HyScreen: A Ground-Based Imaging System for High-Resolution Red and Far-Red Solar-Induced Chlorophyll Fluorescence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hyperspectral Sensors
2.2. Measurement Protocol
2.3. Case Studies
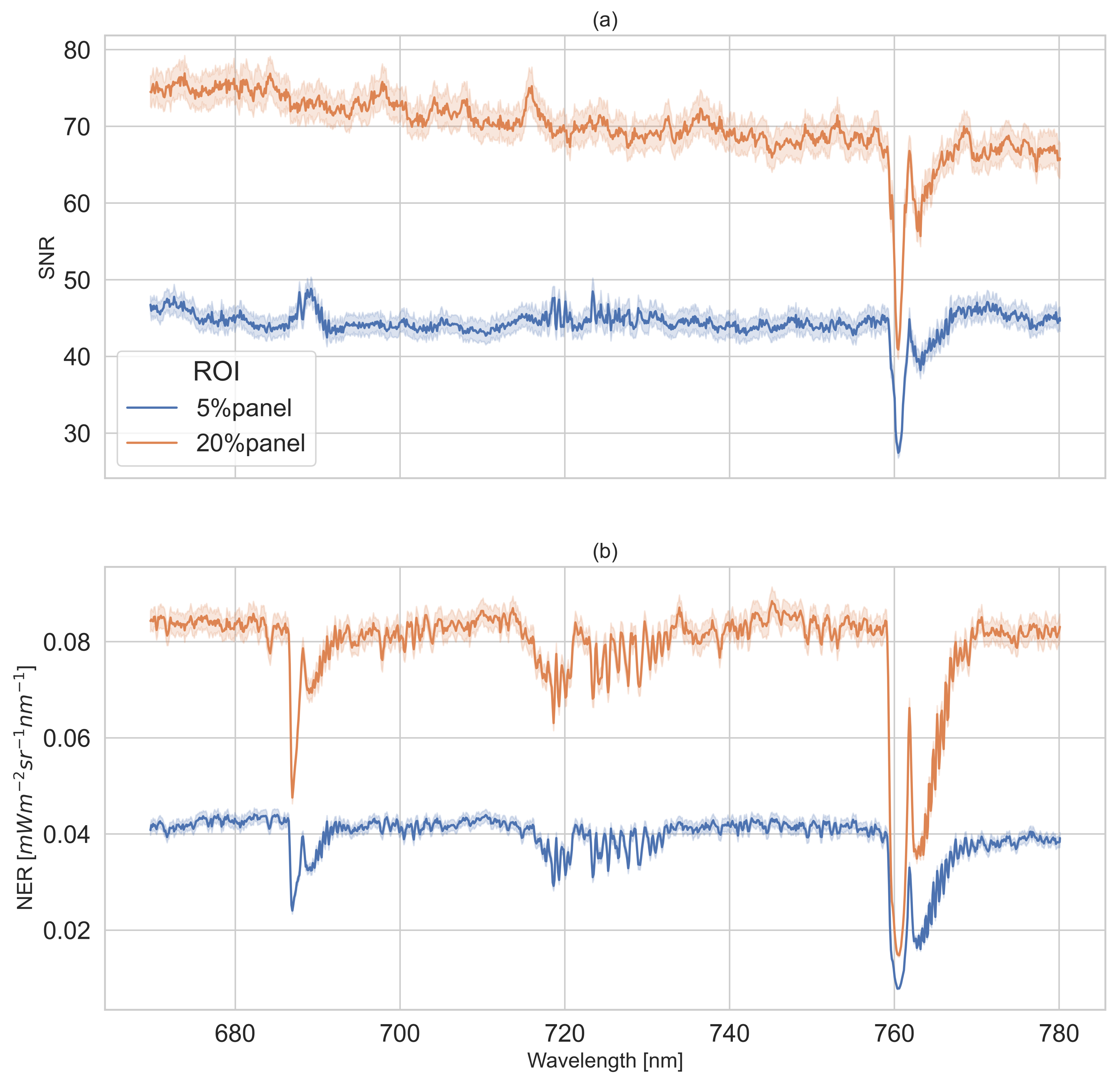
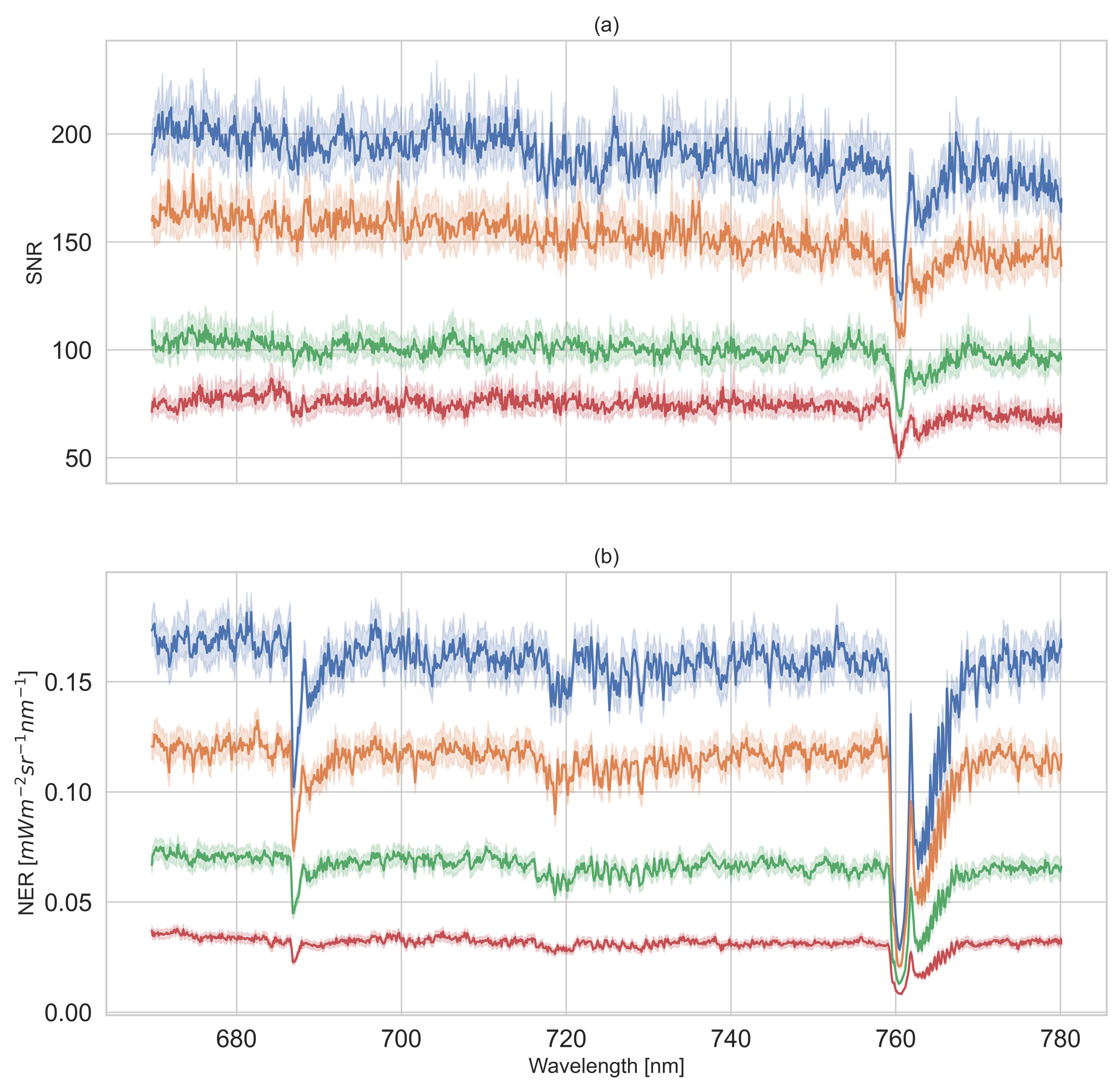
2.3.1. SNR and NER of Reflectance Panels
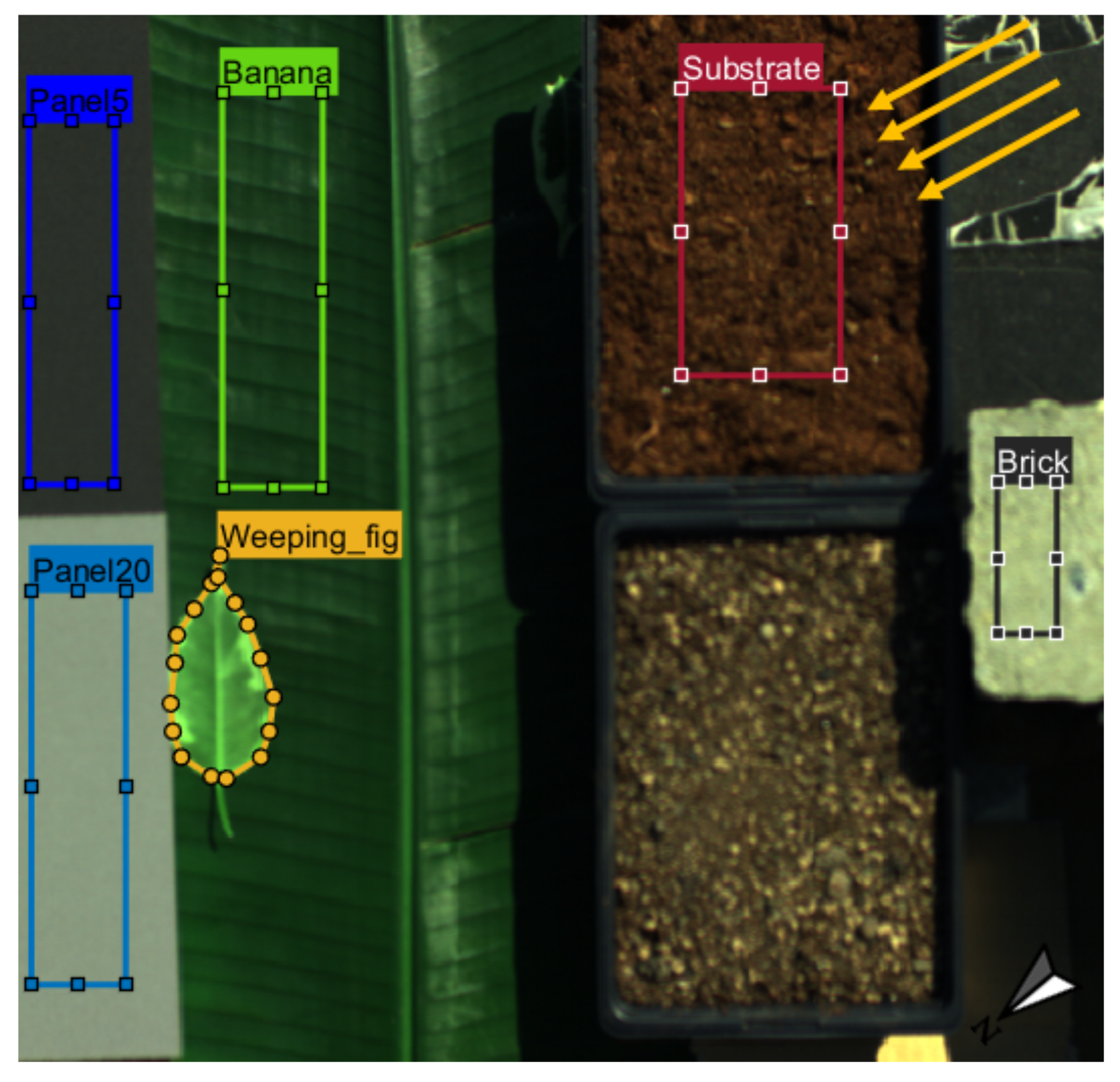
2.3.2. Experiment with Vegetation and Non-Vegetation Objects
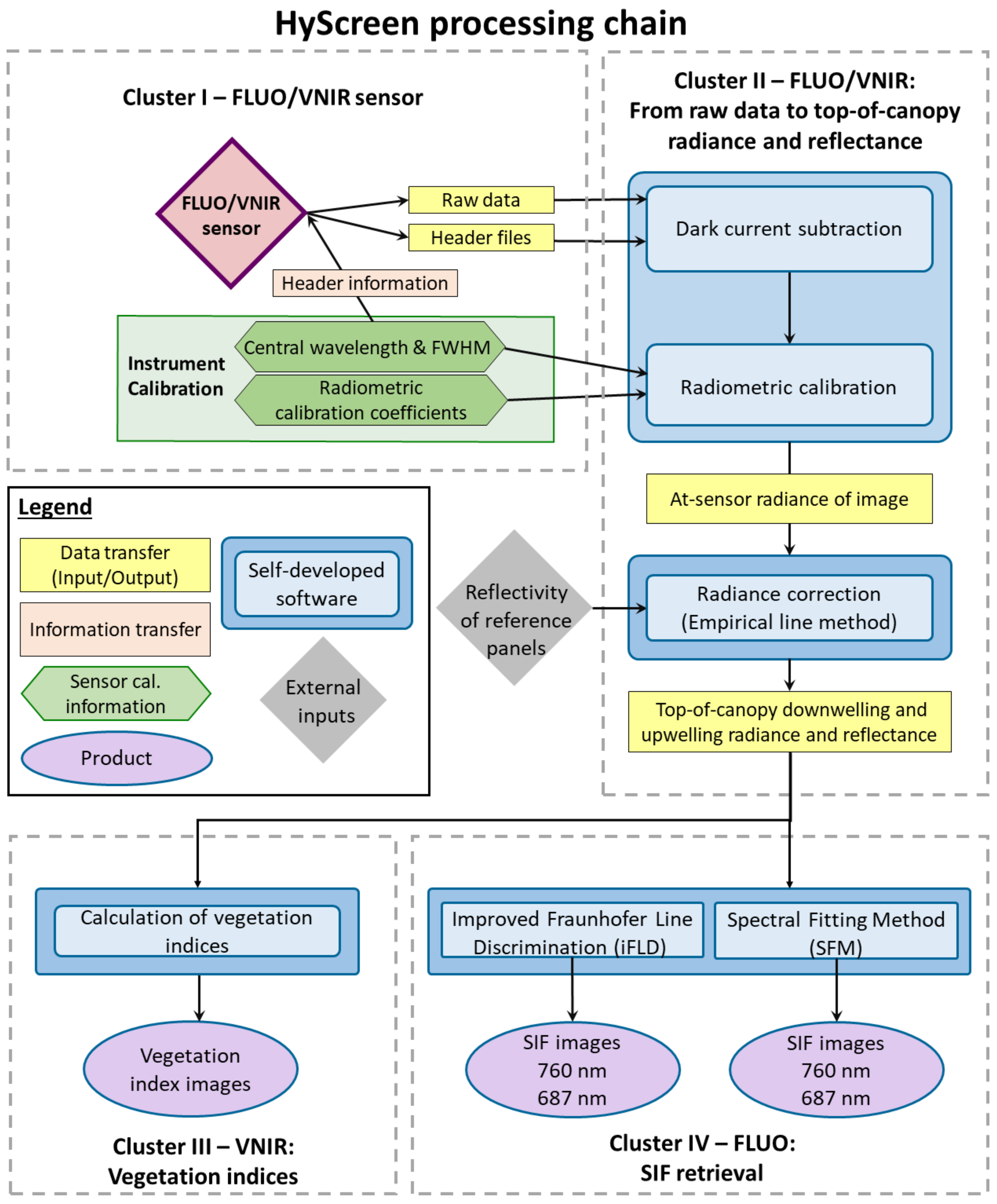
2.4. Image Processing Chain
2.4.1. Raw Data to At-Sensor Radiance
2.4.2. Empirical Line Method for Radiance and Apparent Reflectance
2.4.3. Vegetation Indices
2.4.4. Solar-Induced Chlorophyll Fluorescence Retrieval
2.4.5. SNR and NER Calculation
3. Results
3.1. Results of SNR and NER of Reflectance Panels
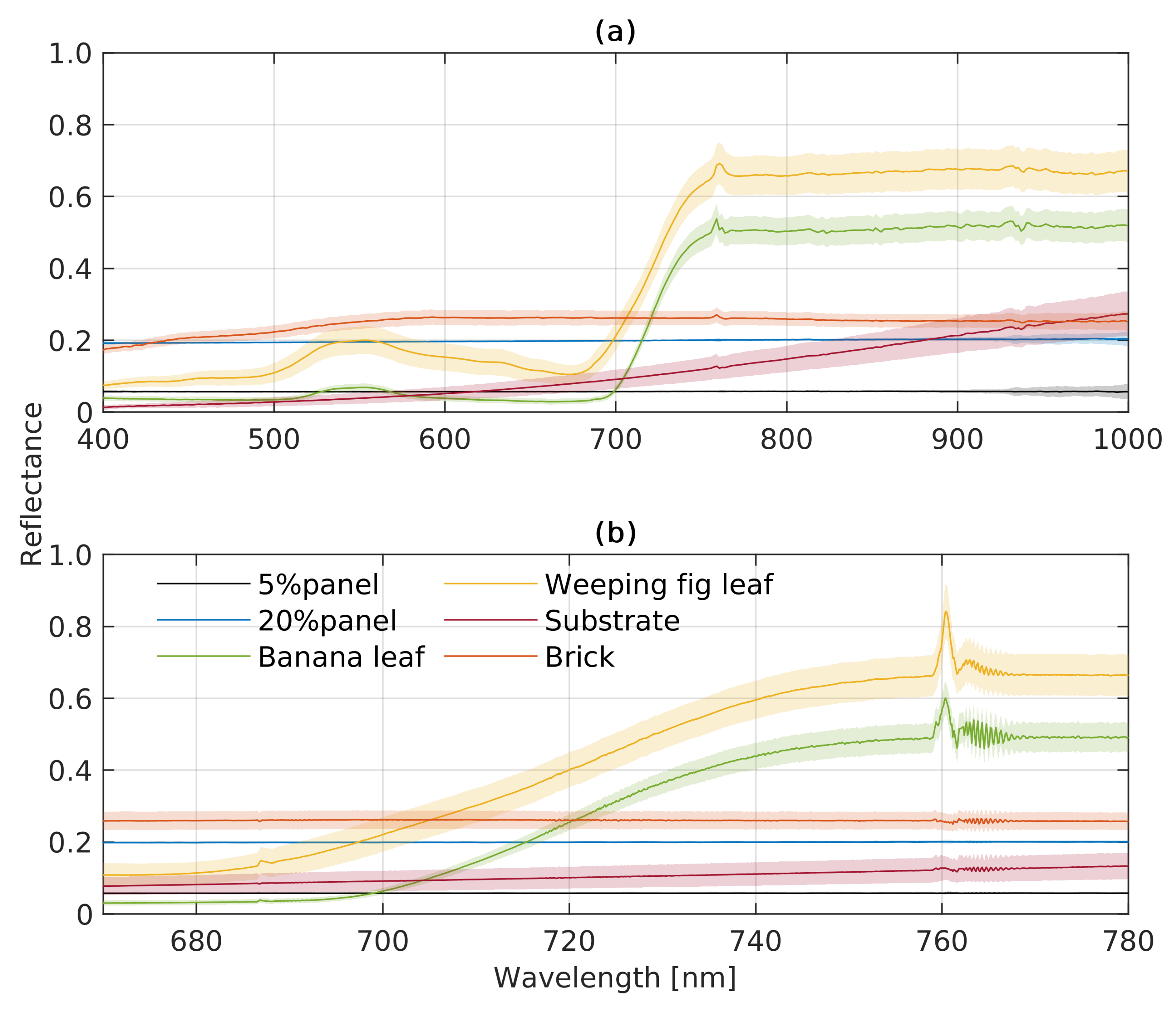
3.2. Results of the Experiment with Vegetation and Non-Vegetation Objects
3.2.1. Radiance and Apparent Reflectance Spectra
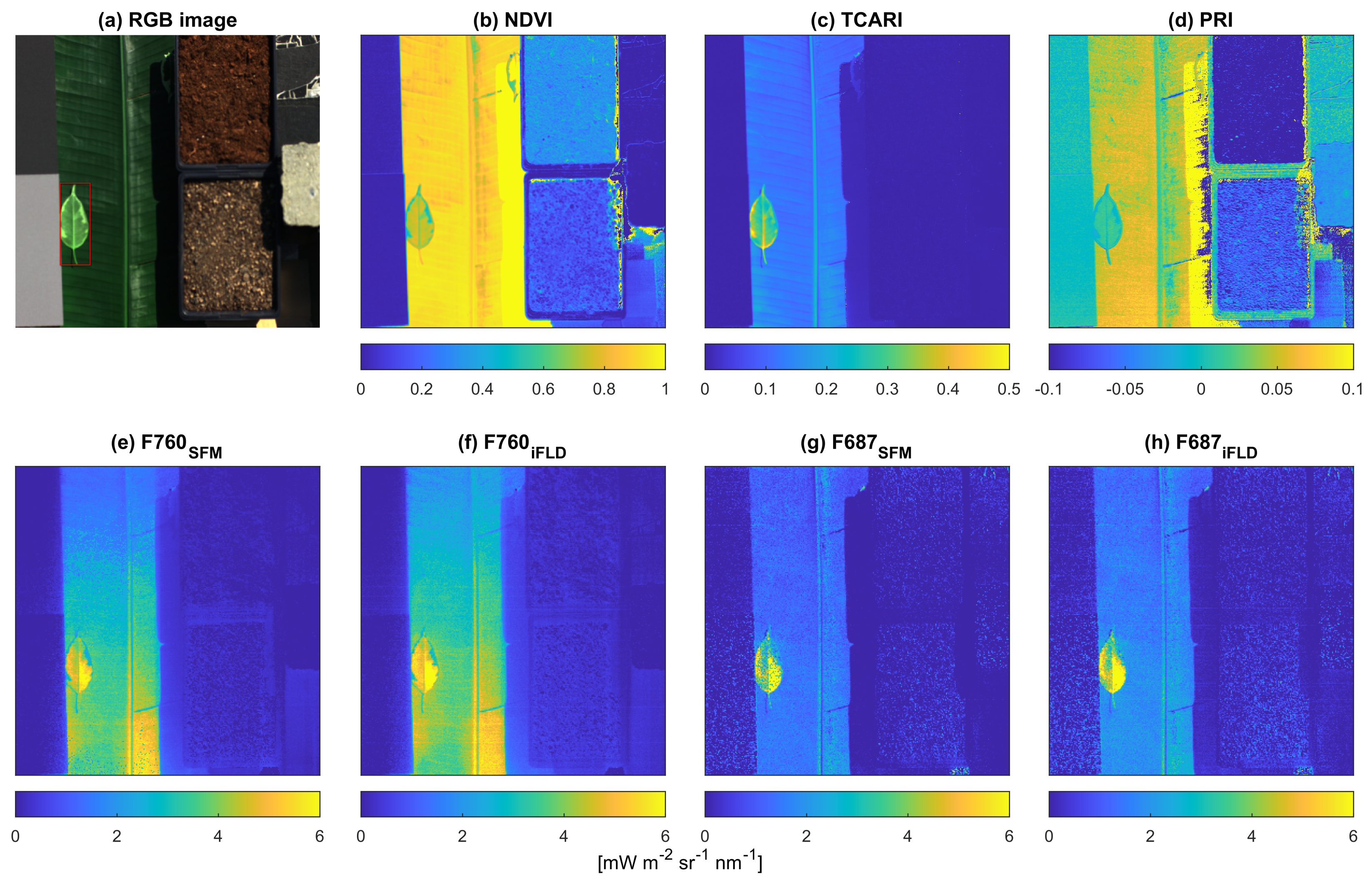
3.2.2. Vegetation Indices and SIF
4. Discussion
4.1. SNR and NER Characterization
4.2. Processing Chain Improvements
4.3. Spatial Distribution of Vegetation Indices and SIF
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. SNR and NER of Two Reference Panels
References
- Schurr, U.; Walter, A.; Rascher, U. Functional dynamics of plant growth and photosynthesis—From steady-state to dynamics—From homogeneity to heterogeneity. Plant Cell Environ. 2006, 29, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Kefauver, S.; Araus, J.L.; Muller, O.; Rascher, U.; Flood, P.J.; Lawson, T. Measuring the dynamic photosynthome. Ann. Bot. 2018, 122, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Porcar-Castell, A.; Malenovskỳ, Z.; Magney, T.; Van Wittenberghe, S.; Fernández-Marín, B.; Maignan, F.; Zhang, Y.; Maseyk, K.; Atherton, J.; Albert, L.P.; et al. Chlorophyll a fluorescence illuminates a path connecting plant molecular biology to Earth-system science. Nat. Plants 2021, 7, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Kolber, Z.; Klimov, D.; Ananyev, G.; Rascher, U.; Berry, J.; Osmond, B. Measuring photosynthetic parameters at a distance: Laser induced fluorescence transient (LIFT) method for remote measurements of photosynthesis in terrestrial vegetation. Photosynth. Res. 2005, 84, 121–129. [Google Scholar] [CrossRef]
