Photostability Enhancement of Dual-Luminophore Pressure-Sensitive Paint by Adding Antioxidants
Abstract
:1. Introductions
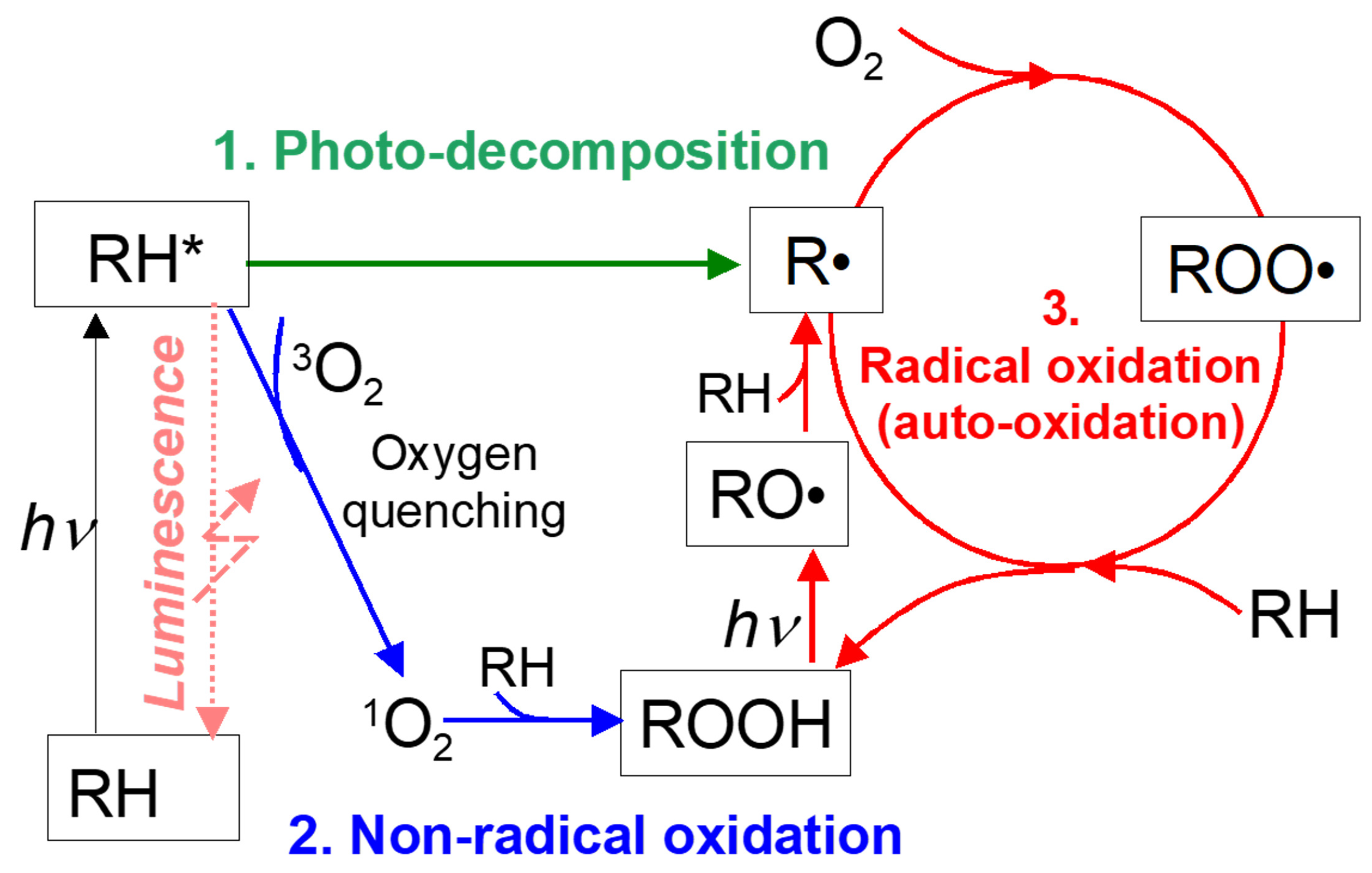
2. Photodegradation and Antioxidants
2.1. Photodegradation Mechanism
2.2. Antioxidants
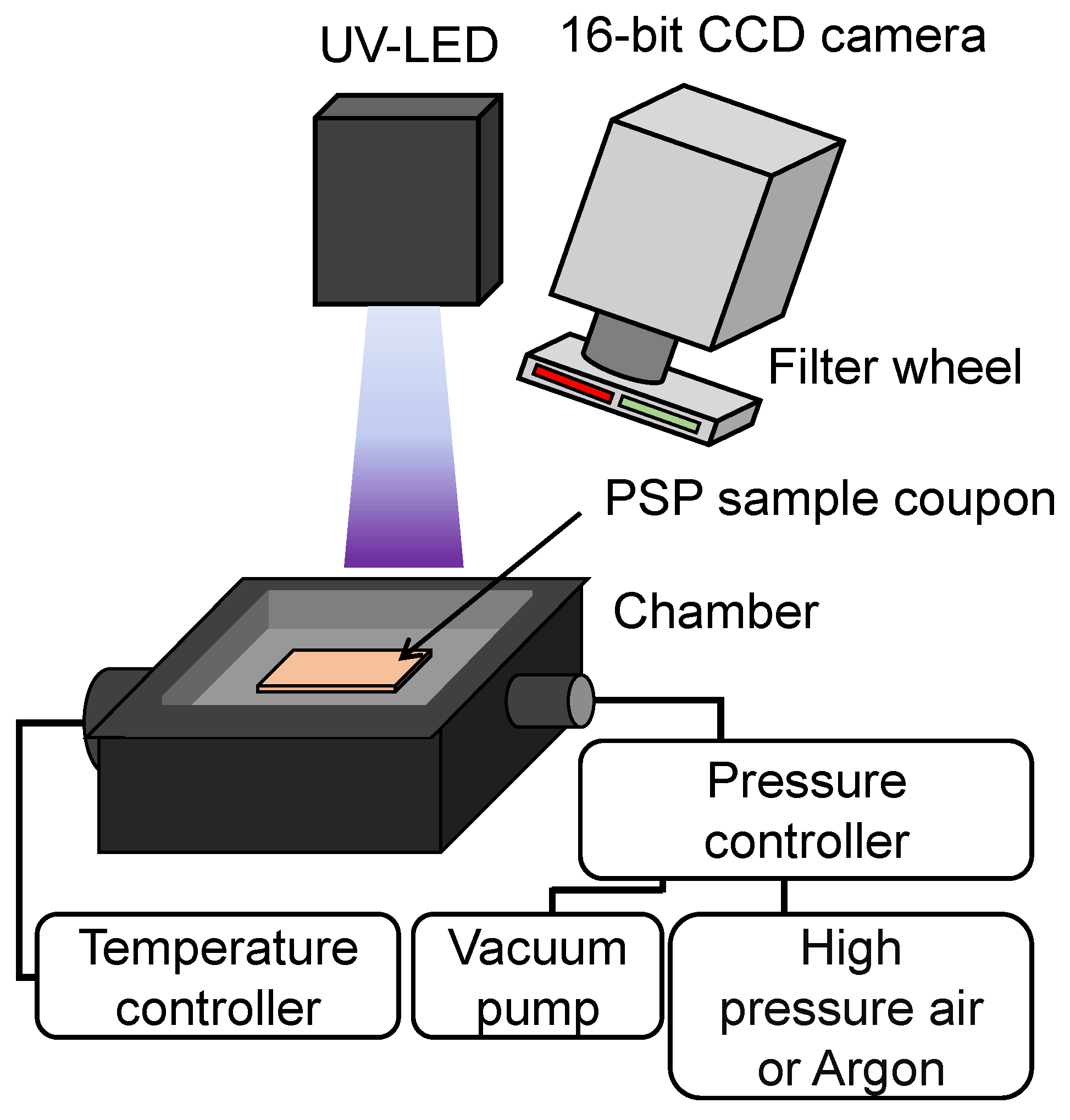
3. Experimental Setup
3.1. Paint Formulation
3.2. PSP Calibration System
3.3. Test Conditions and Procedures
4. Results and Discussions
4.1. Degradation Rates
4.2. Pressure and Temperature Sensitivities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kavandi, J.; Callis, J.; Gouterman, M.; Khalil, G.; Wright, D.; Green, E.; Burns, D.; McLachlan, B. Luminescent barometry in wind tunnels. Rev. Sci. Instruments 1990, 61, 3340–3347. [Google Scholar] [CrossRef]
- McLachlan, B.; Kavandi, J.; Callis, J.; Gouterman, M.; Green, E.; Khalil, G.; Burns, D. Surface pressure field mapping using luminescent coatings. Exp. Fluids 1993, 14, 33–41. [Google Scholar] [CrossRef]
- Liu, T.; Campbell, B.T.; Burns, S.P.; Sullivan, J.P. Temperature-and pressure-sensitive luminescent paints in aerodynamics. Appl. Mech. Rev. 1997, 50, 227–246. [Google Scholar] [CrossRef]
- Bell, J.H.; Schairer, E.T.; Hand, L.A.; Mehta, R.D. Surface pressure measurements using luminescent coatings. Annu. Rev. Fluid Mech. 2001, 33, 155–206. [Google Scholar] [CrossRef]
- Liu, T.; Sullivan, J.P.; Asai, K.; Klein, C.; Egami, Y. Pressure and Temperature Sensitive Paints; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Gregory, J.; Asai, K.; Kameda, M.; Liu, T.; Sullivan, J. A review of pressure-sensitive paint for high-speed and unsteady aerodynamics. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2008, 222, 249–290. [Google Scholar] [CrossRef]
- Gregory, J.W.; Sakaue, H.; Liu, T.; Sullivan, J.P. Fast pressure-sensitive paint for flow and acoustic diagnostics. Annu. Rev. Fluid Mech. 2014, 46, 303–330. [Google Scholar] [CrossRef]
- Peng, D.; Liu, Y. Fast pressure-sensitive paint for understanding complex flows: From regular to harsh environments. Exp. Fluids 2020, 61, 8. [Google Scholar] [CrossRef]
