Assessing Corneal Endothelial Damage Using Terahertz Time-Domain Spectroscopy and Support Vector Machines
Abstract
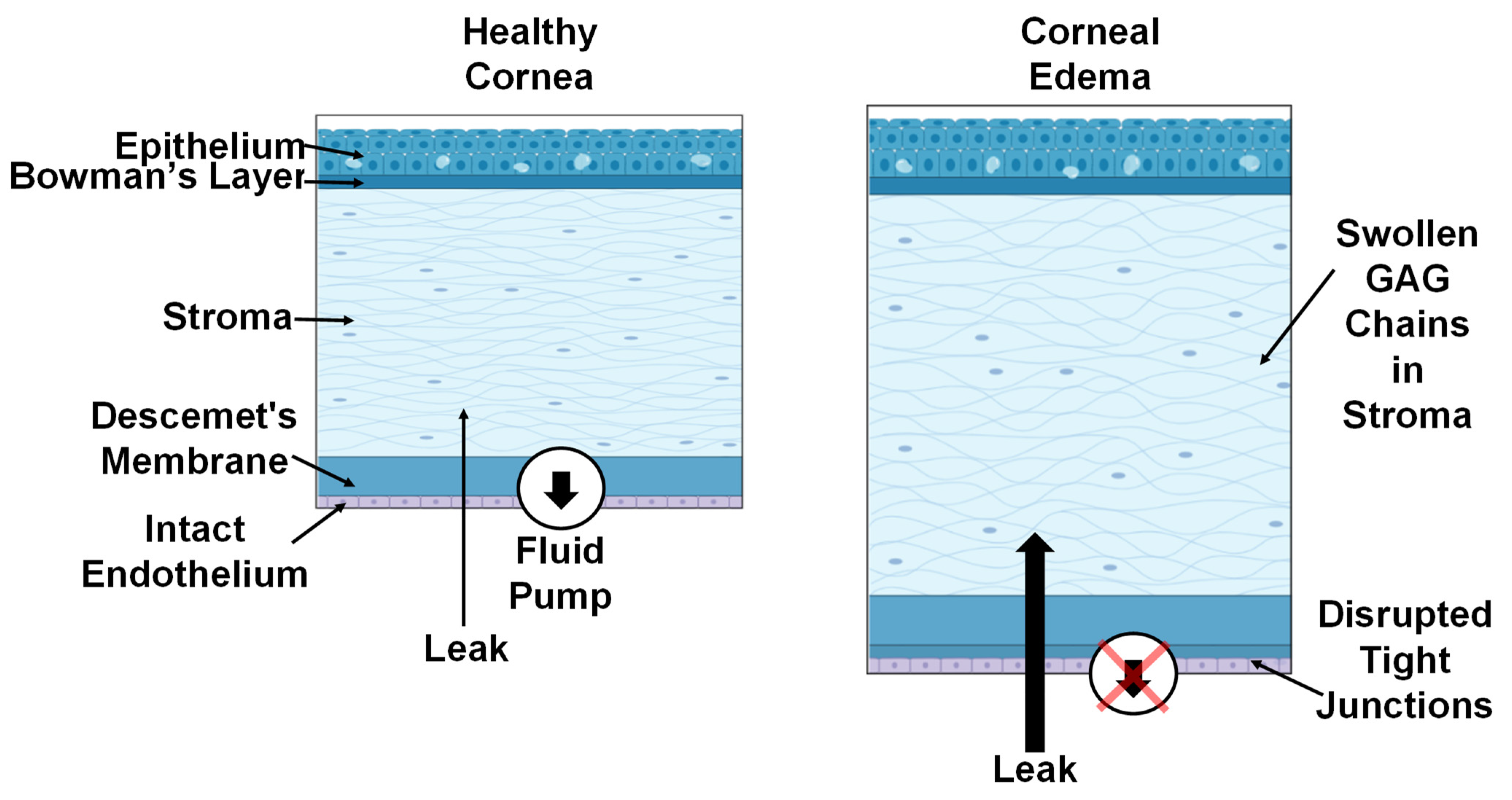
:1. Introduction
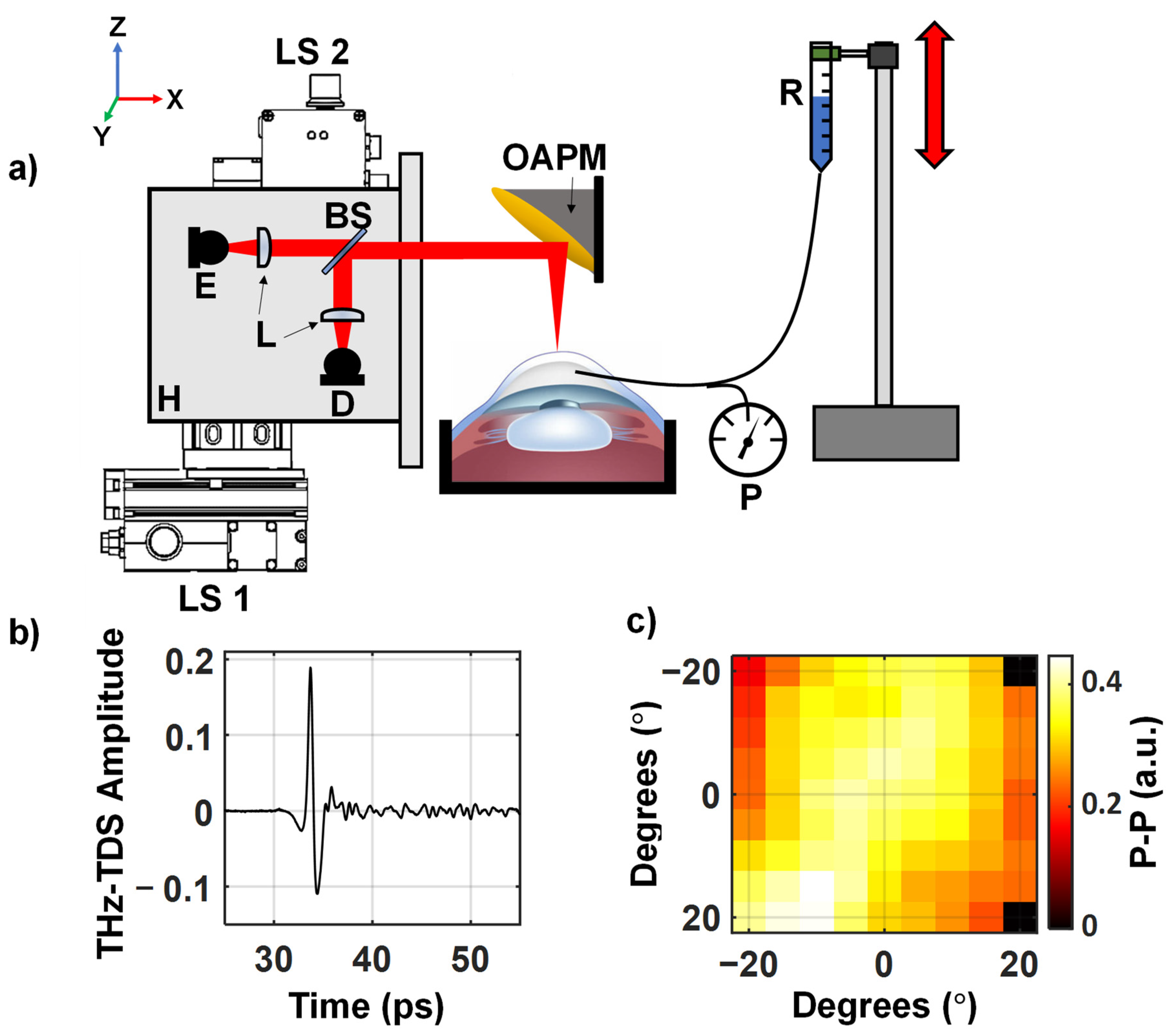
2. Materials and Methods
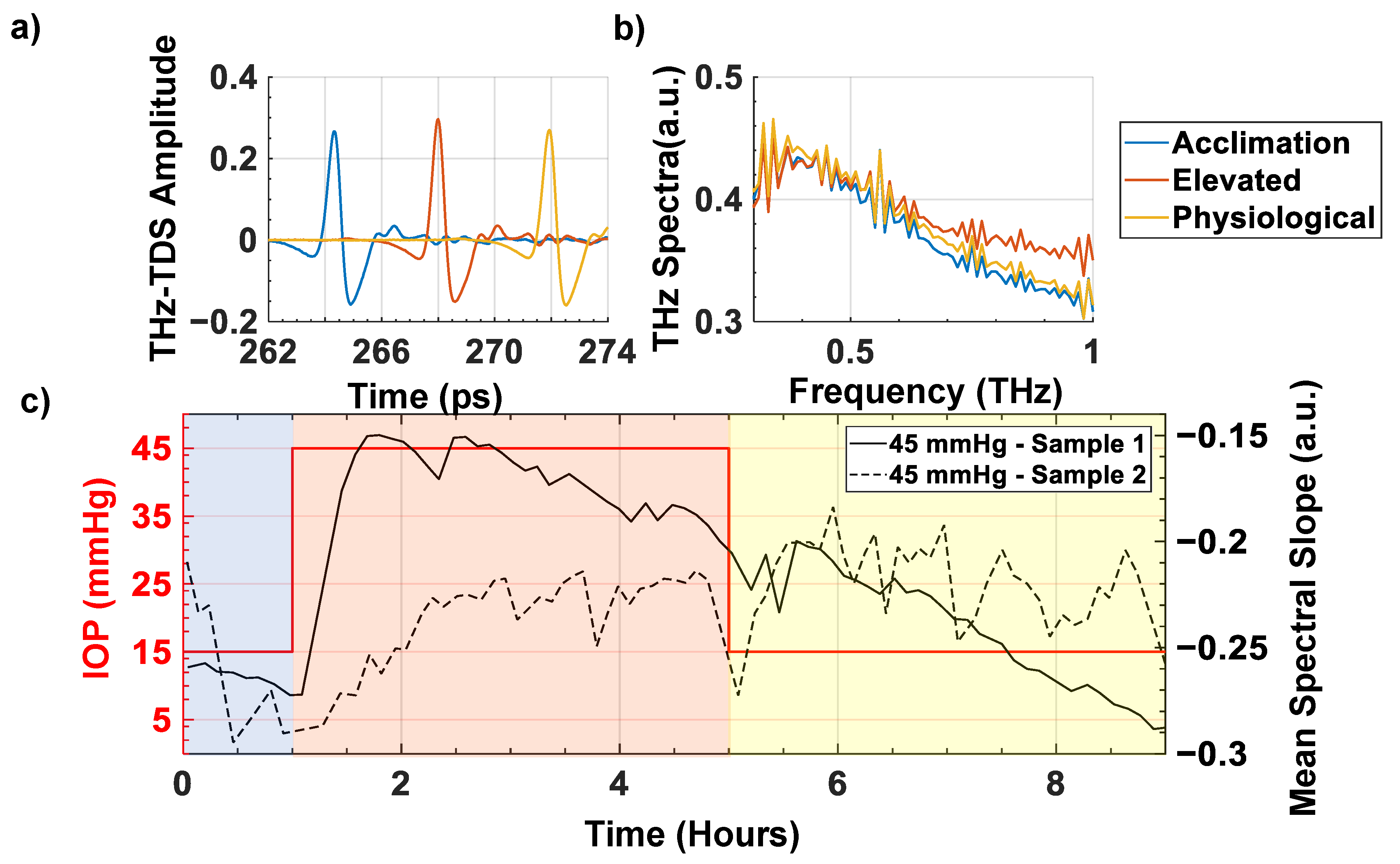
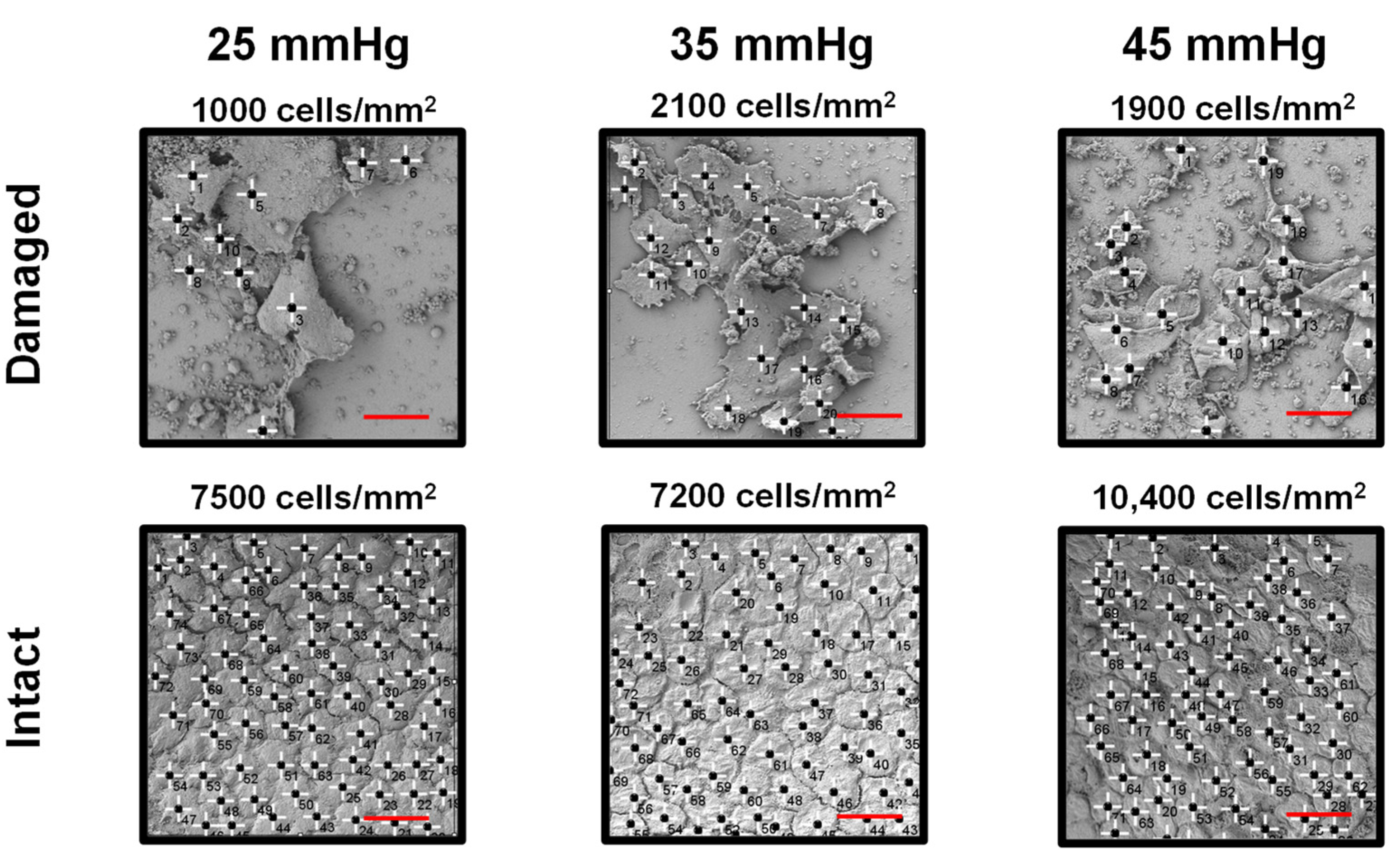
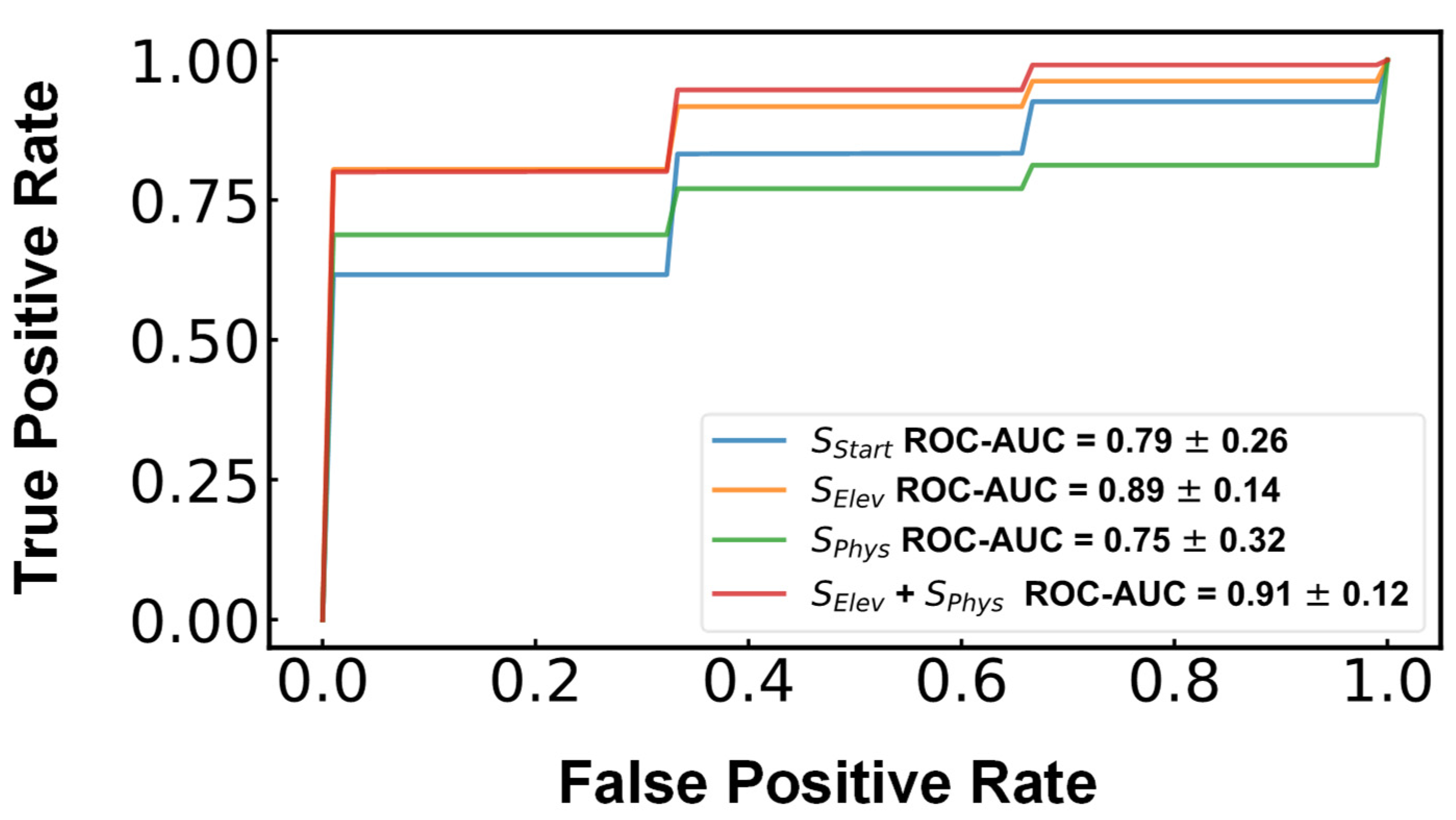
3. Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ytteborg, J.; Dohlman, C.H. Corneal edema and intraocular pressure. II. Clinical results. Arch. Ophthalmol. 1965, 74, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Zucker, B.B. Hydration and Transparency of Corneal Stroma. Arch. Ophthalmol. 1966, 75, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Pinsky, P.M. Mechanisms of self-organization for the collagen fibril lattice in the human cornea. J. R. Soc. Interface 2013, 10, 20130512. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Petsche, S.J.; Pinsky, P.M. A structural model for the in vivo human cornea including collagen-swelling interaction. J. R. Soc. Interface 2015, 12, 20150241. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, S. Cell Signaling in Regulation of the Barrier Integrity of the Corneal Endothelium. Exp. Eye Res. 2011, 95, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Kim, E.; Bonanno, J.A. Fluid transport by the cornea endothelium is dependent on buffering lactic acid efflux. Am. J. Physiol. Cell Physiol. 2016, 311, C116–C126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamis, A.P.; Filatov, V.; Tripathi, B.J.; Tripathi, R.C. Fuchs’ endothelial dystrophy of the cornea. Surv. Ophthalmol. 1993, 38, 149–168. [Google Scholar] [CrossRef]
- Lefebvre, V.; Sowka, J.W.; Frauens, B.J. The clinical spectrum between posterior polymorphous dystrophy and iridocorneal endothelial syndromes. Optometry 2009, 80, 431–436. [Google Scholar] [CrossRef]
