Quantifying Cognitive Workload Using a Non-Contact Magnetocardiography (MCG) Wearable Sensor
Abstract
:1. Introduction
2. Methods
2.1. Study Participants
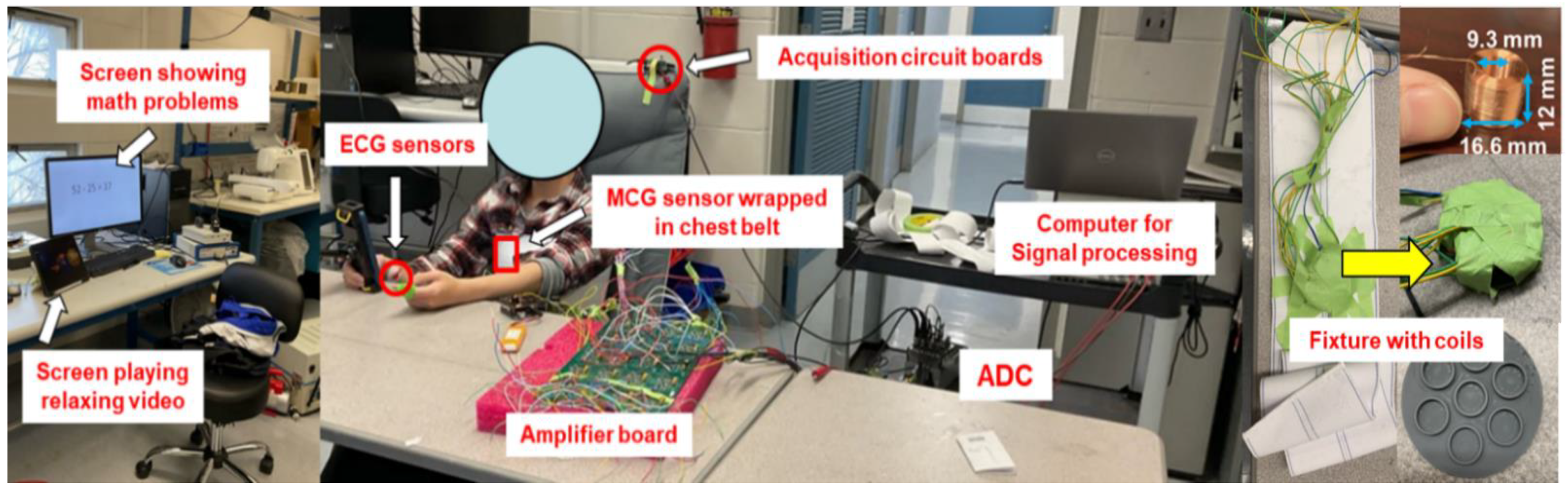
2.2. Experimental Setup
2.3. Low and High Cognitive Workload Test Conditions
2.4. Heart Rate Variability (HRV) Parameters
3. Results
3.1. Validation of MCG Sensor Performance
3.2. Confirmation of Engagement during the High Cognitive Workload Testing Conditions
3.3. Inter-Subject Classification Performance
3.4. Intra-Subject Classification Performance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peterson, L.; Peterson, M.J. Short-term retention of individual verbal items. J. Exp. Psychol. 1595, 58, 193. [Google Scholar] [CrossRef] [PubMed]
- Rozado, D.; Dunser, A. Combining EEG with pupillometry to improve cognitive workload detection. Computer 2015, 48, 425–438. [Google Scholar] [CrossRef]
- Mohammad, A.A.; Anh, H.D.; Wataru, K. Cognitive workload detection from raw EEG-signals of vehicle driver using deep learning. In Proceedings of the IEEE 2019 21st International Conference on Advanced Communication Technology (ICACT), PyeongChang, Korea, 17–20 February 2019. [Google Scholar]
- Dehais, F.; Duprès, A.; Blum, S.; Drougard, N.; Scannella, S.; Raphaëlle, N.R.; Lotte, F. Monitoring pilot’s mental workload using ERPs and spectral power with a six-dry-electrode EEG system in real flight conditions. Sensors 2019, 9, 1324. [Google Scholar] [CrossRef] [Green Version]
- Anthony, A.; Elizabeth, C.; Prithima, M.; Lukasz, M. Classification of EEG Features for Prediction of Working Memory Load. In Advances in The Human Side of Service Engineering; Ahram, T., Karwowski, W., Eds.; Springer: Cham, Switzerland, 2017; pp. 32–58. [Google Scholar]
- Zak, Y.; Parmet, Y.; Oron-Gilad, T. Subjective Workload assessment technique (SWAT) in real time: Affordable methodology to continuously assess human operators’ workload. In Proceedings of the IEEE 2020 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Toronto, ON, Canada, 17–20 October 2020. [Google Scholar]
- Malagoli, A.; Corradini, M.; Corradini, P.; Shuett, T.; Fonda, S. Towards a method for the objective assessment of cognitiveworkload: A pilot study in vessel traffic service (VTS) of maritime domain. In Proceedings of the IEEE 2017 IEEE 3rd International Forum on Research and Technologies for Society and Industry (RTSI), Modena, Italy, 11–13 September 2017. [Google Scholar]
- Vidulich, M.A. The cognitive psychology of subjective mental workload. In Advances in Psychology; Peter, A.H., Najmedin, M., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 219–229. [Google Scholar]
- Antonenko, P.; Paas, F.; Grabner, R.; Van Gog, T. Using electroencephalography to measure cognitive load. Educ. Psychol. Rev. 2010, 22, 425–438. [Google Scholar] [CrossRef]