- Mohammed, G.H.; Colombo, R.; Middleton, E.M.; Rascher, U.; van der Tol, C.; Nedbal, L.; Goulas, Y.; Pérez-Priego, O.; Damm, A.; Meroni, M.; et al. Remote sensing of solar-induced chlorophyll fluorescence (SIF) in vegetation: 50 years of progress. Remote Sens. Environ. 2019, 231, 111177. [Google Scholar] [CrossRef]
- Meroni, M.; Rossini, M.; Guanter, L.; Alonso, L.; Rascher, U.; Colombo, R.; Moreno, J. Remote sensing of solar-induced chlorophyll fluorescence: Review of methods and applications. Remote Sens. Environ. 2009, 113, 2037–2051. [Google Scholar] [CrossRef]
- Cendrero-Mateo, M.P.; Wieneke, S.; Damm, A.; Alonso, L.; Pinto, F.; Moreno, J.; Guanter, L.; Celesti, M.; Rossini, M.; Sabater, N.; et al. Sun-induced chlorophyll fluorescence III: Benchmarking retrieval methods and sensor characteristics for proximal sensing. Remote Sens. 2019, 11, 962. [Google Scholar] [CrossRef] [Green Version]
- Joiner, J.; Yoshida, Y.; Vasilkov, A.; Middleton, E. First observations of global and seasonal terrestrial chlorophyll fluorescence from space. Biogeosciences 2011, 8, 637–651. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Labrador, J.; Hueni, A.; Mihai, L.; Sakowska, K.; Julitta, T.; Kuusk, J.; Sporea, D.; Alonso, L.; Burkart, A.; Cendrero-Mateo, M.P.; et al. Sun-induced chlorophyll fluorescence I: Instrumental considerations for proximal spectroradiometers. Remote Sens. 2019, 11, 960. [Google Scholar] [CrossRef]
- Aasen, H.; Van Wittenberghe, S.; Sabater Medina, N.; Damm, A.; Goulas, Y.; Wieneke, S.; Hueni, A.; Malenovskỳ, Z.; Alonso, L.; Pacheco-Labrador, J.; et al. Sun-induced chlorophyll fluorescence II: Review of passive measurement setups, protocols, and their application at the leaf to canopy level. Remote Sens. 2019, 11, 927. [Google Scholar] [CrossRef] [Green Version]