- Bigar, F.; Witmer, R. Corneal Endothelial Changes in Primary Acute Angle-closure Glaucoma. Ophthalmology 1982, 89, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.P.Y.; Broadway, D.C.; Khawaja, A.P.; Yip, J.L.Y.; Garway-Heath, D.F.; Burr, J.M.; Luben, R.; Hayat, S.; Dalzell, N.; Khaw, K.-T.; et al. Glaucoma and intraocular pressure in EPIC-Norfolk Eye Study: Cross sectional study. BMJ 2017, 358, j3889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagnon, M.M.; Boisjoly, H.M.; Brunette, I.; Charest, M.; Amyot, M. Corneal endothelial cell density in glaucoma. Cornea 1997, 16, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, A.A.; D'Oria, F.; Alio, J.L. Surgery for glaucoma in modern corneal graft procedures. Surv. Ophthalmol. 2021, 66, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.C. Proliferative capacity of corneal endothelial cells. Corneal Endothel. Health Dis. 2012, 95, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sie, N.M.; Yam, G.H.-F.; Soh, Y.Q.; Lovatt, M.; Dhaliwal, D.; Kocaba, V.; Mehta, J.S. Regenerative capacity of the corneal transition zone for endothelial cell therapy. Stem Cell Res. Ther. 2020, 11, 523. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, D.K.; Bahn, C.F.; Lillie, J.H.; Meyer, R.F.; Martonyi, C.L. Evidence for corneal endothelial cell hypertrophy during postnatal growth of the cat cornea. Investig. Ophthalmol. Vis. Sci. 1983, 24, 247–250. [Google Scholar]
- Feizi, S. Corneal endothelial cell dysfunction: Etiologies and management. Ther. Adv. Ophthalmol. 2018, 10, 2515841418815802. [Google Scholar] [CrossRef] [Green Version]
- Taylor, Z.D.; Garritano, J.; Sung, S.; Bajwa, N.; Bennett, D.B.; Nowroozi, B.; Tewari, P.; Sayre, J.W.; Hubschman, J.-P.; Deng, S.X.; et al. THz and mm-Wave Sensing of Corneal Tissue Water Content: In Vivo Sensing and Imaging Results. IEEE Trans. Terahertz Sci. Technol. 2015, 5, 184–196. [Google Scholar] [CrossRef] [Green Version]
