Absorption Spectra Description for T-Cell Concentrations Determination and Simultaneous Measurements of Species during Co-Cultures
Abstract
:1. Introduction
1.1. Context
1.2. Commercial Availabilities for Cell Counting
1.3. Other Biological and Physical Techniques for Cell Qualifications
1.4. Global Methods and Co-Culture Investigations
1.5. Current Needs and Proposed Method
2. Materials and Methods
2.1. CEM Preparation
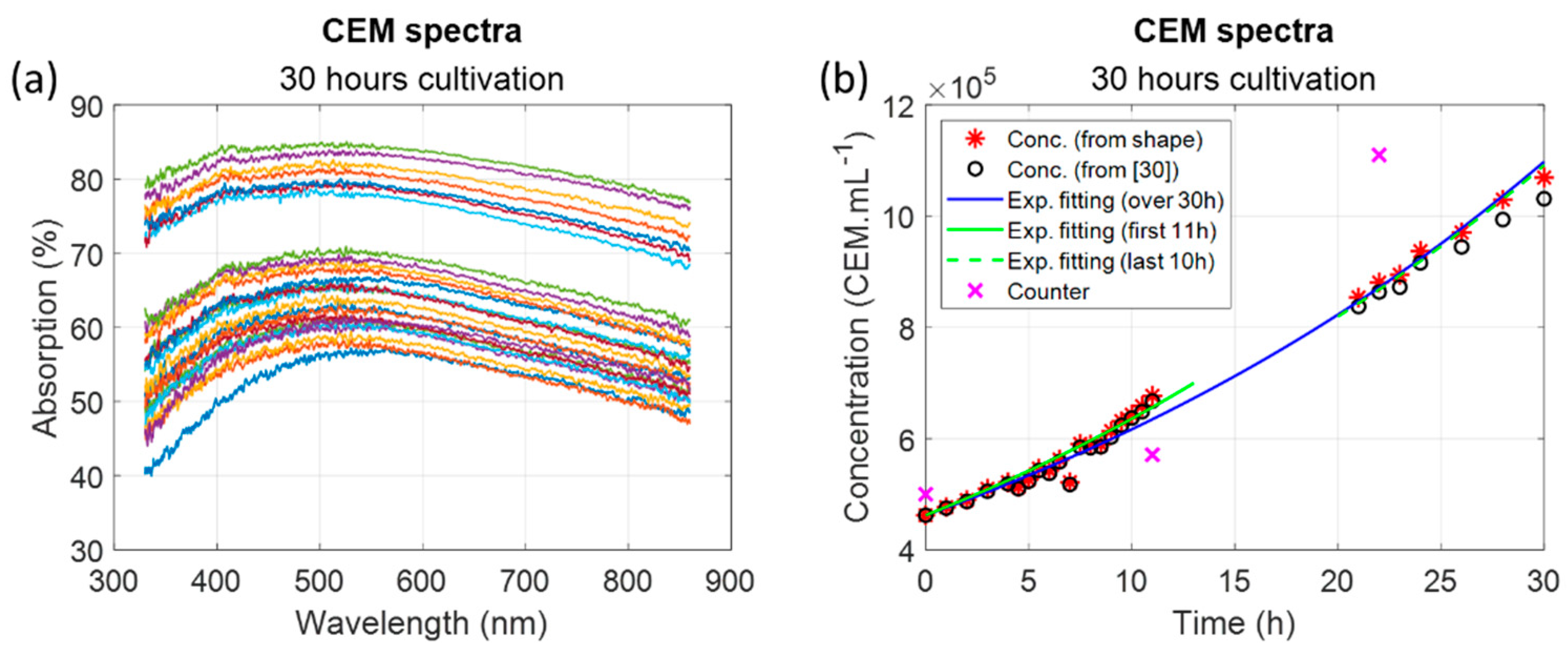
2.2. Cultivation of CEM Cells over 30 Hours
