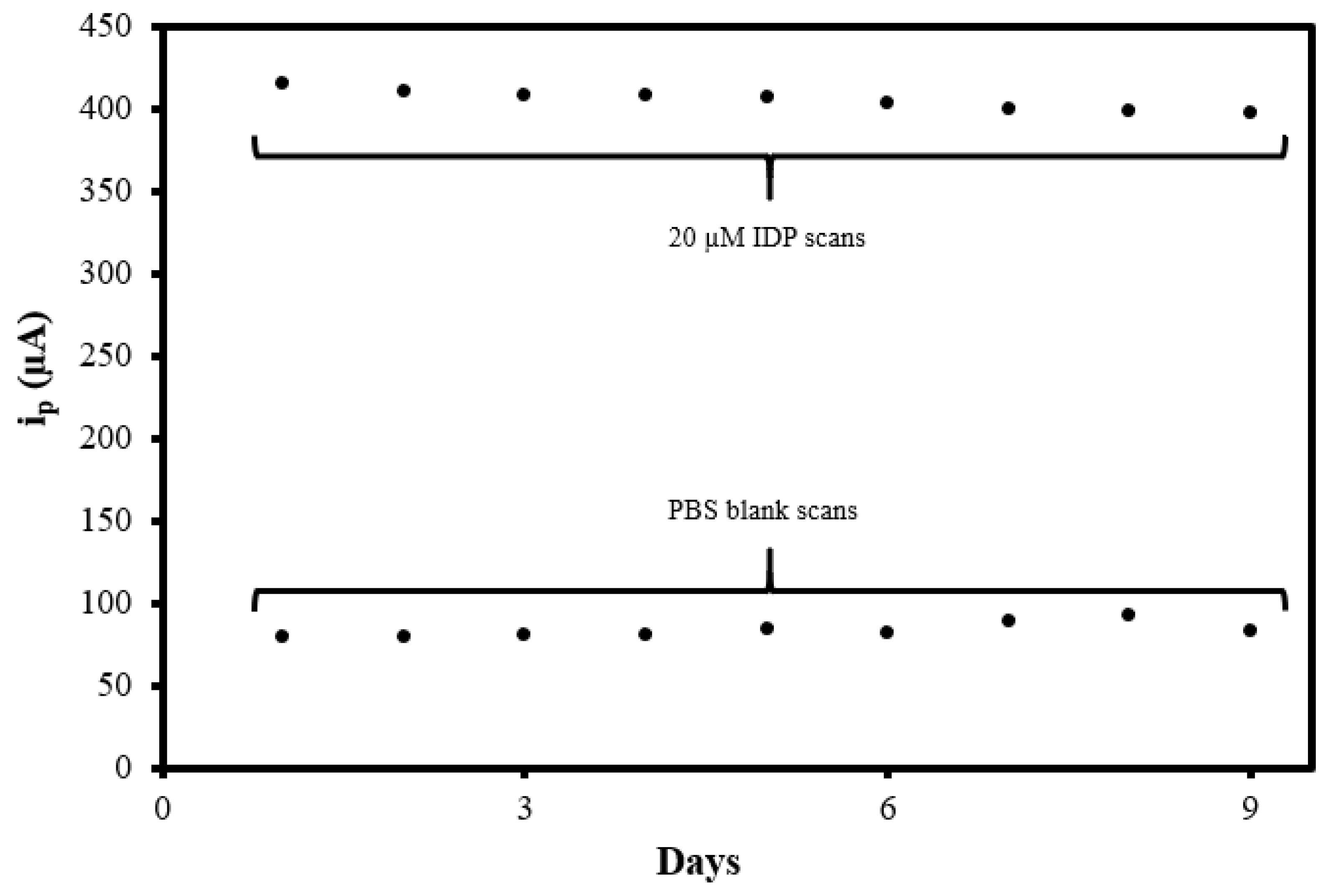
3.7. Sensor Reproducibility and Reusability Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- David, D.; George, I.A.; Peter, J.V. Toxicology of the Newer Neonicotinoid Insecticides: Imidacloprid Poisoning in a Human. Clin. Toxicol. 2007, 45, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Tišler, T.; Jemec, A.; Mozetič, B.; Trebše, P. Hazard Identification of Imidacloprid to Aquatic Environment. Chemosphere 2009, 76, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Bal, R.; Naziroğlu, M.; Türk, G.; Yilmaz, Ö.; Kuloğlu, T.; Etem, E.; Baydas, G. Insecticide Imidacloprid Induces Morphological and DNA Damage through Oxidative Toxicity on the Reproductive Organs of Developing Male Rats. Cell Biochem. Funct. 2012, 30, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Kugathas, S.; Audouze, K.; Ermler, S.; Orton, F.; Rosivatz, E.; Scholze, M.; Kortenkamp, A. Effects of Common Pesticides on Prostaglandin D2 (PGD2) Inhibition in SC5 Mouse Sertoli Cells, Evidence of Binding at the COX-2 Active Site, and Implications for Endocrine Disruption. Environ. Health Perspect. 2016, 124, 452–459. [Google Scholar] [CrossRef]
- Yuan, X.; Shen, J.; Zhang, X.; Tu, W.; Fu, Z.; Jin, Y. Imidacloprid Disrupts the Endocrine System by Interacting with Androgen Receptor in Male Mice. Sci. Total Environ. 2020, 708, 135163. [Google Scholar] [CrossRef]
- Caron-Beaudoin, É.; Viau, R.; Sanderson, J.T. Effects of Neonicotinoid Pesticides on Promoter-Specific Aromatase (CYP19) Expression in Hs578t Breast Cancer Cells and the Role of the VEGF Pathway. Environ. Health Perspect. 2018, 126, 047014. [Google Scholar] [CrossRef]
- Oya, N.; Ito, Y.; Ebara, T.; Kato, S.; Ueyama, J.; Aoi, A.; Nomasa, K.; Sato, H.; Matsuki, T.; Sugiura-Ogasawara, M.; et al. Cumulative Exposure Assessment of Neonicotinoids and an Investigation into Their Intake-Related Factors in Young Children in Japan. Sci. Total Environ. 2021, 750, 141630. [Google Scholar] [CrossRef]
- Naiel, M.A.E.; Shehata, A.M.; Negm, S.S.; Abd El-Hack, M.E.; Amer, M.S.; Khafaga, A.F.; Bin-Jumah, M.; Allam, A.A. The New Aspects of Using Some Safe Feed Additives on Alleviated Imidacloprid Toxicity in Farmed Fish: A Review. Rev. Aquac. 2020, 12, 2250–2267. [Google Scholar] [CrossRef]