- Mohanavelu, K.; Poonguzhali, S.; Ravi, D.; Singh, P.K.; Mahajabin, M.; Ramachandran, K.; Singh, U.K.; Jayaraman, S. Cognitive Workload Analysis of Fighter Aircraft Pilots in Flight Simulator Environment. Def. Sci. J. 2020, 70, 131–139. [Google Scholar] [CrossRef]
- Tao, D.; Tan, H.; Wang, H.; Zhang, X.; Qu, X.; Zhang, T. A systematic review of physiological measures of mental workload. Int. J. Environ. Res. Public Health 2019, 16, 2716. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, J.; Han, C.; Park, K. An instant donning multi-channel EEG headset (with comb-shaped dry electrodes) and BCI applications. Sensors 2019, 19, 1537. [Google Scholar] [CrossRef] [Green Version]
- Pignoni, G.; Komandur, S. Development of a quantitative evaluation tool of cognitive workload in field studies through eye tracking. In Proceedings of the International Conference on Human-Computer Interaction, Orlando, FL, USA, 26–31 July 2019. [Google Scholar]
- Pedrotti, M.; Mirzaei, M.A.; Tedesco, A.; Chardonnet, J.R.; Mérienne, F.; Benedetto, S.; Baccino, T. Automatic stress classification with pupil diameter analysis. Int. J. Hum.-Comput. Interact. 2014, 30, 220–236. [Google Scholar] [CrossRef] [Green Version]
- Domingos, C.; da Silva Caldeira, H.; Miranda, M.; Melício, F.; Rosa, A.C.; Pereira, J.G. The Influence of Noise in the Neurofeedback Training Sessions in Student Athletes. Int. J. Environ. Res. Public Health 2021, 18, 13223. [Google Scholar] [CrossRef]
- Marinescu, A.C.; Sharples, S.; Ritchie, A.C.; Sanchez Lopez, T.; McDowell, M.; Morvan, H.P. Physiological parameter response to variation of mental workload. Hum. Factors 2018, 60, 31–56. [Google Scholar] [CrossRef]
- Aeschbacher, S.; Bossard, M.; Ruperti Repilado, F.J.; Good, N.; Schoen, T.; Zimny, M.; Probst-Hensch, N.M.; Schmidt-Trucksäss, A.; Risch, M.; Risch, L.; et al. Healthy lifestyle and heart rate variability in young adults. Eur. J. Prev. Cardiol. 2016, 23, 1037–1044. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansikka, H.; Virtanen, K.; Harris, D.; Simola, P. Fighter pilots’ heart rate, heart rate variation and performance during an instrument flight rules proficiency test. Appl. Ergon. 2016, 56, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Abe, E.; Kamata, K.; Nakayama, C.; Suzuki, Y.; Yamakawa, T.; Hiraoka, T.; Kano, M.; Sumi, Y.; Masuda, F.; et al. Heart rate variability-based driver drowsiness detection and its validation with EEG. IEEE Trans. Biomed. Eng. 2018, 66, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Tjolleng, A.; Jung, K.; Hong, W.; Lee, W.; Lee, B.; You, H.; Son, J.; Park, S. Classification of a Driver’s cognitive workload levels using artificial neural network on ECG signals. Appl. Ergon. 2017, 59, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Domingos, C.; Silva, C.M.D.; Antunes, A.; Prazeres, P.; Esteves, I.; Rosa, A.C. The influence of an alpha band neurofeedback training in heart rate variability in athletes. Int. J. Environ. Res. Public Health 2021, 18, 12579. [Google Scholar] [CrossRef]