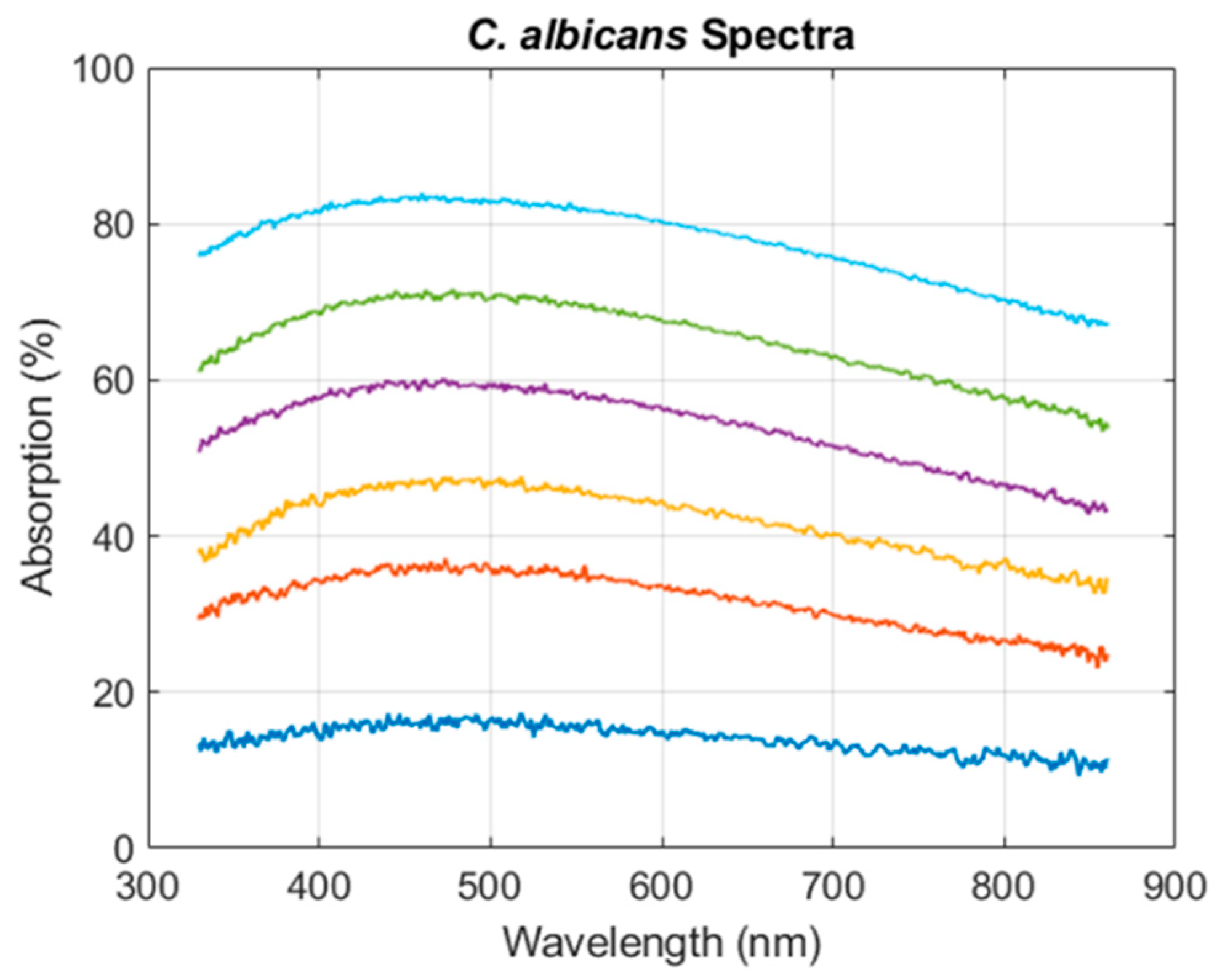
2.3. Concentration Ranges for Optical Absorption Modeling of Candida Albicans
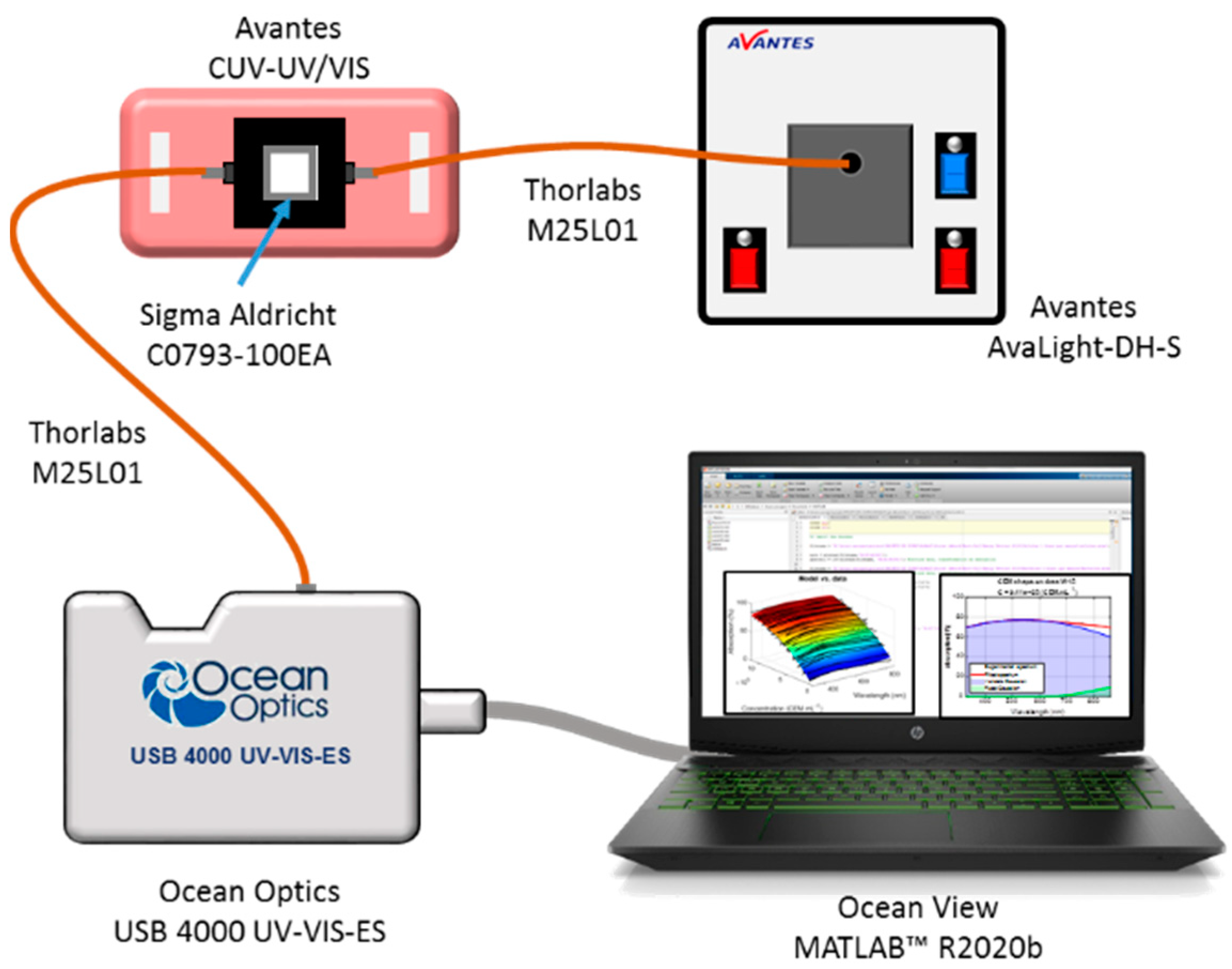
2.4. Spectroscopic Absorption Measurements
2.5. Spectral Data Processing
3. Results
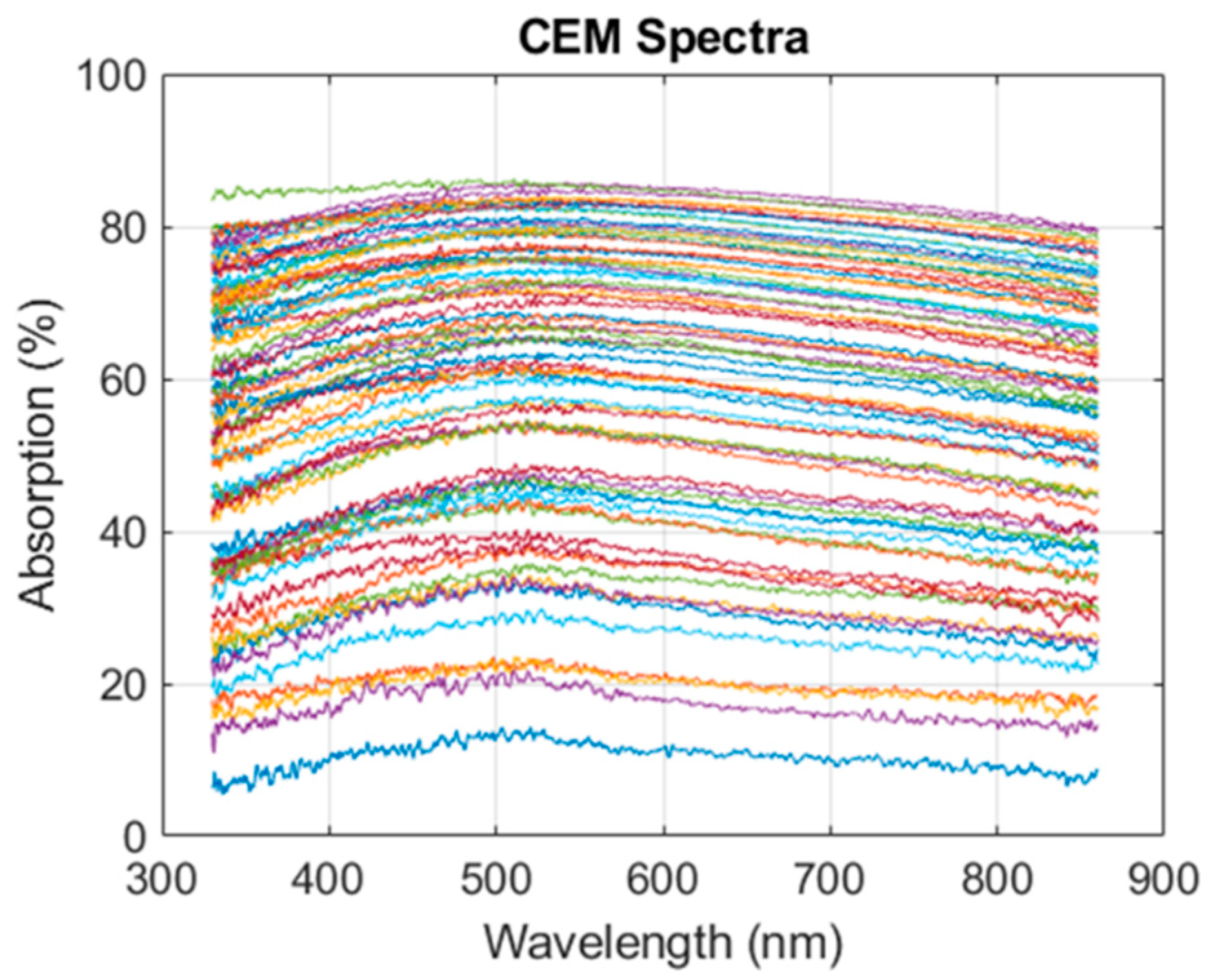