- Siegmann, B.; Alonso, L.; Celesti, M.; Cogliati, S.; Colombo, R.; Damm, A.; Douglas, S.; Guanter, L.; Hanuš, J.; Kataja, K.; et al. The high-performance airborne imaging spectrometer HyPlant—From raw images to top-of-canopy reflectance and fluorescence products: Introduction of an automatized processing chain. Remote Sens. 2019, 11, 2760. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; van der Tol, C. Linking canopy scattering of far-red sun-induced chlorophyll fluorescence with reflectance. Remote Sens. Environ. 2018, 209, 456–467. [Google Scholar] [CrossRef]
- Pinto, F.; Damm, A.; Schickling, A.; Panigada, C.; Cogliati, S.; Müller-Linow, M.; Ballvora, A.; Rascher, U. Sun-induced chlorophyll fluorescence from high-resolution imaging spectroscopy data to quantify spatio-temporal patterns of photosynthetic function in crop canopies. Plant Cell Environ. 2016, 39, 1500–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Liu, L. Influence of the canopy BRDF characteristics and illumination conditions on the retrieval of solar-induced chlorophyll fluorescence. Int. J. Remote Sens. 2018, 39, 1782–1799. [Google Scholar] [CrossRef]
- Kallel, A. FluLCVRT: Reflectance and fluorescence of leaf and canopy modeling based on Monte Carlo vector radiative transfer simulation. J. Quant. Spectrosc. Radiat. Transf. 2020, 253, 107183. [Google Scholar] [CrossRef]
- Rascher, U.; Alonso, L.; Burkart, A.; Cilia, C.; Cogliati, S.; Colombo, R.; Damm, A.; Drusch, M.; Guanter, L.; Hanus, J.; et al. Sun-induced fluorescence—A new probe of photosynthesis: First maps from the imaging spectrometer HyPlant. Glob. Chang. Biol. 2015, 21, 4673–4684. [Google Scholar] [CrossRef] [Green Version]
- Bendig, J.; Malenovskỳ, Z.; Gautam, D.; Lucieer, A. Solar-induced chlorophyll fluorescence measured from an unmanned aircraft system: Sensor etaloning and platform motion correction. IEEE Trans. Geosci. Remote Sens. 2019, 58, 3437–3444. [Google Scholar] [CrossRef]
- Wang, N.; Suomalainen, J.; Bartholomeus, H.; Kooistra, L.; Masiliūnas, D.; Clevers, J.G. Diurnal variation of sun-induced chlorophyll fluorescence of agricultural crops observed from a point-based spectrometer on a UAV. Int. J. Appl. Earth Obs. Geoinf. 2021, 96, 102276. [Google Scholar] [CrossRef]