- Ammar, D.A.; Lei, T.C.; Kahook, M.Y.; Masihzadeh, O. Imaging the Intact Mouse Cornea Using Coherent Anti-Stokes Raman scattering (CARS). Investig. Ophthalmol. Vis. Sci. 2013, 54, 5258–5265. [Google Scholar] [CrossRef] [Green Version]
- Shao, P.; Seiler, T.G.; Eltony, A.M.; Ramier, A.; Kwok, S.J.J.; Scarcelli, G.; Ii, R.P.; Yun, S.-H. Effects of Corneal Hydration on Brillouin Microscopy In Vivo. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3020–3027. [Google Scholar] [CrossRef] [Green Version]
- El-Shenawee, M.; Vohra, N.; Bowman, T.; Bailey, K. Cancer detection in excised breast tumors using terahertz imaging and spectroscopy. Biomed. Spectrosc. Imaging 2019, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Patel, R.; Neel, V.; Giles, R.H.; Yaroslavsky, A.N. Multimodal Optical and Terahertz Biopsy of Nonmelanoma Cancers Skin. In Proceedings of the Biophotonics Congress: Biomedical Optics Congress 2018 (Microscopy/Translational/Brain/OTS), Hollywood, FL, USA, 3–6 April 2018; p. MF4A.4. [Google Scholar]
- George, D.K.; Chen, J.Y.; He, Y.; Knab, J.R.; Markelz, A.G. Functional-State Dependence of Picosecond Protein Dynamics. J. Phys. Chem. B 2021, 125, 11134–11140. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Cardoso, G.G.; Amador-Medina, L.F.; Gutierrez-Torres, G.; Reyes-Reyes, E.S.; Benavides Martínez, C.A.; Cardona Espinoza, C.; Arce Cruz, J.; Salas-Gutierrez, I.; Murillo-Ortíz, B.O.; Castro-Camus, E. Terahertz imaging demonstrates its diagnostic potential and reveals a relationship between cutaneous dehydration and neuropathy for diabetic foot syndrome patients. Sci. Rep. 2022, 12, 3110. [Google Scholar] [CrossRef] [PubMed]