3.1. Modeling CEM Absorption Spectra
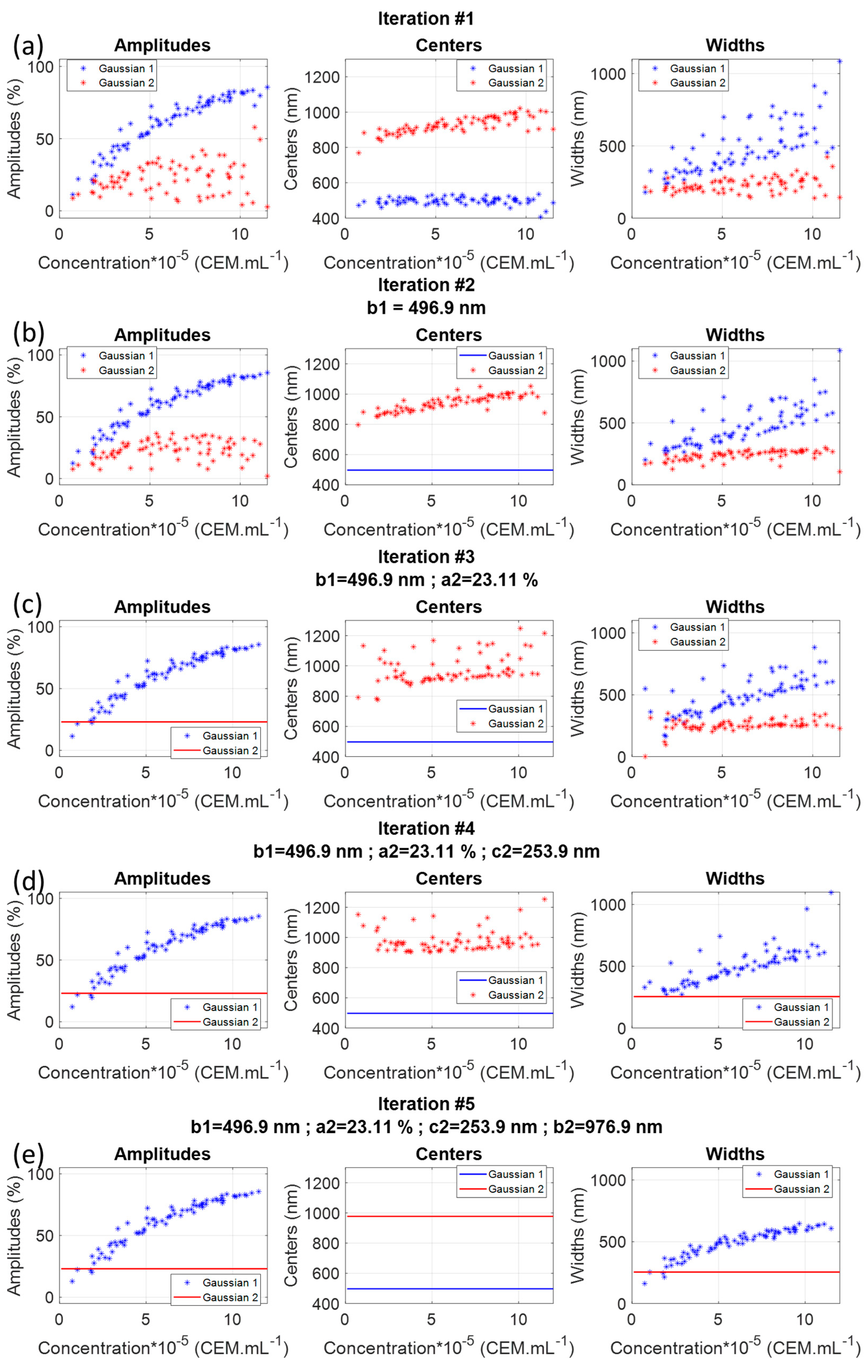
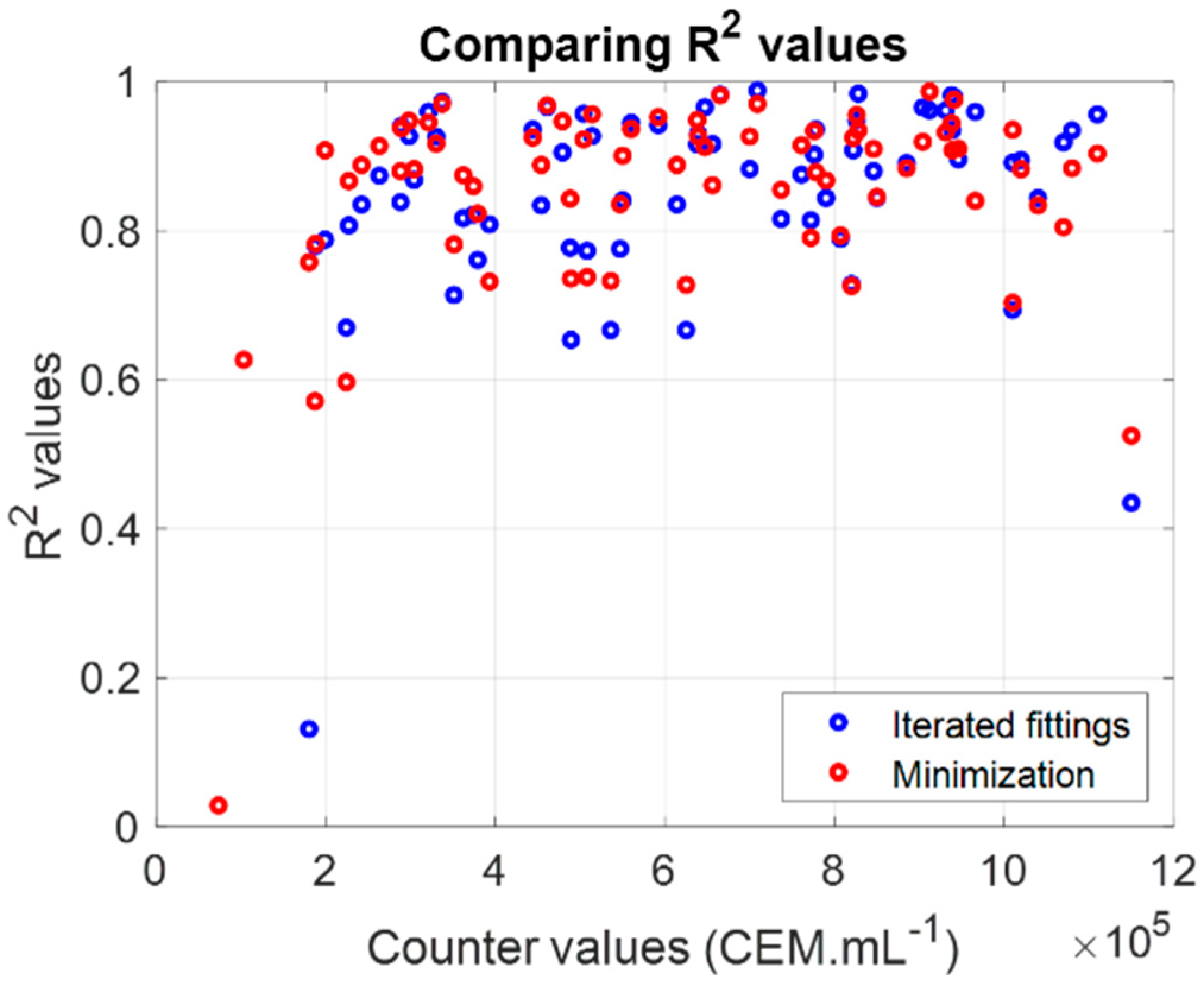
3.1.1. Iterated Fittings Approximation
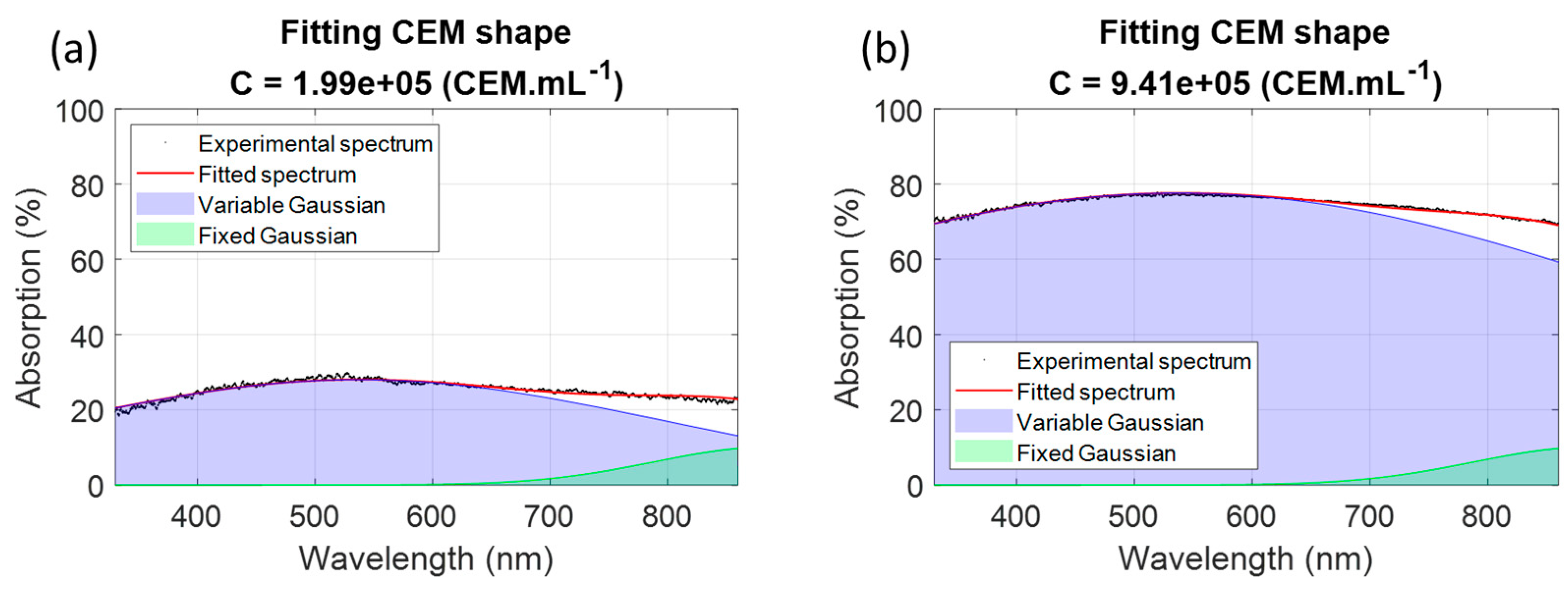
3.1.2. Parameters Calculation Using a Minimization Method