- Julitta, T.; Burkart, A.; Colombo, R.; Rossini, M.; Schickling, A.; Migliavacca, M.; Cogliati, S.; Wutzler, T.; Rascher, U. Accurate measurements of fluorescence in the O2A and O2B band using the FloX spectroscopy system—Results and prospects. In Proceedings of the Potsdam Greenhouse Gas Workshop from Photosystems to Ecosystems, Potsdam, Germany, 24–26 October 2017. [Google Scholar]
- Pinto, F.; Müller-Linow, M.; Schickling, A.; Cendrero-Mateo, M.P.; Ballvora, A.; Rascher, U. Multiangular observation of canopy sun-induced chlorophyll fluorescence by combining imaging spectroscopy and stereoscopy. Remote Sens. 2017, 9, 415. [Google Scholar] [CrossRef]
- Jiang, Y.; Snider, J.L.; Li, C.; Rains, G.C.; Paterson, A.H. Ground based hyperspectral imaging to characterize canopy-level photosynthetic activities. Remote Sens. 2020, 12, 315. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.; Meacham-Hensold, K.; Siebers, M.H.; Bernacchi, C.J. The inverse relationship between solar-induced fluorescence yield and photosynthetic capacity: Benefits for field phenotyping. J. Exp. Bot. 2021, 72, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Meroni, M.; Celesti, M.; Cogliati, S.; Julitta, T.; Panigada, C.; Rascher, U.; Van der Tol, C.; Colombo, R. Analysis of red and far-red sun-induced chlorophyll fluorescence and their ratio in different canopies based on observed and modeled data. Remote Sens. 2016, 8, 412. [Google Scholar] [CrossRef] [Green Version]
- Ač, A.; Malenovskỳ, Z.; Olejníčková, J.; Gallé, A.; Rascher, U.; Mohammed, G. Meta-analysis assessing potential of steady-state chlorophyll fluorescence for remote sensing detection of plant water, temperature and nitrogen stress. Remote Sens. Environ. 2015, 168, 420–436. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Chen, M.; Hao, D.; Damm, A.; Badgley, G.; Rascher, U.; Johnson, J.E.; Dechant, B.; Siegmann, B.; Ryu, Y.; et al. Combining near-infrared radiance of vegetation and fluorescence spectroscopy to detect effects of abiotic changes and stresses. Remote Sens. Environ. 2022, 270, 112856. [Google Scholar] [CrossRef]
- The Mathworks, Inc. MATLAB Version 9.10.0.2015706 (R2021a); The Mathworks, Inc.: Natick, MA, USA, 2021. [Google Scholar]