- Sellers, M. Application of pressure sensitive paint for determining aerodynamic loads on a scale model of the F-16C. In Proceedings of the 21st Aerodynamic Measurement Technology and Ground Testing Conference, Denver, CO, USA, 19–22 June 2000; p. 2528. [Google Scholar]
- Klein, C.; Engler, R.H.; Henne, U.; Sachs, W.E. Application of pressure-sensitive paint for determination of the pressure field and calculation of the forces and moments of models in a wind tunnel. Exp. Fluids 2005, 39, 475–483. [Google Scholar] [CrossRef]
- Vardaki, E.; Stokes, N.; Patel, S.; Gustafsson, P. Pressure sensitive paint measurements on the Gripen model at the ARA transonic wind tunnel. In Proceedings of the 50th AIAA Aerospace Sciences Meeting including the New Horizons Forum and Aerospace Exposition, Nashville, TN, USA, 9–12 January 2012; p. 1188. [Google Scholar]
- Torgerson, S.; Liu, T.; Sullivan, J. Use of pressure sensitive paints in low speed flows. In Proceedings of the Advanced Measurement and Ground Testing Conference, New Orleans, LA, USA, 17–20 June 1996; p. 2184. [Google Scholar]
- Merienne, M.; Bouvier, F. Vortical flow field investigation using a two-component pressure sensitive paint at low speed. In Proceedings of the ICIASF 99. 18th International Congress on Instrumentation in Aerospace Simulation Facilities. Record (Cat. No. 99CH37025), Toulouse, France, 14–17 June 1999; pp. 19/1–19/9. [Google Scholar]
- Engler, R.H.; Mérienne, M.C.; Klein, C.; Le Sant, Y. Application of PSP in low speed flows. Aerosp. Sci. Technol. 2002, 6, 313–322. [Google Scholar] [CrossRef]
- Quinn, M.K.; Gongora-Orozco, N.; Kontis, K.; Ireland, P. Application of pressure-sensitive paint to low-speed flow around a U-bend of strong curvature. Exp. Therm. Fluid Sci. 2013, 48, 58–66. [Google Scholar] [CrossRef]
- Kammeyer, M.; Donovan, J.; Kelble, C.; Benne, M.; Kihlken, T.; Felter, J. Accuracy assessment of a pressure-sensitive paint measurement system. In Proceedings of the 40th AIAA Aerospace Sciences Meeting & Exhibit, Reno, NV, USA, 14–17 January 2002; p. 530. [Google Scholar]