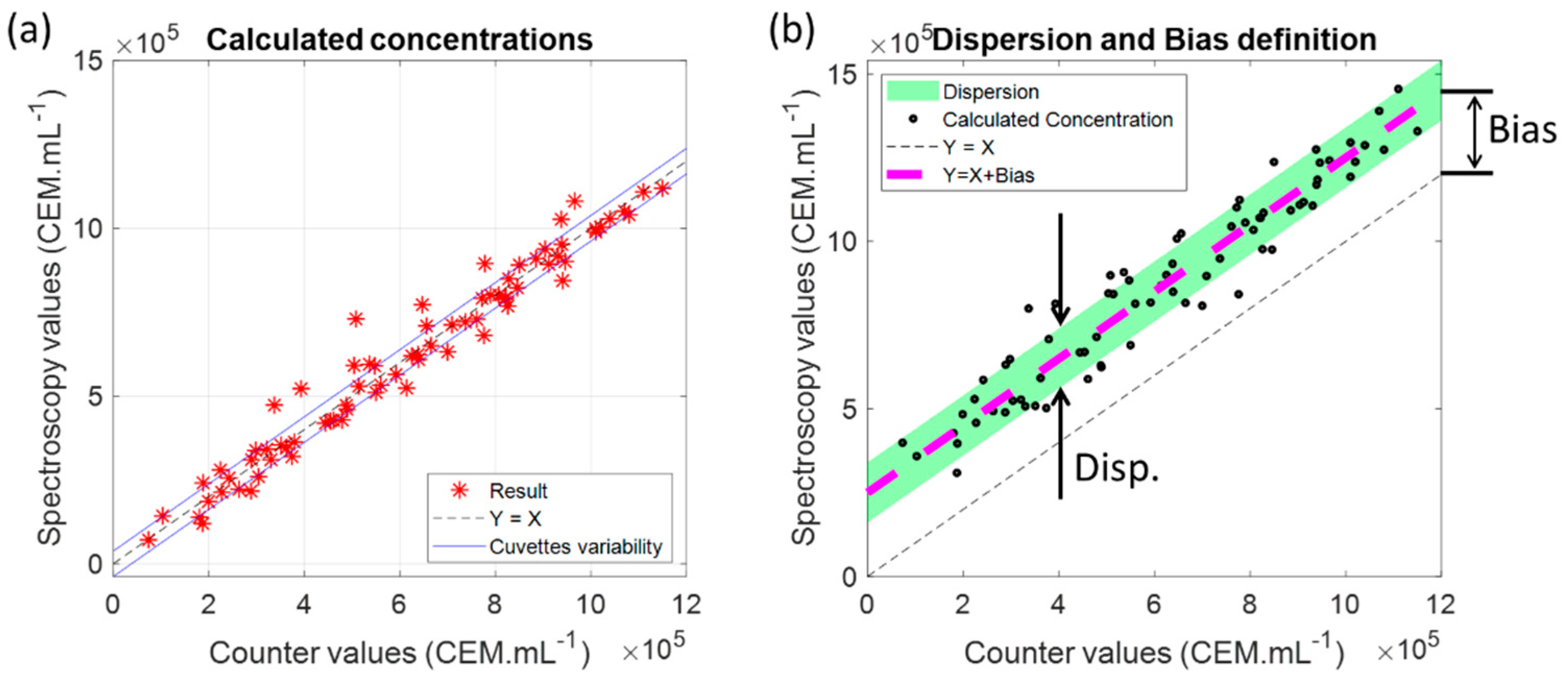
3.1.3. Measuring CEM Concentrations Using the Shape of the Absorption Spectra
3.1.4. Measuring CEM Concentration over 30 Hours
- Disp(0–11)h = 1.9 × 104 cells;
- Disp(21–30)h = 2.2 × 104 cells;
- Disp(0–30)h = 1.8 × 104 cells.