- Harris, Z.B.; Arbab, M.H. Terahertz PHASR Scanner with 2 kHz, 100 picosecond Time-Domain Trace Acquisition Rate and an Extended Field-of-View Based on a Heliostat Design. IEEE Trans. Terahertz Sci. Technol. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Harris, Z.B.; Khani, M.E.; Arbab, M.H. Terahertz Portable Handheld Spectral Reflection (PHASR) Scanner. IEEE Access 2020, 8, 228024–228031. [Google Scholar] [CrossRef]
- Harris, Z.B.; Katletz, S.; Khani, M.E.; Virk, A.; Arbab, M.H. Design and characterization of telecentric f-θ scanning lenses for broadband terahertz frequency systems. AIP Adv. 2020, 10, 125313. [Google Scholar] [CrossRef]
- Stantchev, R.I.; Li, K.; Pickwell-MacPherson, E. Rapid Imaging of Pulsed Terahertz Radiation with Spatial Light Modulators and Neural Networks. ACS Photonics 2021, 8, 3150–3155. [Google Scholar] [CrossRef]
- Arbab, M.H.; Winebrenner, D.P.; Dickey, T.C.; Klein, M.B.; Chen, A.; Mourad, P.D. A Noninvasive Terahertz Assessment of 2nd and 3rd Degree Burn Wounds. In Proceedings of the Conference on Lasers and Electro-Optics 2012, San Jose, CA, USA, 6–11 May 2012; p. CTu3B.3. [Google Scholar]
- Osman, O.B.; Jack Tan, T.; Henry, S.; Warsen, A.; Farr, N.; McClintic, A.M.; Wang, Y.-N.; Arbabi, S.; Arbab, M.H. Differentiation of burn wounds in an in vivo porcine model using terahertz spectroscopy. Biomed. Opt. Express 2020, 11, 6528–6535. [Google Scholar] [CrossRef]
- Khani, M.E.; Osman, O.B.; Harris, Z.B.; Chen, A.; Zhou, J.W.; Singer, A.J.; Arbab, M.H. Accurate and early prediction of the wound healing outcome of burn injuries using the wavelet Shannon entropy of terahertz time-domain waveforms. J. Biomed. Opt. 2022, 27, 116001. [Google Scholar] [CrossRef]
- Osman, O.B.; Harris, Z.B.; Zhou, J.W.; Khani, M.E.; Singer, A.J.; Arbab, M.H. In Vivo Assessment and Monitoring of Burn Wounds Using a Handheld Terahertz Hyperspectral Scanner. Adv. Photonics Res. 2022, 3, 2100095. [Google Scholar] [CrossRef]