- Butcherine, P.; Benkendorff, K.; Kelaher, B.; Barkla, B.J. The Risk of Neonicotinoid Exposure to Shrimp Aquaculture. Chemosphere 2019, 217, 329–348. [Google Scholar] [CrossRef]
- Baskaran, S.; Kookana, R.S.; Naidu, R. Determination of the Insecticide Imidacloprid in Water and Soil Using High-Performance Liquid Chromatography. J. Chromatogr. A 1997, 787, 271–275. [Google Scholar] [CrossRef]
- Tursen, J.; Yang, T.; Bai, L.; Li, D.; Tan, R. Determination of Imidacloprid and Acetamiprid in Bottled Juice by a New DLLME-HPLC. Environ. Sci. Pollut. Res. 2021, 28, 50867–50877. [Google Scholar] [CrossRef] [PubMed]
- Mozzaquatro, J.d.O.; César, I.A.; Pinheiro, A.E.B.; Caldas, E.D. Pesticide Residues Analysis in Passion Fruit and Its Processed Products by LC–MS/MS and GC–MS/MS: Method Validation, Processing Factors and Dietary Risk Assessment. Food Chem. 2022, 375, 131643. [Google Scholar] [CrossRef]
- Sun, Y.; Han, L.; Strasser, P. A Comparative Perspective of Electrochemical and Photochemical Approaches for Catalytic H2O2 Production. Chem. Soc. Rev. 2020, 49, 6605–6631. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, A.; Lamers, D.; Schoettl, J.; Mayer, C.; Jaeger, M. In-Field Detection Method for Imidacloprid by Surface Enhanced Raman Spectroscopy. Toxicol. Environ. Chem. 2022, 104, 36–54. [Google Scholar] [CrossRef]
- Babazadeh, S.; Moghaddam, P.A.; Keshipour, S.; Mollazade, K. Colorimetric Sensing of Imidacloprid in Cucumber Fruits Using a Graphene Quantum Dot/Au (III) Chemosensor. Sci. Rep. 2020, 10, 14327. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, S.; Pang, C.; Wang, M.; Ma, X.; Zhang, C. Highly Selective Fluorescence Probe for Imidacloprid Measurement Based on Fluorescence Resonance Energy Transfer. Microchem. J. 2022, 175, 107172. [Google Scholar] [CrossRef]
- Du, M.; Yang, Q.; Liu, W.; Ding, Y.; Chen, H.; Hua, X.; Wang, M. Development of Immunoassays with High Sensitivity for Detecting Imidacloprid in Environment and Agro-Products Using Phage-Borne Peptides. Sci. Total Environ. 2020, 723, 137909. [Google Scholar] [CrossRef]
