Analysis of Physiological Responses during Pain Induction
Abstract
1. Introduction
Goals and Organization
2. Background
3. Materials and Methods
3.1. Data Collection
3.2. Data Analysis
4. Results
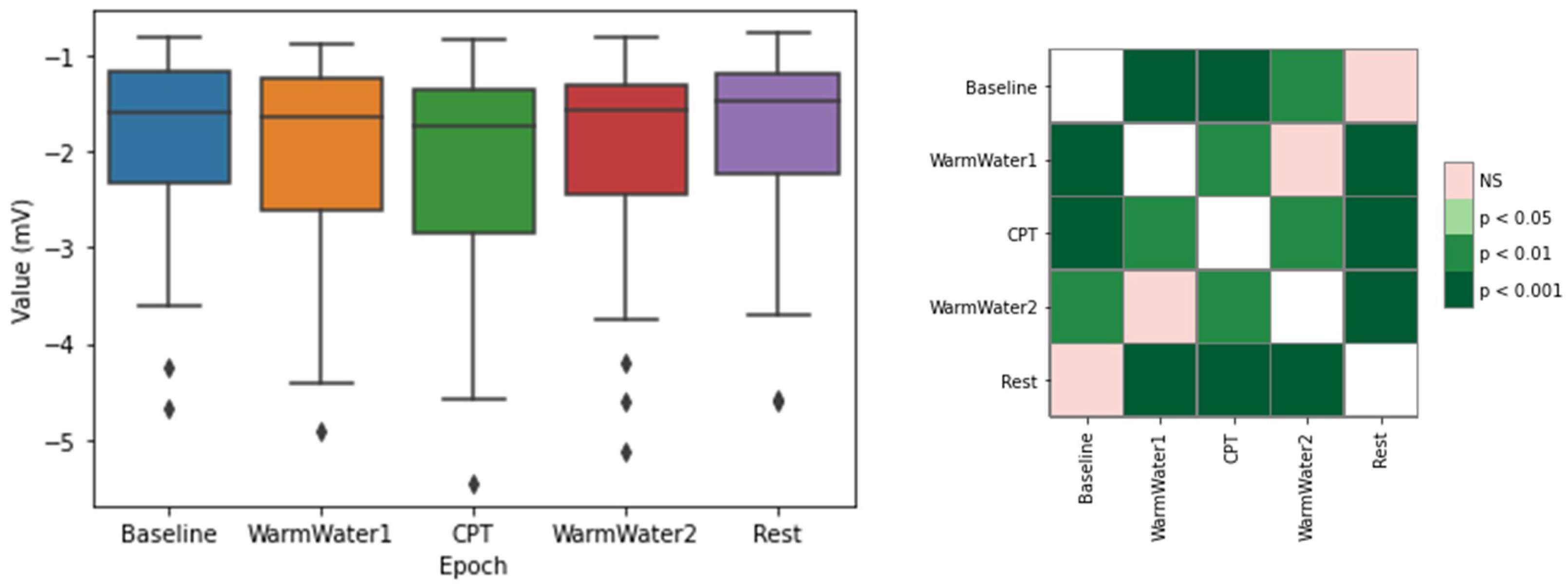
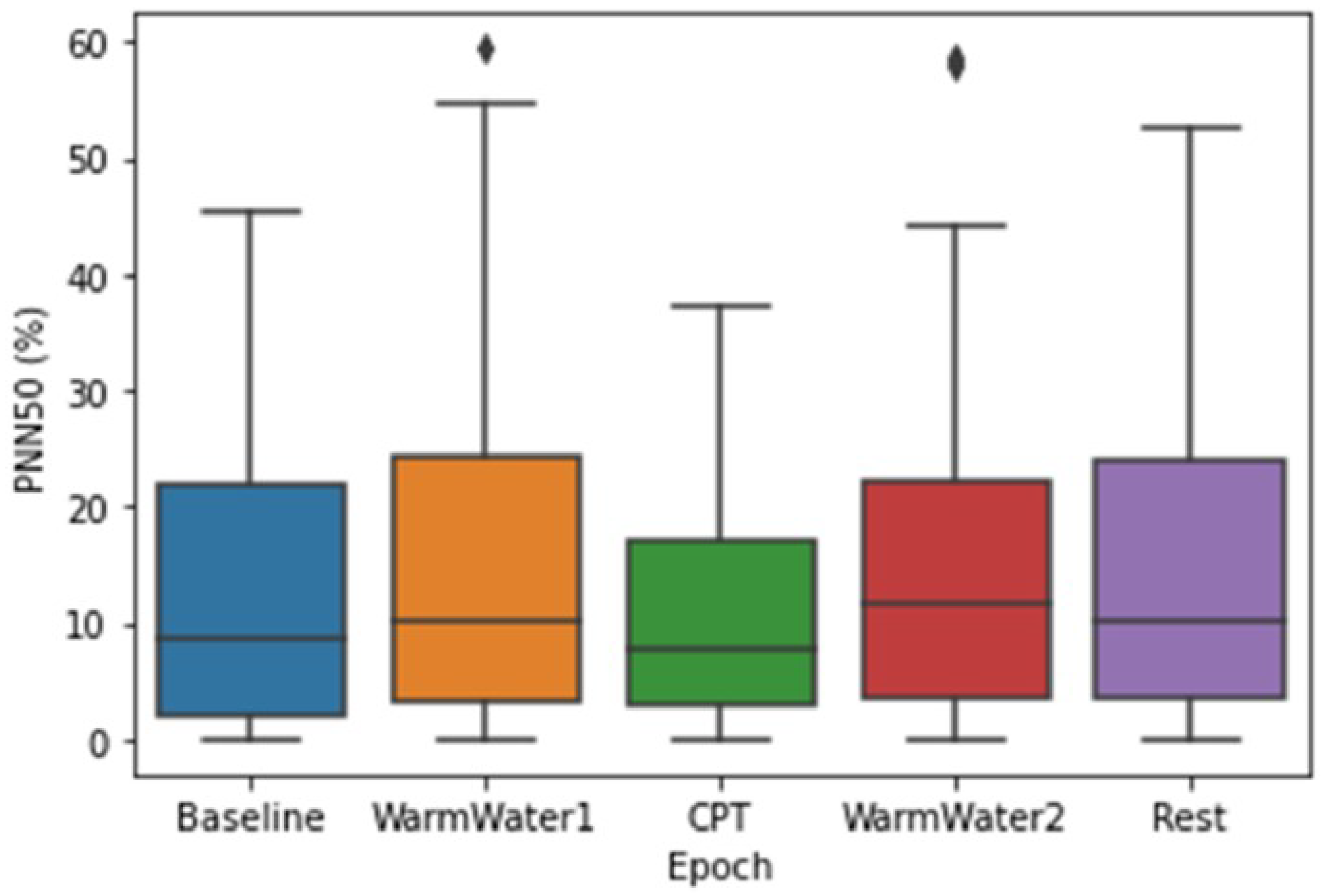
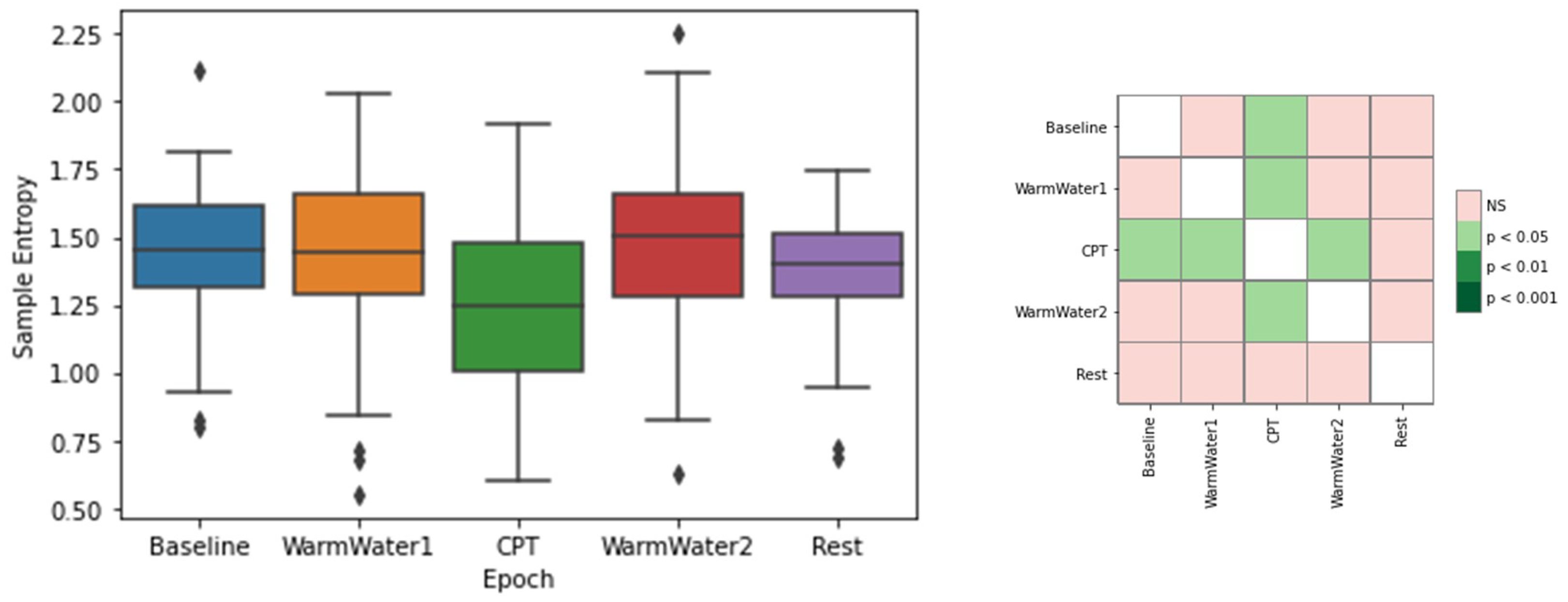
4.1. ECG Processing and Analysis
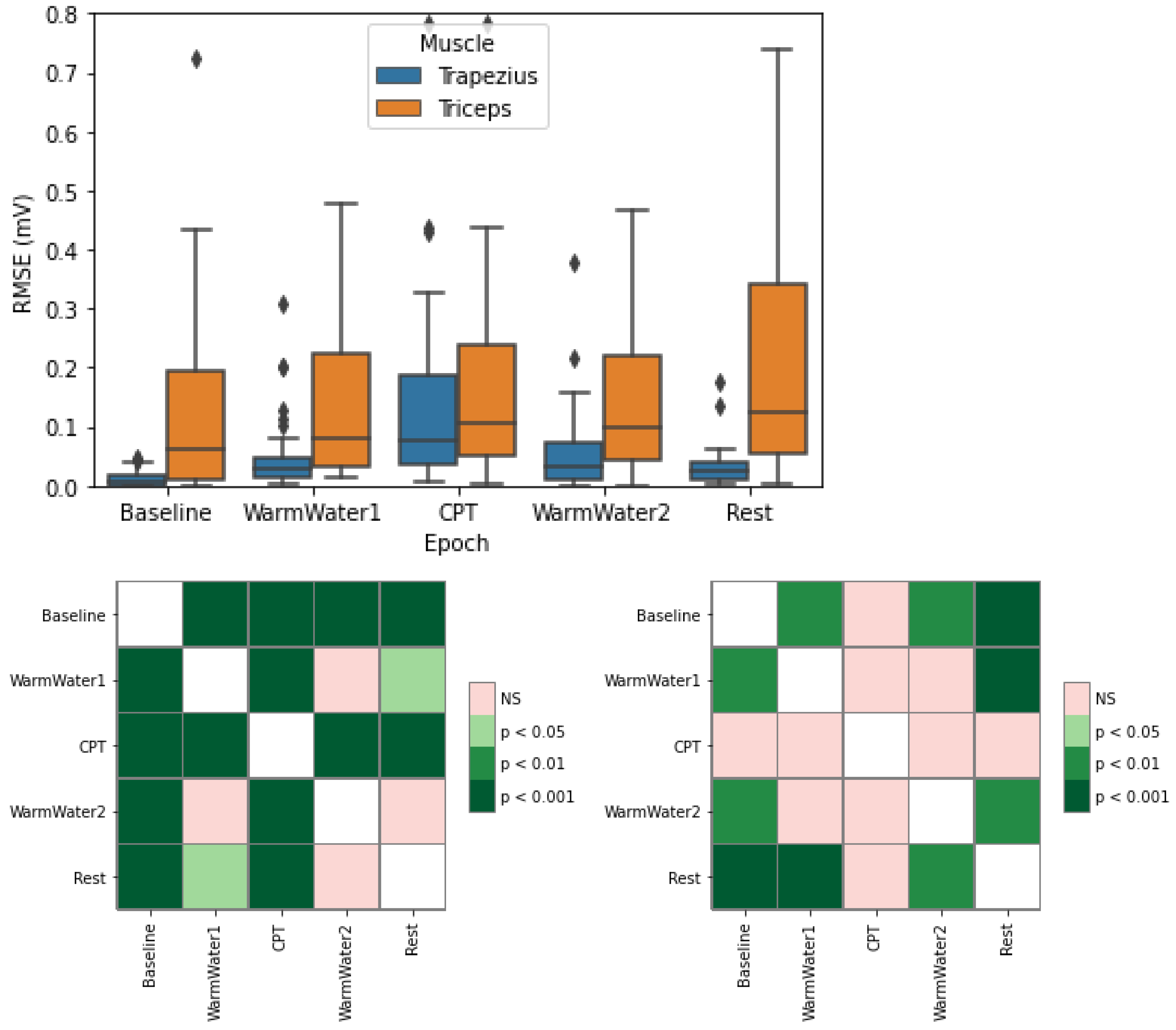
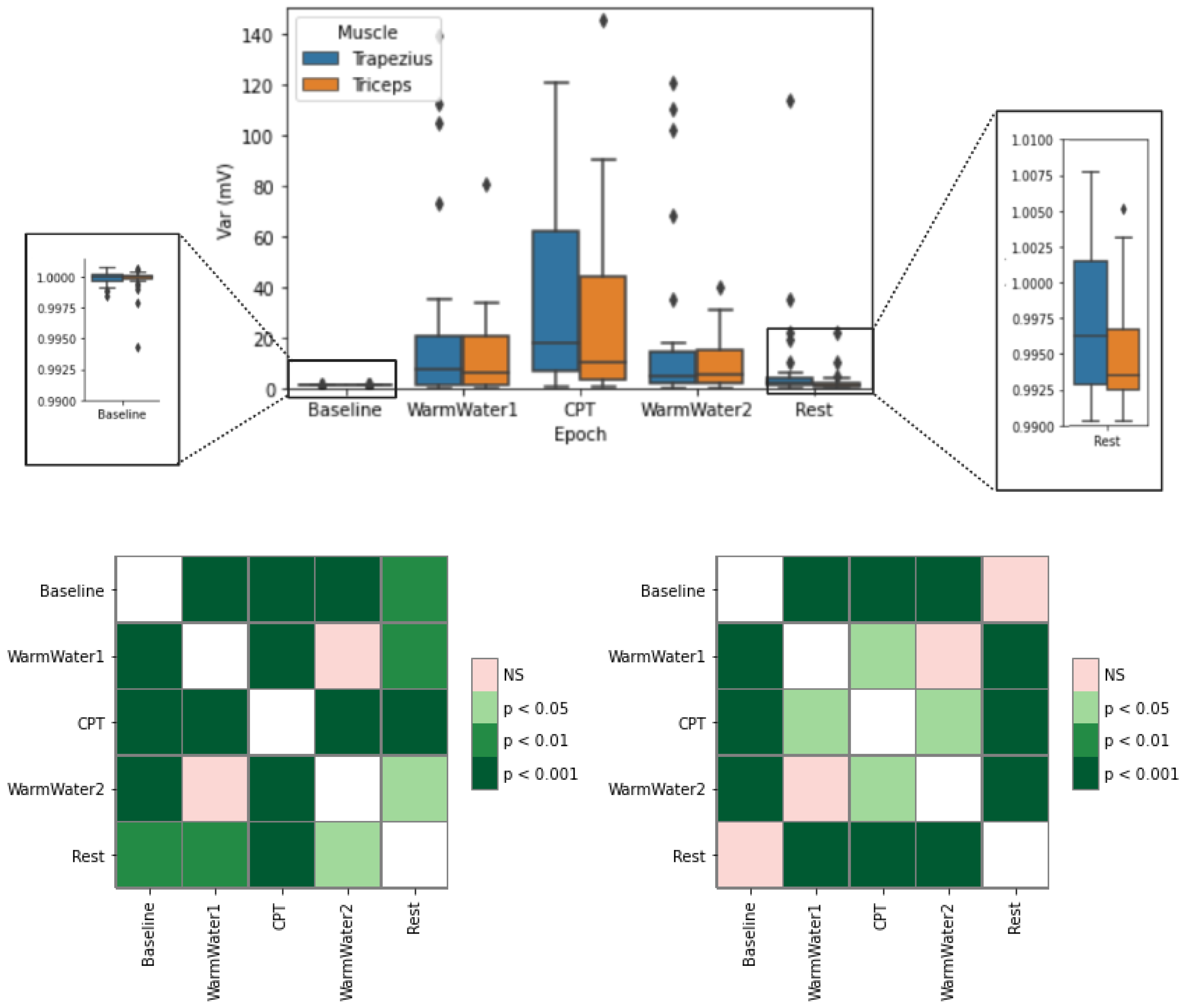
4.2. EMG Processing and Analysis
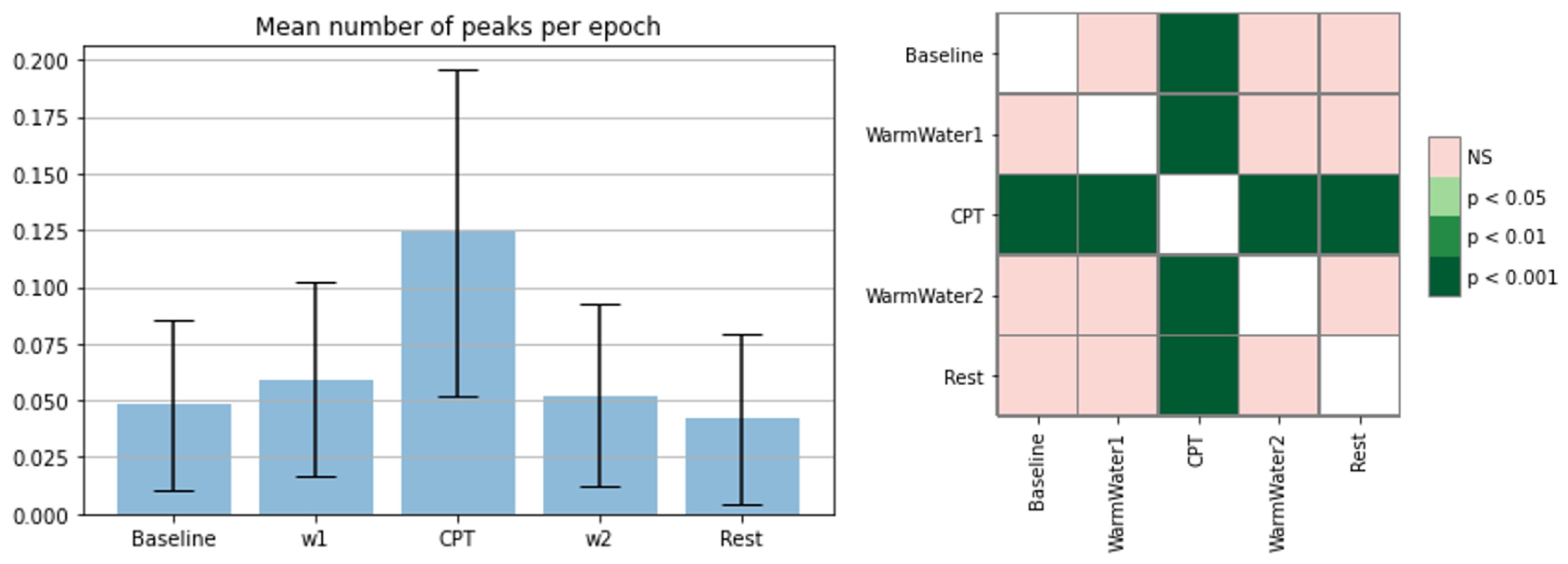
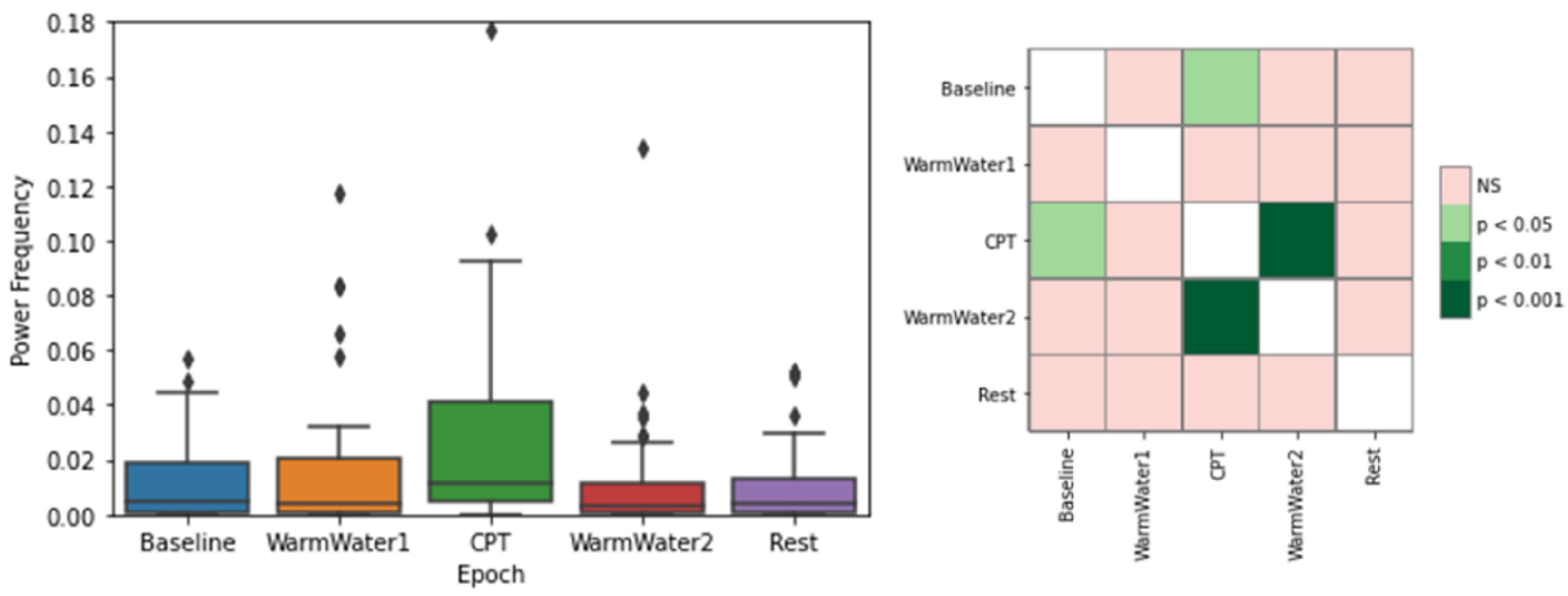
4.3. EDA Processing and Analysis
5. Discussion
6. Conclusions and Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raja, S.; Carr, D.; Cohen, M.; Finnerup, N.; Flor, H.; Gibson, S.; Keefe, F.; Mogil, J.; Ringkamp, M.; Sluka, K.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Loggia, M.L.; Napadow, V. Multi-parameter autonomic-based pain assessment: More is more? Pain 2012, 153, 1779–1780. [Google Scholar] [CrossRef] [PubMed]