3.2. Measuring Concentrations with Reconstructed Co-Cultures
3.2.1. Establishing the Candida Albicans Spectra Shape Equation
- CA spectra could efficiently be fitted with two Gaussian functions;
- Only 4 iterated fittings were required resulting in the existence of three sub-functions describing the evolutions of , and . Each of them could be fitted with a logarithm function;
- The final parameters obtained with the minimization algorithm are given in Table 3.
- Disp. = 2 × 105 cells (about 10% at the center concentration range);
- Bias = 7804 cells.
3.2.2. Examples of Double Concentration Measurements
- CEM: disp. = 1.28 × 105 cells (21% at the center range);
- CEM: bias = 3.2 × 104 cells;
- CA: disp. = 5.8 × 105 cells (29% at the center range);
- CA: bias = −1.6 × 105 cells.
4. Discussion
4.1. Format of the Spectroscopy Data
4.2. Ways to Optically Measure Concentrations
4.3. Successive Fittings
4.4. Considerations about Data Dispersion and Bias
4.5. Remarks about the Cell Multiplication Times
4.6. Concerning the Reconstructed Co-Cultures CEM/Candida Albicans
4.7. Position of Our Studies and Model to Others
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATMP | Advanced Therapy Medicinal Product |
| EFS | Etablissement Français du Sang (French Blood Agency) |
| ELISA | Enzyme-Linked ImmunoSorbent Assay |
| FBS | Fetal Bovine Serum |
| FDA | Food and Drug Administration |
| HEPES | HydroxyEthyl-PiperazineEthane-Sulfonic acid buffer |
| OD | Optical Density |
| PAT | Process Analytical Technology |
| PBS | Phosphate-Buffered Saline |
| RPMI | Roswell Park Memorial Institute medium |
| SAB | Sabouraud Dextrose Agar |
References
- ATMP: Advanced Therapy Medicinal Product. European Medicines Agency. Available online: https://www.ema.europa.eu/en/glossary/advanced-therapy-medicinal-product (accessed on 24 November 2021).
- Wang, X.; Rivière, I. Clinical manufacturing of CAR T cells: Foundation of a promising therapy. Mol. Ther. Oncolytics. 2016, 3, 16015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacogne, B.; Legrand, D.; Azzopardi, C.L.; Pieralli, C.; Frelet-Barrand, A. Optical Spectroscopy Methods to Monitor Cells and Bacteria Concentrations and to Detect Contamination during Cell Culture: Application to the Fabrication of ATMPs. In Biomedical Engineering Systems and Technologies; Communications in Computer and Information Science, Springer Book Series; Ye, X., De Maria, E., Gomez Vilda, P., Cabitza, F., Fred, A., Gamboa, H., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2021; Volume 1400, pp. 53–75. [Google Scholar]
- Wacogne, B.; Legrand, D.; Pieralli, C.; Frelet-Barrand, A. Optical Spectroscopy for the Quality Control of ATMP fabrication: A new method to monitor cell expansion and to detect contaminations. In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020)—BIODEVICES, Valletta, Malta, 24–26 February 2020; Volume 1, pp. 64–72. [Google Scholar]
- Lo, E.K.K.; Lee, P.C.; El-Nezami, H. Low dose of zearalenone elevated colon cancer cell growth through G protein-coupled estrogenic receptor. Sci. Rep. 2021, 11, 7403. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Al-Momin, S.; Vanitha, V.K.; Al-Aqeel, H.; Al-Musallam, L.; Al-Mansour, H.; Shajan, A.B. Evaluation of Various Growth Conditions for the Cultivation of Microalgae Species in the Arid Regions. J. Mech. Cont. Math. Sci. 2019, 4, 255–265. [Google Scholar] [CrossRef]
- Gauchotte, G.; Hergalant, S.; Vigouroux, C.; Casse, J.M.; Houlgatte, R.; Kaoma, T.; Helle, D.; Brochin, L.; Rech, F.; Peyre, M.; et al. Cytoplasmic overexpression of RNA-binding protein HuR is a marker of poor prognosis in meningioma, and HuR knockdown decreases meningioma cell growth and resistance to hypoxia. J. Pathol. 2017, 242, 421–434. [Google Scholar] [CrossRef]
- IPRASENSE. Available online: https://www.iprasense.com/ (accessed on 28 October 2022).