- Van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Smith, G.M.; Milton, E.J. The use of the empirical line method to calibrate remotely sensed data to reflectance. Int. J. Remote Sens. 1999, 20, 2653–2662. [Google Scholar] [CrossRef]
- Arroyo-Mora, J.P.; Kalacska, M.; Løke, T.; Schläpfer, D.; Coops, N.C.; Lucanus, O.; Leblanc, G. Assessing the impact of illumination on UAV pushbroom hyperspectral imagery collected under various cloud cover conditions. Remote Sens. Environ. 2021, 258, 112396. [Google Scholar] [CrossRef]
- Sabater, N.; Vicent, J.; Alonso, L.; Verrelst, J.; Middleton, E.M.; Porcar-Castell, A.; Moreno, J. Compensation of oxygen transmittance effects for proximal sensing retrieval of canopy–leaving sun–induced chlorophyll fluorescence. Remote Sens. 2018, 10, 1551. [Google Scholar] [CrossRef] [Green Version]
- Rouse, J., Jr.; Haas, R.H.; Schell, J.; Deering, D. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite—1 Symposium, NASA SP-351, Washington, DC, USA, 10–14 December 1973; pp. 309–317. [Google Scholar]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Gamon, J.; Penuelas, J.; Field, C. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Alonso, L.; Gomez-Chova, L.; Vila-Frances, J.; Amoros-Lopez, J.; Guanter, L.; Calpe, J.; Moreno, J. Improved Fraunhofer Line Discrimination method for vegetation fluorescence quantification. IEEE Geosci. Remote Sens. Lett. 2008, 5, 620–624. [Google Scholar] [CrossRef]
- Cogliati, S.; Verhoef, W.; Kraft, S.; Sabater, N.; Alonso, L.; Vicent, J.; Moreno, J.; Drusch, M.; Colombo, R. Retrieval of sun-induced fluorescence using advanced spectral fitting methods. Remote Sens. Environ. 2015, 169, 344–357. [Google Scholar] [CrossRef]
- Schaepman, M.E.; Dangel, S. Solid laboratory calibration of a nonimaging spectroradiometer. Appl. Opt. 2000, 39, 3754–3764. [Google Scholar] [CrossRef] [PubMed]
- Granlund, L.; Keinänen, M.; Tahvanainen, T. Identification of peat type and humification by laboratory VNIR/SWIR hyperspectral imaging of peat profiles with focus on fen-bog transition in aapa mires. Plant Soil 2021, 460, 667–686. [Google Scholar] [CrossRef]