- Mitsuo, K.; Kurita, M.; Nakakita, K.; Watanabe, S. Temperature correction of PSP measurement for low-speed flow using infrared camera. In Proceedings of the ICIASF 2005 Record International Congress on Instrumentation in Aerospace Simulation Facilities, Sendai, Japan, 29 August–1 September 2005; pp. 214–220. [Google Scholar]
- Nakakita, K.; Kurita, M.; Mitsuo, K. Development of the Pressure-Sensitive Paint Measurement for Large Wind Tunnels at Japan Aerospace Exploration Agency. In Proceedings of the 24th International Congress of the Aeronautical Sciences, Yokohama, Japan, 29 August–3 September 2004; Volume 3, p. 2004. [Google Scholar]
- Kameda, M.; Seki, H.; Makoshi, T.; Amao, Y.; Nakakita, K. Unsteady measurement of a transonic delta wing flow by a novel PSP. In Proceedings of the 26th AIAA Applied Aerodynamics Conference, Honolulu, HI, USA, 18–21 August 2008; p. 6418. [Google Scholar]
- Peng, D.; Jiao, L.; Sun, Z.; Gu, Y.; Liu, Y. Simultaneous PSP and TSP measurements of transient flow in a long-duration hypersonic tunnel. Exp. Fluids 2016, 57, 188. [Google Scholar] [CrossRef]
- Sugioka, Y.; Koike, S.; Nakakita, K.; Numata, D.; Nonomura, T.; Asai, K. Experimental analysis of transonic buffet on a 3D swept wing using fast-response pressure-sensitive paint. Exp. Fluids 2018, 59, 108. [Google Scholar] [CrossRef]
- Sugioka, Y.; Nakakita, K.; Koike, S.; Nakajima, T.; Nonomura, T.; Asai, K. Characteristic unsteady pressure field on a civil aircraft wing related to the onset of transonic buffet. Exp. Fluids 2021, 62, 20. [Google Scholar] [CrossRef]
- Hradil, J.; Davis, C.; Mongey, K.; McDonagh, C.; MacCraith, B.D. Temperature-corrected pressure-sensitive paint measurements using a single camera and a dual-lifetime approach. Meas. Sci. Technol. 2002, 13, 1552. [Google Scholar] [CrossRef]
- Zelelow, B.; Khalil, G.E.; Phelan, G.; Carlson, B.; Gouterman, M.; Callis, J.B.; Dalton, L.R. Dual luminophor pressure sensitive paint: II. Lifetime based measurement of pressure and temperature. Sens. Actuators B Chem. 2003, 96, 304–314. [Google Scholar] [CrossRef]