- Bruzaca, E.E.S.; de Oliveira, R.C.; Duarte, M.S.S.; Sousa, C.P.; Morais, S.; Correia, A.N.; de Lima-Neto, P. Electrochemical Sensor Based on Multi-Walled Carbon Nanotubes for Imidacloprid Determination. Anal. Methods 2021, 13, 2124–2136. [Google Scholar] [CrossRef]
- Gee, S.J.; Hammock, B.D.; van Emon, J.M. Introduction. In Environmental Immunochemical Analysis Detection of Pesticides and Other Chemicals; Elsevier: Amsterdam, The Netherlands, 1996; pp. 1–6. [Google Scholar]
- Li, K.; Li, Q.X. Development of an Enzyme-Linked Immunosorbent Assay for the Insecticide Imidacloprid. J. Agric. Food Chem. 2000, 48, 3378–3382. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, H.T.; Xie, T.J.; Yang, X.; Dong, A.J.; Zhang, H.; Wang, J.; Wang, Z.Y. Molecularly Imprinted Polymer on Graphene Surface for Selective and Sensitive Electrochemical Sensing Imidacloprid. Sens. Actuators B Chem. 2017, 252, 991–1002. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, G.; Zhu, A.; Zhao, Z.; Ren, C.; Nie, L.; Kan, X. A Multiporous Electrochemical Sensor for Epinephrine Recognition and Detection Based on Molecularly Imprinted Polypyrrole. RSC Adv. 2012, 2, 7803. [Google Scholar] [CrossRef]
- Mugo, S.M.; Alberkant, J. Flexible Molecularly Imprinted Electrochemical Sensor for Cortisol Monitoring in Sweat. Anal. Bioanal. Chem. 2020, 412, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Idil, N.; Hedström, M.; Denizli, A.; Mattiasson, B. Whole Cell Based Microcontact Imprinted Capacitive Biosensor for the Detection of Escherichia Coli. Biosens. Bioelectron. 2017, 87, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.; Mugo, S.M. A MIP-Enabled Stainless-Steel Hypodermic Needle Sensor for Electrochemical Detection of Aflatoxin B1. Anal. Methods 2022, 14, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Selahle, S.K.; Mpupa, A.; Nomngongo, P.N. A Review of Extraction, Analytical, and Advanced Methods for the Determination of Neonicotinoid Insecticides in Environmental Water Matrices. Rev. Anal. Chem. 2021, 40, 187–203. [Google Scholar] [CrossRef]
- Peng, S.; Yang, S.; Zhang, X.; Jia, J.; Chen, Q.; Lian, Y.; Wang, A.; Zeng, B.; Yang, H.; Li, J.; et al. Analysis of Imidacloprid Residues in Mango, Cowpea and Water Samples Based on Portable Molecular Imprinting Sensors. PLoS ONE 2021, 16, e0257042. [Google Scholar] [CrossRef]