- Damm, A.; Erler, A.; Hillen, W.; Meroni, M.; Schaepman, M.E.; Verhoef, W.; Rascher, U. Modeling the impact of spectral sensor configurations on the FLD retrieval accuracy of sun-induced chlorophyll fluorescence. Remote Sens. Environ. 2011, 115, 1882–1892. [Google Scholar] [CrossRef]
- Cendrero-Mateo, M.; Bennertz, S.; Burkart, A.; Julitta, T.; Cogliati, S.; Scharr, H.; Rademske, P.; Alonso, L.; Pinto, F.; Rascher, U. Sun induced fluorescence calibration and validation for field phenotyping. In Proceedings of the IGARSS 2018–2018 IEEE International Geoscience and Remote Sensing Symposium, Valencia, Spain, 22–27 July 2018; IEEE: Valencia, Spain, 2018; pp. 8248–8251. [Google Scholar] [CrossRef]
- Nehir, M.; Frank, C.; Aßmann, S.; Achterberg, E.P. Improving optical measurements: Non-linearity compensation of compact charge-coupled device (CCD) spectrometers. Sensors 2019, 19, 2833. [Google Scholar] [CrossRef] [Green Version]
- Albert, L.P.; Cushman, K.; Allen, D.W.; Zong, Y.; Alonso, L.; Kellner, J.R. Stray light characterization in a high-resolution imaging spectrometer designed for solar-induced fluorescence. In Proceedings of the Algorithms, Technologies, and Applications for Multispectral and Hyperspectral Imagery XXV, Baltimore, MA, USA, 16–18 April 2019; Volume 10986, pp. 116–124. [Google Scholar] [CrossRef]
- Scharr, H.; Rademske, P.; Alonso, L.; Cogliati, S.; Rascher, U. Spatio-spectral deconvolution for high resolution spectral imaging with an application to the estimation of sun-induced fluorescence. Remote Sens. Environ. 2021, 267, 112718. [Google Scholar] [CrossRef]
- Lysenko, V. Fluorescence kinetic parameters and cyclic electron transport in guard cell chloroplasts of chlorophyll-deficient leaf tissues from variegated weeping fig (Ficus benjamina L.). Planta 2012, 235, 1023–1033. [Google Scholar] [CrossRef]
- Shih, T.H.; Lin, S.H.; Huang, M.Y.; Huang, W.D.; Yang, C.M. Transcriptome profile of the variegated Ficus microcarpa c.v. Milky stripe fig leaf. Int. J. Mol. Sci. 2019, 20, 1338. [Google Scholar] [CrossRef] [Green Version]
- Gastellu-Etchegorry, J.P.; Lauret, N.; Yin, T.; Landier, L.; Kallel, A.; Malenovskỳ, Z.; Al Bitar, A.; Aval, J.; Benhmida, S.; Qi, J.; et al. DART: Recent advances in remote sensing data modeling with atmosphere, polarization, and chlorophyll fluorescence. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2017, 10, 2640–2649. [Google Scholar] [CrossRef]