- Mitsuo, K.; Asai, K.; Hayasaka, M.; Kameda, M. Temperature correction of PSP measurement using dual-luminophor coating. J. Vis. 2003, 6, 213–223. [Google Scholar] [CrossRef]
- Gouterman, M.; Callis, J.; Dalton, L.; Khalil, G.; Mebarki, Y.; Cooper, K.R.; Grenier, M. Dual luminophor pressure-sensitive paint: III. Application to automotive model testing. Meas. Sci. Technol. 2004, 15, 1986. [Google Scholar] [CrossRef]
- Mitsuo, K.; Kurita, M.; Nakakita, K.; Watanabe, S.; Wada, Y. Temperature Correction of PSP Measurement Using Bi-luminophore Dyes. In Proceedings of the 26th AIAA Aerodynamic Measurement Technology and Ground Testing Conference, Seattle, WA, USA, 23–26 June 2008; p. 3945. [Google Scholar]
- Crafton, J.; Fonov, S. Development of pressure-sensitive paint systems for low speed flows and large wind tunnels. In Proceedings of the 51st AIAA Aerospace Sciences Meeting including the New Horizons Forum and Aerospace Exposition, Grapevine, TX, USA, 7–10 January 2013; p. 482. [Google Scholar]
- Quinn, M.K. Binary pressure-sensitive paint measurements using miniaturised, colour, machine vision cameras. Meas. Sci. Technol. 2018, 29, 055107. [Google Scholar] [CrossRef] [Green Version]
- Kameya, T.; Matsuda, Y.; Egami, Y.; Yamaguchi, H.; Niimi, T. Combined pressure and temperature sensor using pressure-and temperature-sensitive paints. In Proceedings of the 2012 International Symposium on Micro-NanoMechatronics and Human Science (MHS), Nagoya, Japan, 4–7 November 2012; pp. 473–475. [Google Scholar]
- Katagiri, S.; Manseki, K.; Tsukahara, Y.; Mitsuo, K.; Wada, Y. Luminescent polymer film containing tetranuclear Eu (III) complex as temperature-sensing device. J. Alloys Compd. 2008, 453, L1–L3. [Google Scholar] [CrossRef]
- Basu, B.J.; Anandan, C.; Rajam, K. Study of the mechanism of degradation of pyrene-based pressure sensitive paints. Sens. Actuators B Chem. 2003, 94, 257–266. [Google Scholar] [CrossRef]
- Egami, Y.; Asai, K. Effects of antioxidants on photodegradation of porous pressure-sensitive paint. In Proceedings of the 22nd AIAA Aerodynamic Measurement Technology and Ground Testing Conference, St. Louis, MO, USA, 24–26 June 2002; p. 2905. [Google Scholar]