- Kalevo, L.; Miettinen, T.; Leino, A.; Kainulainen, S.; Korkalainen, H.; Myllymaa, K.; Töyräs, J.; Leppänen, T.; Laitinen, T.; Myllymaa, S. Effect of sweating on electrode-skin contact impedances and artifacts in EEG recordings with various screen-printed Ag/Agcl electrodes. IEEE Access 2020, 8, 50934–50943. [Google Scholar] [CrossRef]
- Rodrigues, E.; Lima, D.; Barbosa, P.; Gonzaga, K.; Guerra, R.O.; Pimentel, M.; Barbosa, H.; Maciel, Á. HRV Monitoring Using Commercial Wearable Devices as a Health Indicator for Older Persons during the Pandemic. Sensors 2022, 22, 2001. [Google Scholar] [CrossRef]
- Zhu, K.; Kiourti, A. Detection of Extremely Weak and Wideband Bio-Magnetic Signals in Non-Shielded Environments Using Passive Coil Sensors. IEEE J. Electromagn. RF Microw. Med. Biol. 2022, 1–8. [Google Scholar] [CrossRef]
- Zhu, K.; Shah, A.M.; Berkow, J.; Kiourti, A. Miniature Coil Array for Passive Magnetocardiography in Non-Shielded Environments. IEEE J. Electromagn. RF Microw. Med. Biol. 2020, 5, 124–131. [Google Scholar] [CrossRef]
- Bauemschmitt, R.; Feuerstein, M.; Schirmbeck, E.U.; Traub, J.; Klinker, G.; Wildhirt, S.M.; Lange, R. Improved preoperative planning in robotic heart surgery. In Proceedings of the Computers in Cardiology, Chicago, IL, USA, 19–22 September 2004; pp. 773–776. [Google Scholar]
- Puurtinen, M.; Viik, J.; Hyttinen, J. Best electrode locations for a small bipolar ECG device: Signal strength analysis of clinical data. Ann. Biomed. Eng. 2009, 37, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Costley, J.; Fanguy, M.; Lange, C.; Baldwin, M. The effects of video lecture viewing strategies on cognitive load. J. Comput. High. Educ. 2021, 33, 19–38. [Google Scholar] [CrossRef] [Green Version]
- Aalioui, L.; Gouzi, F.; Tricot, A. Reducing cognitive load during video lectures in physiology with eye movement modeling and pauses: A randomized controlled study. Adv. Physiol. Educ. 2022, 46, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Rubio, S.; Diaz, E.; Martin, J.; Puente, J. Evaluation of Subjective Mental Workload: A Comparison of SWAT, NASA-TLX, and Workload Profile Methods. Appl. Psychol. 2004, 53, 61–86. [Google Scholar] [CrossRef]
- Turner, J.R.; Hewitt, J.K.; Morgan, R.K.; Sims, J.; Carroll, D.; Kelly, K.A. Graded mental arithmetic as an active psychological challenge. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 1986, 3, 307–309. [Google Scholar] [CrossRef]
- Novak, D. Biomechatronic applications of brain-computer interfaces. In Handbook of biomechatronics, 1st ed.; Jacob, S., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 129–175. [Google Scholar]
- Evrengül, H.; Tanriverdi, H.; Dursunoglu, D.; Kaftan, A.; Kuru, O.; Unlu, U.; Kilic, M. Time and frequency domain analyses of heart rate variability in patients with epilepsy. Epilepsy Res. 2005, 63, 131–139. [Google Scholar] [CrossRef]