- Van der Tol, C.; Verhoef, W.; Timmermans, J.; Verhoef, A.; Su, Z. An integrated model of soil-canopy spectral radiances, photosynthesis, fluorescence, temperature and energy balance. Biogeosciences 2009, 6, 3109–3129. [Google Scholar] [CrossRef] [Green Version]
- Acebron, K.; Matsubara, S.; Jedmowski, C.; Emin, D.; Muller, O.; Rascher, U. Diurnal dynamics of nonphotochemical quenching in Arabidopsis npq mutants assessed by solar-induced fluorescence and reflectance measurements in the field. New Phytol. 2021, 229, 2104–2119. [Google Scholar] [CrossRef]
- Van Wittenberghe, S.; Sabater, N.; Cendrero-Mateo, M.; Tenjo, C.; Moncholi, A.; Alonso, L.; Moreno, J. Towards the quantitative and physically-based interpretation of solar-induced vegetation fluorescence retrieved from global imaging. Photosynthetica 2021, 59, 438–457. [Google Scholar] [CrossRef]



| Sensor | VNIR | FLUO |
|---|---|---|
| Sensor type | CMOS | sCMOS |
| Dynamic range (bit) | 12 | 14 |
| Spectral range (nm) | 400.00–1000.00 | 669.68–780.22 |
| Mean spectral sampling interval (nm) | 0.79 | 0.06 |
| Mean spectral resolution (FWHM) (nm) | 3.21 | 0.31 |
| Field of view (FOV) (°) | 32.71 | 32.24 |
| Spatial pixels | 1312 | 1512 |
| Standard measurement setup | ||
| Spectral binning | 2 | 2 |
| Spectral sampling interval (nm) | 1.46–1.61 | 0.10–0.12 |
| Spectral resolution (FWHM) (nm) | 2.42–4.35 | 0.36 at /0.40 at |
| Number of bands | 384 | 1004 |
| Spatial binning | 2 | 4 |
| Spatial pixels | 656 | 378 |
| Swath width of the sensor mounted 1 m above canopy (mm) | 587 | 578 |
| Frame rate (fps) | 20 | 10 |
| Exposure time range (ms) | 0.1–50 | 0.1–100 |
| Power consumption (W) | 80 | 115 |
| Input voltage (V) | 12 | 12 |
| Index | Equation | Reference |
|---|---|---|
| NDVI | [32] | |
| TCARI | [33] | |
| PRI | [34] |
| iFLD | ||||||
| Method | and R Interpolation WI | Abs. Feature WI | Interpolation Method | |||
| OA | 750–780 nm | 759.3–768.0 nm | : polynomial 2nd grade | |||
| R: linear smoothing spline | ||||||
| OB | 665–716 nm | 683.3–696.9 nm | : polynomial 2nd grade | |||