- Empower Your Research with IncuCyte® Live-Cell Analysis Systems. Available online: https://www.sartorius.com/en/products/cell-analysis/incucyte-live-cell-analysis-system (accessed on 28 October 2022).
- IncuCyte® S3 Live-Cell Analysis System. Available online: https://www.youtube.com/watch?v=efCIIPPcdhY (accessed on 28 October 2022).
- HoloMonitor. Available online: https://phiab.com (accessed on 28 October 2022).
- Engvall, E.; Perlman, P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Goldsby, R.A.; Kindt, T.J.; Osborne, B.A.; Kuby, J. Enzyme-Linked Immunosorbent Assay. In Immunology, 5th ed.; Freeman, W.H.: New York, NY, USA, 2003; pp. 148–150. [Google Scholar]
- Nguyen, T.T.; Trinh, K.T.L.; Yoon, W.J.; Lee, N.Y.; Ju, H. Integration of a microfluidic polymerase chain reaction device and surface plasmon resonance fiber sensor into an inline all-in-one platform for pathogenic bacteria detection. Sens. Actuators B Chem. 2016, 242, 1–8. [Google Scholar] [CrossRef]
- Karlsson, R.; Stahlberg, R. Surface plasmon resonance detection and multispot sensing for direct monitoring of interactions involving low-molecular-weight analytes and for determination of low affinities. Anal. Biochem. 1995, 228, 274–280. [Google Scholar] [CrossRef]
- Tsong-Rong, Y.; Chao-Fa, L.; Hung-Che, C. QCM as Cell-Based Biosensor. In Chemical Biology; Ekinci, D., Ed.; Intechopen: London, UK, 2012; pp. 414–434. [Google Scholar]
- Wudy, F.; Multerer, M.; Stock, C.; Schmeer, G.; Gores, H.J. Rapid impedance scanning QCM for electrochemical applications based on miniaturized hardware and high-performance curve fitting. Electrochim. Acta 2008, 53, 6568–6574. [Google Scholar] [CrossRef]
- Cole, H.; Demont, A.; Marison, I. The Application of Dielectric Spectroscopy and Biocalorim-etry for the Monitoring of Biomass in Immobilized Mammalian Cell Cultures. Processes 2015, 3, 384–405. [Google Scholar] [CrossRef] [Green Version]
- Downey, B.J.; Graham, L.J.; Breit, J.F.; Glutting, N.K.A. Novel approach for using dielectric spectroscopy to predict viable cell volume (VCV) in early process development. Biotechnol Prog. 2014, 30, 479–487. [Google Scholar] [CrossRef]
- Musmann, C.; Joeris, K.; Markert, S.; Solle, D.; Scheper, T. Spectroscopic methods and their applicability for high-throughput characterization of mammalian cell cultures in automated cell culture systems. Eng. Life Sci. 2016, 16, 405–416. [Google Scholar] [CrossRef]
- Chen, G.; Hu, J.; Qin, Y.; Zhou, W. Viable cell density on-line auto-control in perfusion cell culture aided by in-situ Raman spectroscopy. Biochem. Eng. J. 2021, 172, 108063. [Google Scholar] [CrossRef]
- Calvet, A.; Ryder, A.G. Monitoring Cell culture media degradation using surface enhanced Raman scattering (SERS) spectroscopy. Anal. Chim. Acta 2014, 840, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romo-Herrera, J.M.; Juarez-Moreno, K.; Guerrini, L.; Kang, Y.; Feliu, N.; Parak, W.J.; Alvarez-Pueblan, R.A. Paper-based plasmonic substrates as surface-enhanced Raman scattering spectroscopy platforms for cell culture applications. Mater. Today Bio. 2021, 11, 100125. [Google Scholar] [CrossRef] [PubMed]
- Esmonde-White, K.A.; Cuellar, M.; Uerpmann, C.; Lenain, B.; Lewis, I.R. Raman spectroscopy as a process analytical technology for pharmaceutical manufacturing and bioprocessing. Anal. Bio-Anal. Chem. 2017, 409, 637–649. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.M.; Lee, S.Y. Optical Biosensors for the Detection of Pathogenic Microorganisms. Trends Biotechnol. 2016, 34, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Maryamchik, E.; Gallagher, K.M.; Preffer, F.I.; Kadauke, S.; Maus, M.V. New directions in chimeric antigen receptor T cell CAR-T therapy and related flow cytometry. Cytom. Part B Clin. Cytom. 2020, 98, 299–327. [Google Scholar] [CrossRef] [PubMed]
- Pitoiset, F.; Cassard, L.; El Soufi, K.; Boselli, L.; Grivel, J.; Roux, A.; Klatzmann, D.; Chaput, N.; Rosenzwajg, M. Deep phenotyping of immune cell populations by optimized and standardized flow cytometry analyses. Cytom. Part A 2018, 93A, 793–802. [Google Scholar] [CrossRef] [Green Version]
- van de Geijn, G.J.; Van Gent, M.; van Pul-Bom, N.; Beunis, M.H.; Van Tilburg, A.J.; Njo, T.L. A New Flow Cytometric Method for Differential Cell Counting in Ascitic Fluid. Cytom. Part B 2016, 90B, 506–511. [Google Scholar] [CrossRef]
- Tahir, M.A.; Dina, N.E.; Cheng, H.; Valev, V.K.; Zhang, L. Surface-enhanced Raman spectroscopy for bioanalysis and diagnosis. Nanoscale 2021, 13, 11593–11634. [Google Scholar] [CrossRef]
- Vaccari, N.; Wacogne, B.; Koubevi, C.; Podevin, M.; Rouleau, A.; Frelet-Barrand, A. White light spectroscopy for T-cell culture growth monitoring: Towards a real-time and sampling free device for ATMPs production. J. Transl. Sci. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Aijaz, A.; Trawinski, D.; McKirgan, S.; Parekkadan, B. Non-invasive cell counting of adherent, suspended and encapsulated mammalian cells using optical density. BioTechniques 2020, 68, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilapwar, S.M.; Nardelli, M.; Westerhoff, H.V.; Verma, M. Chapter four—Absorption Spectroscopy. Methods Enzymol. 2011, 500, 59–75. [Google Scholar]
- Available online: https://en.wikipedia.org/wiki/Absorption_spectroscopy (accessed on 28 October 2022).