- Khani, M.E.; Harris, Z.B.; Osman, O.B.; Zhou, J.W.; Chen, A.; Singer, A.J.; Arbab, M.H. Supervised machine learning for automatic classification of in vivo scald and contact burn injuries using the terahertz Portable Handheld Spectral Reflection (PHASR) Scanner. Sci. Rep. 2022, 12, 5096. [Google Scholar] [CrossRef] [PubMed]
- Khani, M.E.; Winebrenner, D.P.; Arbab, M.H. Phase Function Effects on Identification of Terahertz Spectral Signatures Using the Discrete Wavelet Transform. IEEE Trans. Terahertz Sci. Technol. 2020, 10, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Khani, M.E.; Arbab, M.H. Chemical Identification in the Specular and Off-Specular Rough-Surface Scattered Terahertz Spectra Using Wavelet Shrinkage. IEEE Access 2021, 9, 29746–29754. [Google Scholar] [CrossRef]
- Osman, O.B.; Arbab, M.H. Mitigating the effects of granular scattering using cepstrum analysis in terahertz time-domain spectral imaging. PLoS ONE 2019, 14, e0216952. [Google Scholar] [CrossRef]
- Khani, M.E.; Osman, O.B.; Arbab, M.H. Diffuse terahertz spectroscopy in turbid media using a wavelet-based bimodality spectral analysis. Sci. Rep. 2021, 11, 22804. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Bayati, E.; Oguchi, K.; Watanabe, S.; Winebrenner, D.P.; Hassan Arbab, M. Terahertz time-domain polarimetry (THz-TDP) based on the spinning E-O sampling technique: Determination of precision and calibration. Opt. Express 2020, 28, 13482–13496. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Osman, O.B.; Harris, Z.B.; Abazri, A.; Honkanen, R.; Arbab, M.H. Investigation of water diffusion dynamics in corneal phantoms using terahertz time-domain spectroscopy. Biomed. Opt. Express 2020, 11, 1284–1297. [Google Scholar] [CrossRef]