- Cowen, R.; Stasiowska, M.K.; Laycock, H.; Bantel, C. Assessing pain objectively: The use of physiological markers. Anaesthesia 2015, 70, 828–847. [Google Scholar] [CrossRef] [PubMed]
- Hampf, G. Influence of cold pain in the hand on skin impedance, heart rate and skin temperature. Physiol. Behav. 1990, 47, 217–218. [Google Scholar] [CrossRef] [PubMed]
- Kregel, K.C.; Seals, D.R.; Callister, R. Sympathetic nervous system activity during skin cooling in humans: Relationship to stimulus intensity and pain sensation. J. Physiol. 1992, 454, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Younger, J.; Mccue, R.; Mackey, S. Pain Outcomes: A Brief Review of Instruments and Techniques. Curr. Pain Headache Rep. 2009, 13, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Birnie, K.A.; Caes, L.; Wilson, A.C.; Williams, S.E.; Chambers, C.T. A practical guide and perspectives on the use of experimental pain modalities with children and adolescents. Pain Manag. 2014, 4, 97–111. [Google Scholar] [CrossRef] [PubMed]
- McCaul, K.D.; Monson, N.; Maki, R.H. Does distraction reduce pain-produced distress among college students? Health Psychol. 1992, 11, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.; Robinson, M.; Riley, J.; Sheffield, D. Sex, Gender, and Blood Pressure: Contributions to Experimental Pain Report. Psychosom. Med. 2001, 63, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Tousignant-Laflamme, Y.; Rainville, P.; Marchand, S. Establishing a Link Between Heart Rate and Pain in Healthy Subjects: A Gender Effect. J. Pain Off. J. Am. Pain Soc. 2005, 6, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Schestatsky, P.; Valls-Solé, J.; Costa, J.; León, L.; Veciana, M.; Chaves, M.L. Skin