- Jarusintanakorn, S.; Phechkrajang, C.; Khongkaew, P.; Mastrobattista, E.; Yamabhai, M. Determination of Chinese hamster ovary (CHO) cell densities and antibody titers from small volumes of cell culture supernatants using multivariate analysis and partial least squares regression of UV-Vis spectra. Anal. Bioanal. Chem. 2021, 413, 5743–5753. [Google Scholar] [CrossRef] [PubMed]
- Drieschner, T.; Ostertag, E.; Boldrini, B.; Lorenz, A.; Brecht, M.; Rebner, K. Direct optical detection of cell density and viability of mammalian cells by means of UV/VIS spectroscopy. Anal. Bioanal. Chem. 2020, 412, 3359–3371. [Google Scholar] [CrossRef]
- Chang, K.S.; Jang, H.D.; Lee, C.F.; Lee, Y.G.; Yuan, C.J.; Lee, S.H. Series quartz crystal sensor for remote bacteria population monitoring in raw milk via the Internet. Biosens. Bioelectron. 2006, 21, 1581–1590. [Google Scholar] [CrossRef]
- Safavieh, M.; Ahmed, M.U.; Ng, A.; Zourob, M. High-throughput real-time electrochemical monitoring of LAMP for pathogenic bacteria detection. Biosens. Bioelectron. 2014, 58, 101–106. [Google Scholar] [CrossRef]
- Theint, H.T.; Walsh, J.E.; Wong, S.T.; Voon, K.; Shitan, M. Development of an optical biosensor for the detection of Trypanosoma evansi and Plasmodium berghei. Spectrochim. Acta A Mol. Biomol Spectrosc. 2019, 218, 348–358. [Google Scholar] [CrossRef]
- Geinitz, B.; Rehmann, L.; Büchs, J.; Regestein, L. Noninvasive tool for optical online monitoring of individual biomass concentrations in a defined coculture. Biotechnol. Bioeng. 2020, 117, 999–1011. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Gonzalez, E.; Hitchins, V.; Ilev, I. Detecting bacteria contamination on medical device surfaces using anintegrated fiber-optic mid-infrared spectroscopy sensing method. Sens. Actuators B 2013, 231, 646–654. [Google Scholar] [CrossRef]
- Liu, Y.-J.; André, S.; Saint Cristau, L.; Lagresle, S.; Hannas, Z.; Calvosa, E.; Devos, O.; Duponchel, L. Multivariate statistical process control (MSPC) using Raman spectroscopy for in-line culture cell monitoring considering time-varying batches synchronized with correlation optimized warping (COW). Anal. Chim. Acta 2017, 952, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Costariol, E.; Rotondi, M.C.; Amini, A.; Hewitt, C.J.; Nienow, A.W.; Heathman, T.R.J.; Rafiq, Q.A. Demonstrating the Manufacture of Human CAR-T Cells in an Automated Stirred-Tank Bioreactor. Biotechnol. J. 2020, 15, 2000177. [Google Scholar] [CrossRef] [PubMed]






| Approximated Parameters | p1a1 | b1 | p1c1 | p2c1 | a2 | b2 | c2 |
|---|---|---|---|---|---|---|---|
| Value | 7.45 × 10−7 | 496.9 | 2.14 | 0.41 | 23.11 | 976.9 | 253.9 |
| Parameters | p1a1 | b1 | p1c1 | p2c1 | a2 | b2 | c2 |
|---|---|---|---|---|---|---|---|
| Value | 7.67 × 10−7 | 533.7 | 6.32 | 0.34 | 12.21 | 936.1 | 177.2 |
| Parameters | p1a1 | p2a1 | p1b1 | p2b1 | c1 | p1a2 | p2a2 | b2 | c2 |
|---|---|---|---|---|---|---|---|---|---|
| Value | −218.6 | 41.1 | −7884 | 1130 | 2036 | −182 | 33.36 | 562.3 | 479.5 |
| Parameters | ||||||
|---|---|---|---|---|---|---|
| Value | −7.5 × 104 | 1.029 | 0.04 | 3.6 × 105 | −0.17 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wacogne, B.; Vaccari, N.; Koubevi, C.; Belinger-Podevin, M.; Robert-Nicoud, M.; Rouleau, A.; Frelet-Barrand, A. Absorption Spectra Description for T-Cell Concentrations Determination and Simultaneous Measurements of Species during Co-Cultures. Sensors 2022, 22, 9223. https://doi.org/10.3390/s22239223
Wacogne B, Vaccari N, Koubevi C, Belinger-Podevin M, Robert-Nicoud M, Rouleau A, Frelet-Barrand A. Absorption Spectra Description for T-Cell Concentrations Determination and Simultaneous Measurements of Species during Co-Cultures. Sensors. 2022; 22(23):9223. https://doi.org/10.3390/s22239223
Chicago/Turabian StyleWacogne, Bruno, Naïs Vaccari, Claudia Koubevi, Marine Belinger-Podevin, Marjorie Robert-Nicoud, Alain Rouleau, and Annie Frelet-Barrand. 2022. "Absorption Spectra Description for T-Cell Concentrations Determination and Simultaneous Measurements of Species during Co-Cultures" Sensors 22, no. 23: 9223. https://doi.org/10.3390/s22239223