- Chen, A.; Virk, A.; Harris, Z.; Abazari, A.; Honkanen, R.; Arbab, M.H. Non-contact terahertz spectroscopic measurement of the intraocular pressure through corneal hydration mapping. Biomed. Opt. Express 2021, 12, 3438–3449. [Google Scholar] [CrossRef]
- Zhou, B.; Sit, A.J.; Zhang, X. Noninvasive measurement of wave speed of porcine cornea in ex vivo porcine eyes for various intraocular pressures. Ultrasonics 2017, 81, 86–92. [Google Scholar] [CrossRef]
- Stockslager, M.; Samuels, B.; Allingham, R.R.; Klesmith, Z.; Schwaner, S.; Forest, C.; Ethier, C. System for Rapid, Precise Modulation of Intraocular Pressure, toward Minimally-Invasive In Vivo Measurement of Intracranial Pressure. PLoS ONE 2016, 11, e0147020. [Google Scholar] [CrossRef]
- Ruiz-Ederra, J.; García, M.; Hernandez, M.; Urcola, H.; Hernández-Barbáchano, E.; Araiz, J.; Vecino, E. The pig eye as a novel model of glaucoma. Exp. Eye Res. 2005, 81, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Jones, J.; Tyrer, J.R.; Marshall, J. An interferometric ex vivo study of corneal biomechanics under physiologically representative loading, highlighting the role of the limbus in pressure compensation. Eye Vis. 2020, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Virk, A.S.; Harris, Z.B.; Arbab, M.H. Development of a terahertz time-domain scanner for topographic imaging of spherical targets. Opt. Lett. 2021, 46, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Selvin, S.; Bajwa, N.; Chantra, S.; Nowroozi, B.; Garritano, J.; Goell, J.; Li, A.; Deng, S.X.; Brown, E.; et al. THz imaging system for in vivo human cornea. IEEE Trans. Terahertz. Sci. Technol. 2018, 8, 27–37. [Google Scholar] [CrossRef]