- Zidarič, T.; Finšgar, M.; Maver, U.; Maver, T. Artificial Biomimetic Electrochemical Assemblies. Biosensors 2022, 12, 44. [Google Scholar] [CrossRef]
- Yuan, Z.; Yang, Y.; Yang, Y.; Wang, G.; Huo, Y. Preparation and Electrochemical Properties of Carbon/PANI Composite Mesh Electrode Materials. J. Electron. Mater. 2022, 51, 2289–2297. [Google Scholar] [CrossRef]
- Oechsle, A.-L.; Lewis, L.; Hamad, W.Y.; Hatzikiriakos, S.G.; MacLachlan, M.J. CO2 -Switchable Cellulose Nanocrystal Hydrogels. Chem. Mater. 2018, 30, 376–385. [Google Scholar] [CrossRef]
- Regasa, M.B.; Soreta, T.R.; Femi, O.E.; Ramamurthy, P.C.; Subbiahraj, S. Novel Multifunctional Molecular Recognition Elements Based on Molecularly Imprinted Poly (Aniline-Co-Itaconic Acid) Composite Thin Film for Melamine Electrochemical Detection. Sens. Biosensing Res. 2020, 27, 100318. [Google Scholar] [CrossRef]
- Nandeshwar, R.; Malik, M.; D’Costa, M.; Mangat, M.; Bhaganagare, M.A.; Date, M.P.; Tallur, S. Molecular Imprinting with Polyaniline on ENIG Finish PCB Electrodes for Electrochemical Detection of Melamine. IEEE Sens. J. 2022, 22, 1898–1904. [Google Scholar] [CrossRef]
- Dhanjai; Mugo, S.M.; Lu, W. Modified Stainless Steel Microneedle Electrode for Polyphenolics Detection. Anal. Bioanal. Chem. 2020, 412, 7063–7072. [Google Scholar] [CrossRef] [PubMed]
- Habibi, B.; Jahanbakhshi, M. A Novel Nonenzymatic Hydrogen Peroxide Sensor Based on the Synthesized Mesoporous Carbon and Silver Nanoparticles Nanohybrid. Sens. Actuators B Chem. 2014, 203, 919–925. [Google Scholar] [CrossRef]
- Hou, J.; Xie, W.; Hong, D.; Zhang, W.; Li, F.; Qian, Y.; Han, C. Simultaneous Determination of Ten Neonicotinoid Insecticides and Two Metabolites in Honey and Royal-Jelly by Solid−phase Extraction and Liquid Chromatography−tandem Mass Spectrometry. Food Chem. 2019, 270, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, I.; Ławniczak, P.; Pogorzelec-Glaser, K.; Łapiński, A.; Pankiewicz, R.; Tritt-Goc, J. Cellulose Microfibers Surface Treated with Imidazole as New Proton Conductors. Mater. Chem. Phys. 2020, 239, 122056. [Google Scholar] [CrossRef]