- Kurosaka, C.E.; Kuraoka, H.; Miyake, S. Poincaré Plot Indexes of Heart Rate Variability: Pattern II Responses and Mental Workload. In International Conference on Human-Computer Interaction; Springer: Cham, Switzerland, 2019; pp. 238–243. [Google Scholar]
- Kumar, P.; Das, A.K.; Halder, S. Time-domain HRV analysis of ECG signal under different body postures. Procedia Comput. Sci. 2020, 167, 1705–1710. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Chen, S.; Li, X.; Jin, H.; Du, J. Frequency domain indices of heart rate variability are useful for differentiating vasovagal syncope and postural tachycardia syndrome in children. J. Pediatrics 2019, 207, 59–63. [Google Scholar] [CrossRef]
- Baek, H.J.; Cho, C.H.; Cho, J.; Woo, J.M. Reliability of ultra-short-term analysis as a surrogate of standard 5-min analysis of heart rate variability. Telemed. E-Health 2015, 21, 404–414. [Google Scholar] [CrossRef]
- Castaldo, R.; Montesinos, L.; Melillo, P.; James, C.; Pecchia, L. Ultra-short term HRV features as surrogates of short term HRV: A case study on mental stress detection in real life. BMC Med. Inform. Decis. Mak. 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, B.H.; Allen, M.T.; Christie, I.C.; Santucci, A.K. Validity concerns of common heart-rate variability indices. IEEE Eng. Med. Biol. Mag. 2022, 21, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.M.; Woo, J.M. Determinants for heart rate variability in a normal Korean population. J. Korean Med. Sci. 2011, 26, 1293–1298. [Google Scholar] [CrossRef] [Green Version]
- Sammito, S.; Böckelmann, I. Reference values for time-and frequency-domain heart rate variability measures. Heart Rhythm 2016, 13, 1309–1316. [Google Scholar] [CrossRef]
- Ernst, G. Heart-rate variability—More than heart beats? Front. Public Health 2017, 5, 240. [Google Scholar] [CrossRef] [Green Version]
- Reermann, J.; Elzenheimer, E.; Schmidt, G. Real-time biomagnetic signal processing for uncooled magnetometers in cardiology. IEEE Sens. J. 2019, 519, 4237–4249. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, C.; Zhang, X.; Luo, H.; Zhang, W. Assessment of mental workload based on multi-physiological signals. Technol. Health Care 2020, 28, 67–80. [Google Scholar] [CrossRef]
- Lanfranchi, P.A.; Somers, V.K. Cardiovascular physiology: Autonomic control in health and in sleep disorders. In Principles and Practice of Sleep Medicine, 5th ed.; Meir, H.K., Thomas, R., William, C.D., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2010; Volume 1, pp. 226–236. [Google Scholar]
- Aygun, A.; Nguyen, T.; Haga, Z.; Aeron, S.; Scheutz, M. Investigating Methods for Cognitive Workload Estimation for Assistive Robots. Sensors 2022, 22, 6834. [Google Scholar] [CrossRef]
- Matuz, A.; van der Linden, D.; Kisander, Z.; Hernádi, I.; Kazmer, K.; Csatho, A. Enhanc cariac vagal ton in mntal fatig: Analysis of hart rat variability in Tim-on-Task, rcovry, an ractivity. PLoS ONE 2021, 16, 0238670. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Challapalli, S.; Tung, R.; Parker, M.A.; Kadish, A.H. Relationship of heart rate variability to parasympathetic effect. Circlation 2001, 103, 1977–1983. [Google Scholar] [CrossRef]