- Feldman, D. Polymer weathering: Photo-oxidation. J. Polym. Environ. 2002, 10, 163–173. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene. SpringerPlus 2013, 2, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroe, B.M.; Mrowca, J.J. Quenching of singlet oxygen by nickel complexes. J. Phys. Chem. 1979, 83, 591–595. [Google Scholar] [CrossRef]
- Guillory, J.; Cook, C. Energy transfer processes involving ultraviolet stabilizers. Quenching of excited states of ketones. J. Am. Chem. Soc. 1973, 95, 4885–4891. [Google Scholar] [CrossRef]
- Salvador, M.; Gasparini, N.; Perea, J.D.; Paleti, S.H.; Distler, A.; Inasaridze, L.N.; Troshin, P.A.; Lüer, L.; Egelhaaf, H.J.; Brabec, C. Suppressing photooxidation of conjugated polymers and their blends with fullerenes through nickel chelates. Energy Environ. Sci. 2017, 10, 2005–2016. [Google Scholar] [CrossRef] [Green Version]
- Sugioka, Y.; Numata, D.; Asai, K.; Koike, S.; Nakakita, K.; Nakajima, T. Polymer/ceramic pressure-sensitive paint with reduced roughness for unsteady measurement in transonic flow. AIAA J. 2018, 56, 2145–2156. [Google Scholar] [CrossRef]
- Mitsuo, K.; Kurita, M.; Nakakita, K.; Fujii, K.; Watanabe, S.; Katagiri, S.; Wada, Y. Development of bi-luminophore pressure-sensitive paint systems. In Proceedings of the 2007 22nd International Congress on Instrumentation in Aerospace Simulation Facilities, Pacific Grove, CA, USA, 10–14 June 2007; pp. 1–9. [Google Scholar]
- Mochizuki, D.; Tamura, S.; Yasutake, H.; Kataoka, T.; Mitsuo, K.; Wada, Y. A photostable bi-luminophore pressure-sensitive paint measurement system developed with mesoporous silica nanoparticles. J. Nanosci. Nanotechnol. 2013, 13, 2777–2781. [Google Scholar] [CrossRef]






| Polymer | Particle | Solvent |
|---|---|---|
| Poly(IBM) | Toluene | |
| 0.500 g | 6.64 mg | 30 mL |
| Pressure Sensor PtTFPP | Temperature Sensor Tetranuclear Eu(III) Complex | Antioxidant | Solvent Toluene | |
|---|---|---|---|---|