| R: cubic smoothing spline | ||||||
| SFM | ||||||
| Method | F and R Interpolation WI | Abs. Feature WI | Model function | Gaussian function parameters | ||
| a | c | b | ||||
| OA | 750–780 nm | 759.3–768.0 nm | F: Gaussian R: Cubic spline | iFLD retrieved fluorescence ub = 15, lb = 0 | 740 nm | 24 |
| = +Inf | ||||||
| = −Inf | ||||||
| OB | 684–700 nm | 686.5–690.0 nm | 680 nm | 8 | ||
| = +Inf | ||||||
| = −Inf | ||||||
| ROIs | NDVI | TCARI | PRI | |||
|---|---|---|---|---|---|---|
| Mean | Std. | Mean | Std. | Mean | Std. | |
| Banana | 0.89 | 0.02 | 0.12 | 0.01 | 0.05 | 0.01 |
| Weeping fig | 0.72 | 0.05 | 0.30 | 0.04 | 0.01 | 0.01 |
| Substrate | 0.32 | 0.04 | 0.00 | 0.00 | −0.13 | 0.04 |
| Brick | −0.01 | 0.01 | −0.01 | 0.01 | −0.03 | 0.01 |
| ROIs | iFLD687 | iFLD760 | SFM687 | SFM760 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Std. | Mean | Std. | Mean | Std. | Mean | Std. | |
| Banana | 1.44 | 0.27 | 2.59 | 0.42 | 1.13 | 0.26 | 1.96 | 0.59 |
| Weeping fig | 4.90 | 1.63 | 5.17 | 0.96 | 4.24 | 1.76 | 4.62 | 0.91 |
| Substrate | −0.14 | 0.55 | 0.19 | 0.15 | .05 | 0.43 | 0.07 | 0.11 |
| Brick | −0.69 | 0.95 | −0.18 | 0.16 | −0.37 | 0.74 | −0.11 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Cendrero-Mateo, M.P.; Bendig, J.; Siegmann, B.; Acebron, K.; Kneer, C.; Kataja, K.; Muller, O.; Rascher, U. HyScreen: A Ground-Based Imaging System for High-Resolution Red and Far-Red Solar-Induced Chlorophyll Fluorescence. Sensors 2022, 22, 9443. https://doi.org/10.3390/s22239443
Peng H, Cendrero-Mateo MP, Bendig J, Siegmann B, Acebron K, Kneer C, Kataja K, Muller O, Rascher U. HyScreen: A Ground-Based Imaging System for High-Resolution Red and Far-Red Solar-Induced Chlorophyll Fluorescence. Sensors. 2022; 22(23):9443. https://doi.org/10.3390/s22239443
Chicago/Turabian StylePeng, Huaiyue, Maria Pilar Cendrero-Mateo, Juliane Bendig, Bastian Siegmann, Kelvin Acebron, Caspar Kneer, Kari Kataja, Onno Muller, and Uwe Rascher. 2022. "HyScreen: A Ground-Based Imaging System for High-Resolution Red and Far-Red Solar-Induced Chlorophyll Fluorescence" Sensors 22, no. 23: 9443. https://doi.org/10.3390/s22239443