- Ibrahim, K.A. Synthesis and Characterization of Polyaniline and Poly(Aniline-Co-o-Nitroaniline) Using Vibrational Spectroscopy. Arab. J. Chem. 2017, 10, S2668–S2674. [Google Scholar] [CrossRef]
- Ajeel, K.I.; Kareem, Q.S. Synthesis and Characteristics of Polyaniline (PANI) Filled by Graphene (PANI/GR) Nano-Films. J. Phys. Conf. Ser. 2019, 1234, 012020. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface Modification of Cellulose Nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef]
- Kong, B.; Selomulya, C.; Zheng, G.; Zhao, D. New Faces of Porous Prussian Blue: Interfacial Assembly of Integrated Hetero-Structures for Sensing Applications. Chem. Soc. Rev. 2015, 44, 7997–8018. [Google Scholar] [CrossRef]
- El-Akaad, S.; Mohamed, M.A.; Abdelwahab, N.S.; Abdelaleem, E.A.; de Saeger, S.; Beloglazova, N. Capacitive Sensor Based on Molecularly Imprinted Polymers for Detection of the Insecticide Imidacloprid in Water. Sci. Rep. 2020, 10, 14479. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Lei, W.; Hao, Q.; Xia, X.; Zhang, H.; Li, J.; Li, Q.; Cong, R. Facile Synthesis of Nitrogen-Doped Graphene Derived from Graphene Oxide and Vitamin B3 as High-Performance Sensor for Imidacloprid Determination. Electrochim. Acta 2016, 212, 784–790. [Google Scholar] [CrossRef]
- Liu, L.; Guo, J.; Ding, L. Polyaniline Nanowire Arrays Deposited on Porous Carbon Derived from Raffia for Electrochemical Detection of Imidacloprid. Electroanalysis 2021, 33, 2048–2052. [Google Scholar] [CrossRef]
- Xie, W.; Ju, Y.; Zhang, J.; Yang, Y.; Zeng, Y.; Wang, H.; Li, L. Highly Sensitive and Specific Determination of Imidacloprid Pesticide by a Novel Fe3O4@SiO2@MIPIL Fluorescent Sensor. Anal. Chim. Acta 2022, 1195, 339449. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.C.; Scott-Dupree, C.D.; Drexler, D.M. Honey Bees, Neonicotinoids and Bee Incident Reports: The Canadian Situation. Pest Manag. Sci. 2014, 70, 779–783. [Google Scholar] [CrossRef]


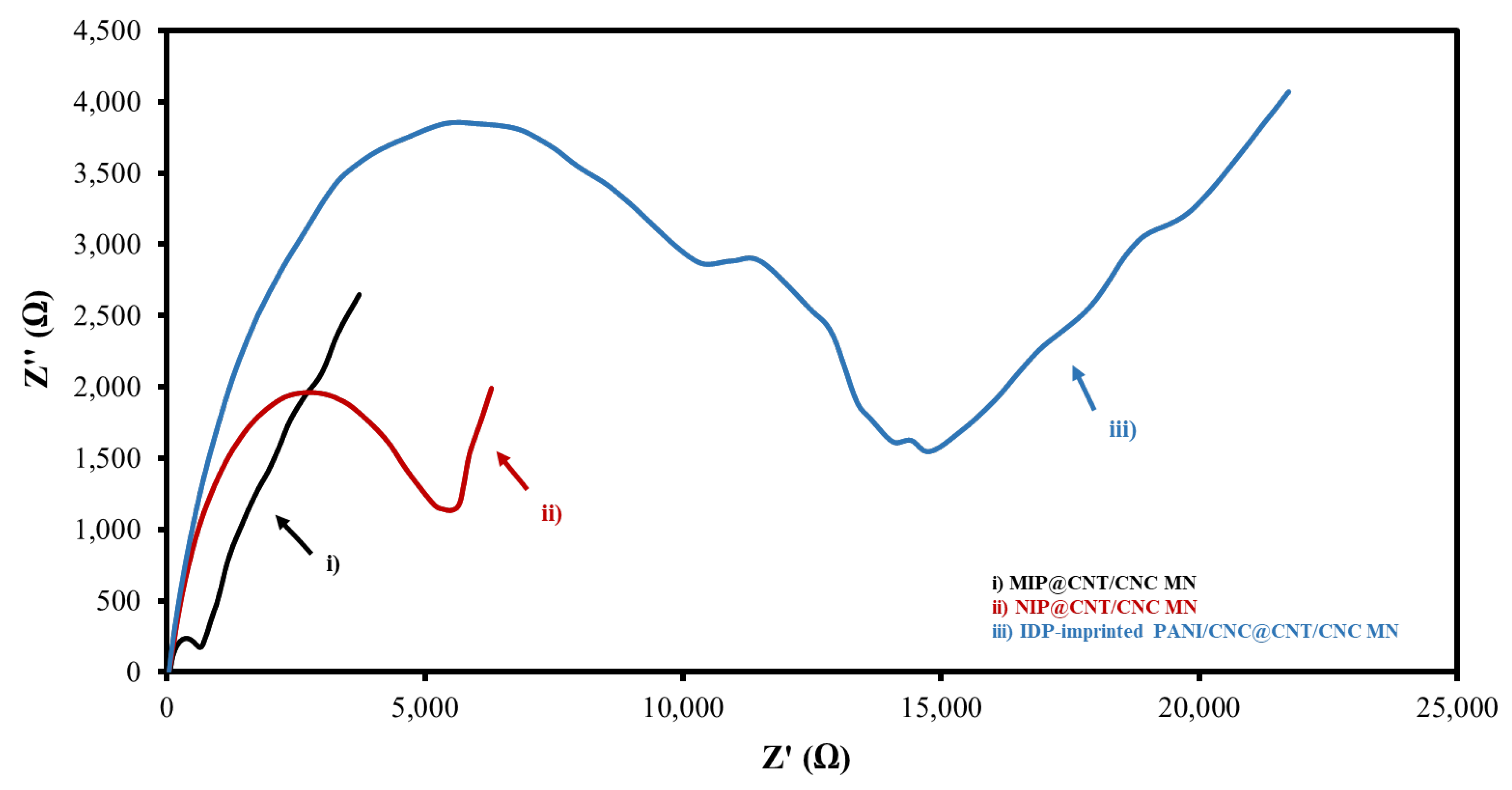
| Sensor Type | Electroactive Surface Area (cm2) | Rct (kΩ) |
|---|---|---|
| MIP@CNT/CNC MN | 0.049 | 0.42 |
| NIP@CNT/CNC MN | 0.034 | 4.5 |