- Sung, S.; Dabironezare, S.; Llombart, N.; Selvin, S.; Bajwa, N.; Chantra, S.; Nowroozi, B.; Garritano, J.; Goell, J.; Li, A.; et al. Optical System Design for Noncontact, Normal Incidence, THz Imaging of in vivo Human Cornea. IEEE Trans. Terahertz Sci. Technol. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.; Garritano, J.; Bajwa, N.; Deng, S.; Hubschman, J.-P.; Grundfest, W.; Taylor, Z. Preliminary results of non-contact THz imaging of cornea. In Proceedings of the Conference on Terahertz, RF, Millimeter, and Submillimeter-Wave Technology and Applications VIII, San Francisco, CA, USA, 10–12 February 2015; Volume 9362. [Google Scholar] [CrossRef] [Green Version]
- Jonuscheit, S.; Doughty, M.J.; Ramaesh, K. In vivo confocal microscopy of the corneal endothelium: Comparison of three morphometry methods after corneal transplantation. Eye (Lond.) 2011, 25, 1130–1137. [Google Scholar] [CrossRef]
- Bourne, W.M. Biology of the corneal endothelium in health and disease. Eye 2003, 17, 912–918. [Google Scholar] [CrossRef]
- Jiang, W.; Simon, R. A comparison of bootstrap methods and an adjusted bootstrap approach for estimating the prediction error in microarray classification. Stat. Med. 2007, 26, 5320–5334. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Ye, L.; Meng, J.; Zhao, Z.; Liu, Z.; Hu, J. Acute ocular hypertension disrupts barrier integrity and pump function in rat corneal endothelial cells. Sci. Rep. 2017, 7, 6951. [Google Scholar] [CrossRef] [Green Version]
- Acar, B.T.; Vural, E.T.; Acar, S. Changes in endothelial cell density following penetrating keratoplasty and deep anterior lamellar keratoplasty. Int. J. Ophthalmol. 2011, 4, 644–647. [Google Scholar] [CrossRef]
- Lee, S.E.; Mehra, R.; Fujita, M.; Roh, D.; Long, C.; Lee, W.; Funderburgh, J.; Ayares, D.; Cooper, D.; Hara, H. Characterization of Porcine Corneal Endothelium for Xenotransplantation. Semin. Ophthalmol. 