| Pt | 4 mg | N/A | N/A | 20 mL |
| Eu | N/A | 41 mg | N/A | 20 mL |
| Dual | 4 mg | 41 mg | N/A | 20 mL |
| +FRS | 4 mg | 41 mg | FRS 0.4 mg | 20 mL |
| +HPD | 4mg | 41 mg | HPD 0.4 mg | 20 mL |
| +SOQ | 4 mg | 41 mg | SOQ 0.4 mg | 20 mL |
| Pressure Sensor | Temperature Sensor | Binder | Degradation Rate of Temperature Sensor | |
|---|---|---|---|---|
| Present study | PtTFPP | tetranuclear Eu(III) complex | Polymer/ceramic [39] | 46% for 30 min |
| Mitsuo et al. [40] | PdTFPP | Poly-IBM-co-TFEM | Ten times higher than alone | |
| Mochizuki et al. [41] | 55% for 30 min |
| Pressure Sensor PtTFPP | Temperature Sensor Tetranuclear Eu(III) Complex | Antioxidant | Solvent Toluene | |
|---|---|---|---|---|
| +2SOQ | 4 mg | 41 mg | SOQ 4 mg | 20 mL |
| +3SOQ | 4 mg | 41 mg | SOQ 40 mg | 20 mL |
| (a) Before Aging Tests | ||||
|---|---|---|---|---|
| Pressure Sensitivity [%/kPa] | Temperature Sensitivity [%/°C] | |||
| Pressure Sensor | Temperature Sensor | Pressure Sensor | Temperature Sensor | |
| Dual | 0.53 | 0.023 | 2.0 | 3.4 |
| +2SOQ | 0.41 | 0.052 | 1.8 | 3.0 |
| +3SOQ | 0.13 | 0.054 | 1.2 | 1.7 |
| (b) After Aging Tests | ||||
| Pressure Sensitivity [%/kPa] | Temperature Sensitivity [%/°C] | |||
| Pressure Sensor | Temperature Sensor | Pressure Sensor | Temperature Sensor | |
| Dual | 0.50 | 0.062 | 2.0 | 2.3 |
| +2SOQ | 0.42 | 0.051 | 1.7 | 2.8 |
| +3SOQ | 0.13 | 0.048 | 1.2 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, K.; Ozawa, Y.; Asai, K.; Nonomura, T. Photostability Enhancement of Dual-Luminophore Pressure-Sensitive Paint by Adding Antioxidants. Sensors 2022, 22, 9470. https://doi.org/10.3390/s22239470
Uchida K, Ozawa Y, Asai K, Nonomura T. Photostability Enhancement of Dual-Luminophore Pressure-Sensitive Paint by Adding Antioxidants. Sensors. 2022; 22(23):9470. https://doi.org/10.3390/s22239470
Chicago/Turabian StyleUchida, Kazuki, Yuta Ozawa, Keisuke Asai, and Taku Nonomura. 2022. "Photostability Enhancement of Dual-Luminophore Pressure-Sensitive Paint by Adding Antioxidants" Sensors 22, no. 23: 9470. https://doi.org/10.3390/s22239470