| ID | Age | Sex | Height (m) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|---|
| Subject 1 | 28 | Female | 1.63 | 48.5 | 18.3 |
| Subject 2 | 24 | Male | 1.70 | 55 | 19.0 |
| Subject 3 | 24 | Male | 1.65 | 56 | 20.6 |
| Subject 4 | 25 | Male | 1.75 | 80 | 26.1 |
| Subject 5 | 23 | Male | 1.78 | 65 | 20.5 |
| Subject 6 | 35 | Female | 1.67 | 56.5 | 20.3 |
| Subject 7 | 30 | Male | 1.76 | 81 | 26.1 |
| Subject 8 | 23 | Male | 1.92 | 82 | 22.2 |
| Subject 9 | 25 | Male | 1.79 | 64 | 20.0 |
| Subject 10 | 20 | Female | 1.75 | 50 | 17.3 |
| Subject 11 | 23 | Female | 1.63 | 52.6 | 19.8 |
| Subject 12 | 20 | Female | 1.75 | 50 | 17.3 |
| Subject 13 | 23 | Female | 1.63 | 52.6 | 19.8 |
| Trial Number | Signal Type | SDRR (ms) | RMSSD (ms) | MeanRR (ms) |
|---|---|---|---|---|
| 1 | MCG | 34.4007 | 22.5954 | 806.0518 |
| ECG | 33.9442 | 21.0884 | 806.051 | |
| 2 | MCG | 35.2044 | 27.6404 | 841.4077 |
| ECG | 36.1201 | 26.0375 | 842.4552 | |
| 3 | MCG | 30.5217 | 31.3984 | 877.2591 |
| ECG | 34.794 | 30.7943 | 877.2374 | |
| 4 | MCG | 32.2313 | 32.9698 | 863.9058 |
| ECG | 36.202 | 31.8829 | 863.9223 | |
| 5 | MCG | 39.0674 | 39.0887 | 848.5608 |
| ECG | 39.9489 | 29.8374 | 848.5273 | |
| 6 | MCG | 41.3109 | 44.9424 | 782.5284 |
| ECG | 37.9880 | 31.5640 | 783.4569 | |
| 7 | MCG | 42.4284 | 42.5028 | 868.6371 |
| ECG | 44.8875 | 28.6951 | 868.6302 |
| ID | Self-Reported Difficulty Level | Math Performance |
|---|---|---|
| Subject 1 | Difficult | 92.4% |
| Subject 2 | Difficult | 92.4% |
| Subject 3 | Difficult | 91.67% |
| Subject 4 | Difficult | 91.4% |
| Subject 5 | Difficult | 98.33% |
| Subject 6 | Difficult | 93.67% |
| Subject 7 | Difficult | 91.139% |
| Subject 8 | Difficult | 96.25% |
| Subject 9 | Difficult | 98.33% |
| Subject 10 | Difficult | 93.44% |
| Subject 11 | Difficult | 90.00% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhu, K.; Kaur, A.; Recker, R.; Yang, J.; Kiourti, A. Quantifying Cognitive Workload Using a Non-Contact Magnetocardiography (MCG) Wearable Sensor. Sensors 2022, 22, 9115. https://doi.org/10.3390/s22239115
Wang Z, Zhu K, Kaur A, Recker R, Yang J, Kiourti A. Quantifying Cognitive Workload Using a Non-Contact Magnetocardiography (MCG) Wearable Sensor. Sensors. 2022; 22(23):9115. https://doi.org/10.3390/s22239115
Chicago/Turabian StyleWang, Zitong, Keren Zhu, Archana Kaur, Robyn Recker, Jingzhen Yang, and Asimina Kiourti. 2022. "Quantifying Cognitive Workload Using a Non-Contact Magnetocardiography (MCG) Wearable Sensor" Sensors 22, no. 23: 9115. https://doi.org/10.3390/s22239115
APA StyleWang, Z., Zhu, K., Kaur, A., Recker, R., Yang, J., & Kiourti, A. (2022). Quantifying Cognitive Workload Using a Non-Contact Magnetocardiography (MCG) Wearable Sensor. Sensors, 22(23), 9115. https://doi.org/10.3390/s22239115