autonomic reactivity to thermoalgesic stimuli. Clin. Auton. Res. 2007, 17, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Hallman, D.; Lindberg, L.G.; Arnetz, B.; Lyskov, E. Effects of static contraction and cold stimulation on cardiovascular autonomic indices, trapezius blood flow and muscle activity in chronic neck–shoulder pain. Eur. J. Appl. Physiol. 2011, 111, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Brusselmans, G.; Nogueira, H.; De Schamphelaere, E.; Devulder, J.; Crombez, G. Skin Temperature during Cold Pressor Test in Fibromyalgia: An Evaluation of the Autonomic Nervous System? Acta Anaesthesiol. Belg. 2015, 66, 19–27. [Google Scholar]
- Jiang, M.; Rosio, R.; Syrjälä, E.; Anzanpour, A.; Terävä, V.; Rahmani, A.M.; Salanterä, S.; Aantaa, R.; Hagelberg, N.; Liljeberg, P. Acute pain intensity monitoring with the classification of multiple physiological parameters. J. Clin. Monit. Comput. 2019, 33, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Gouverneur, P.; Li, F.; Adamczyk, W.M.; Szikszay, T.M.; Luedtke, K.; Grzegorzek, M. Comparison of Feature Extraction Methods for Physiological Signals for Heat-Based Pain Recognition. Sensors 2021, 21, 4838. [Google Scholar] [CrossRef] [PubMed]
- MATLAB. Version 9.10.0.1684407 (R2021a); The MathWorks Inc.: Natick, MA, USA, 2021. [Google Scholar]
- Posada-Quintero, H.F.; Florian, J.P.; Orjuela-Cañón, A.D.; Aljama-Corrales, T.; Charleston-Villalobos, S.; Chon, K.H. Power Spectral Density Analysis of Electrodermal Activity for Sympathetic Function Assessment. Ann. Biomed. Eng. 2016, 44, 3124–3135. [Google Scholar] [CrossRef] [PubMed]
- Streff, A.; Kuehl, L.K.; Michaux, G.; Anton, F. Differential physiological effects during tonic painful hand immersion tests using hot and ice water. Eur. J. Pain 