| IDP-imprinted PANI/CNC@CNT/CNC MN | 0.022 | 9.5 |
| Sensor Type | 2.0–9.1 µM CV Linear Equation | 9.1–99 µM CV Linear Equation |
|---|---|---|
| MIP@CNT/CNC MN | y = 0.12x + 1.02 R2 = 0.97 | y = 0.0100x + 2.03 R2 = 0.99 |
| NIP@CNT/CNC MN | y = 0.023x + 0.11 R2 = 0.99 | y = 0.0032x + 0.33 R2 = 0.98 |
| IDP-imprinted PANI/CNC@CNT/CNC MN | y = 0.007x + 0.072 R2 = 0.94 | y = 0.00051x + 0.127 R2 = 0.96 |
| Sensor Type | 0.20–0.89 µM DPV Linear Equation | 0.89–92 µM DPV Linear Equation |
|---|---|---|
| MIP@CNT/CNC MN | y = 0.056x + 0.036 R2 = 0.97 | y = 0.00142x + 0.099 R2 = 0.96 |
| NIP@CNT/CNC MN | y = −0.038x + 0.007 R2 = 0.99 | y = −0.0006x−0.045 R2 = 0.81 |
| Sensor Name | Detection Range (µM) | LOD (µM) | Reference |
|---|---|---|---|
| PoPD at reduced graphene oxide (RGO) modified electrode | 0.75–70 | 0.40 | [41] |
| MAA-EGDMA MIP sensor | 5–100 | 4.61 | [42] |
| Nitrogen-doped graphene (NGE) | 5–100 | 0.55 | [43] |
| fMWCNT-Nafion®0.5%/GCE | 0.2–1.77 | 0.0374 | [19] |
| RPC@PANI/GCE | 0.400–274 | 0.117 | [44] |
| Fe3O4@SiO2@MIPIL fluorescent sensor | 0.001–0.01 | 0.0003 | [45] |
| IDP-imprinted PANI-co-CNC-Im@CNT/CNC MN sensor | 2.0–99 (CV) 0.20–92 (DPV) | 0.35 (CV) 0.06 (DPV) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mugo, S.M.; Lu, W.; Robertson, S.V. Molecularly Imprinted Polymer-Modified Microneedle Sensor for the Detection of Imidacloprid Pesticides in Food Samples. Sensors 2022, 22, 8492. https://doi.org/10.3390/s22218492
Mugo SM, Lu W, Robertson SV. Molecularly Imprinted Polymer-Modified Microneedle Sensor for the Detection of Imidacloprid Pesticides in Food Samples. Sensors. 2022; 22(21):8492. https://doi.org/10.3390/s22218492
Chicago/Turabian StyleMugo, Samuel M., Weihao Lu, and Scott V. Robertson. 2022. "Molecularly Imprinted Polymer-Modified Microneedle Sensor for the Detection of Imidacloprid Pesticides in Food Samples" Sensors 22, no. 21: 8492. https://doi.org/10.3390/s22218492
APA StyleMugo, S. M., Lu, W., & Robertson, S. V. (2022). Molecularly Imprinted Polymer-Modified Microneedle Sensor for the Detection of Imidacloprid Pesticides in Food Samples. Sensors, 22(21), 8492. https://doi.org/10.3390/s22218492