2013, 29, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.F.; McGhee, C.N.J.; Lee, W.R. A Scanning Electron Microscope Study of Porcine Corneal Endothelium Stored in Chondroitin Sulphate. Cornea 1992, 11, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Nikara, S.; Ahmadi, E.; Nia, A.A. Effects of different preparation techniques on the microstructural features of biological materials for scanning electron microscopy. J. Agric. Food Res. 2020, 2, 100036. [Google Scholar] [CrossRef]
- Müller, A.; Craig, J.P.; Grupcheva, C.N.; McGhee, C.N. The effects of corneal parameters on the assessment of endothelial cell density in the elderly eye. Br. J. Ophthalmol. 2004, 88, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, K.; Hatou, S.; Inagaki, E.; Higa, K.; Tsubota, K.; Shimmura, S. A Rabbit Corneal Endothelial Dysfunction Model Using Endothelial-Mesenchymal Transformed Cells. Sci. Rep. 2018, 8, 16868. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.; Harris, Z.B.; Virk, A.; Abazari, A.; Varadaraj, K.; Honkanen, R.; Arbab, M.H. Assessing Corneal Endothelial Damage Using Terahertz Time-Domain Spectroscopy and Support Vector Machines. Sensors 2022, 22, 9071. https://doi.org/10.3390/s22239071
Chen A, Harris ZB, Virk A, Abazari A, Varadaraj K, Honkanen R, Arbab MH. Assessing Corneal Endothelial Damage Using Terahertz Time-Domain Spectroscopy and Support Vector Machines. Sensors. 2022; 22(23):9071. https://doi.org/10.3390/s22239071
Chicago/Turabian StyleChen, Andrew, Zachery B. Harris, Arjun Virk, Azin Abazari, Kulandaiappan Varadaraj, Robert Honkanen, and Mohammad Hassan Arbab. 2022. "Assessing Corneal Endothelial Damage Using Terahertz Time-Domain Spectroscopy and Support Vector Machines" Sensors 22, no. 23: 9071. https://doi.org/10.3390/s22239071
APA StyleChen, A., Harris, Z. B., Virk, A., Abazari, A., Varadaraj, K., Honkanen, R., & Arbab, M. H. (2022). Assessing Corneal Endothelial Damage Using Terahertz Time-Domain Spectroscopy and Support Vector Machines. Sensors, 22(23), 9071. https://doi.org/10.3390/s22239071