2010, 14, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Wijsman, J.; Grundlehner, B.; Penders, J.; Hermens, H. Trapezius Muscle EMG as Predictor of Mental Stress. ACM Trans. Embed. Comput. Syst. 2013, 12, 1–20. [Google Scholar] [CrossRef]
- Lovallo, W. The Cold Pressor Test and Autonomic Function: A Review and Integration. Psychophysiology 1975, 12, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Weise, F.; Laude, D.; Girard, A.; Zitoun, P.; Siché, J.P.; Elghozi, J.L. Effects of the cold pressor test on short-term fluctuations of finger arterial blood pressure and heart rate in normal subjects. Clin. Auton. Res. 1993, 3, 303–310. [Google Scholar] [CrossRef] [PubMed]







| Feature | Description |
|---|---|
| Mean HR | Number of beats per minute (mean) |
| R peaks | Maximum value of the ECG cycles (upward deflections) |
| S peaks | Minimum value of the ECG cycles (downward deflections) |
| RMSSD | Root Mean Square of Successive Differences between normal heartbeats. It is a reflection of the beat-to-beat difference in the HR and is used to estimate the alteration of the HRV caused by the vagus nerve. |
| pNN50 | Percentage of successive RR intervals that are greater than 50 ms, associated with the Parasympathetic Nervous System (PNS) activity |
| SampEn | Measures the regularity and complexity of a given signal. Smaller values indicate a regular and predictable signal |
| Features | Description |
|---|---|
| RMSE and RMSA | Root Mean Square for Electromyogram and Amplitude, respectively. Related to the constant force and not-fatiguing contractions of the muscles. |
| VAR | Variance. It allows for expressing the power of the EMG signal. |
| Features | Description |
|---|---|
| SCR peaks | The number of SCR peaks (cumulative calculation of SCR peaks for each participant and epoch averaged and normalized by time in seconds). |
| EDASymp | Indexes of the sympathetic nervous system for the frequency band of 0.045–0.25 Hz [17]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastião, R.; Bento, A.; Brás, S. Analysis of Physiological Responses during Pain Induction. Sensors 2022, 22, 9276. https://doi.org/10.3390/s22239276
Sebastião R, Bento A, Brás S. Analysis of Physiological Responses during Pain Induction. Sensors. 2022; 22(23):9276. https://doi.org/10.3390/s22239276
Chicago/Turabian StyleSebastião, Raquel, Ana Bento, and Susana Brás. 2022. "Analysis of Physiological Responses during Pain Induction" Sensors 22, no. 23: 9276. https://doi.org/10.3390/s22239276
APA StyleSebastião, R., Bento, A., & Brás, S. (2022). Analysis of Physiological Responses during Pain Induction. Sensors, 22(23), 9276. https://doi.org/10.3390/s22239276








