The Effects of Isometric Fatigue on Trunk Muscle Stiffness: Implications for Shear-Wave Elastography Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
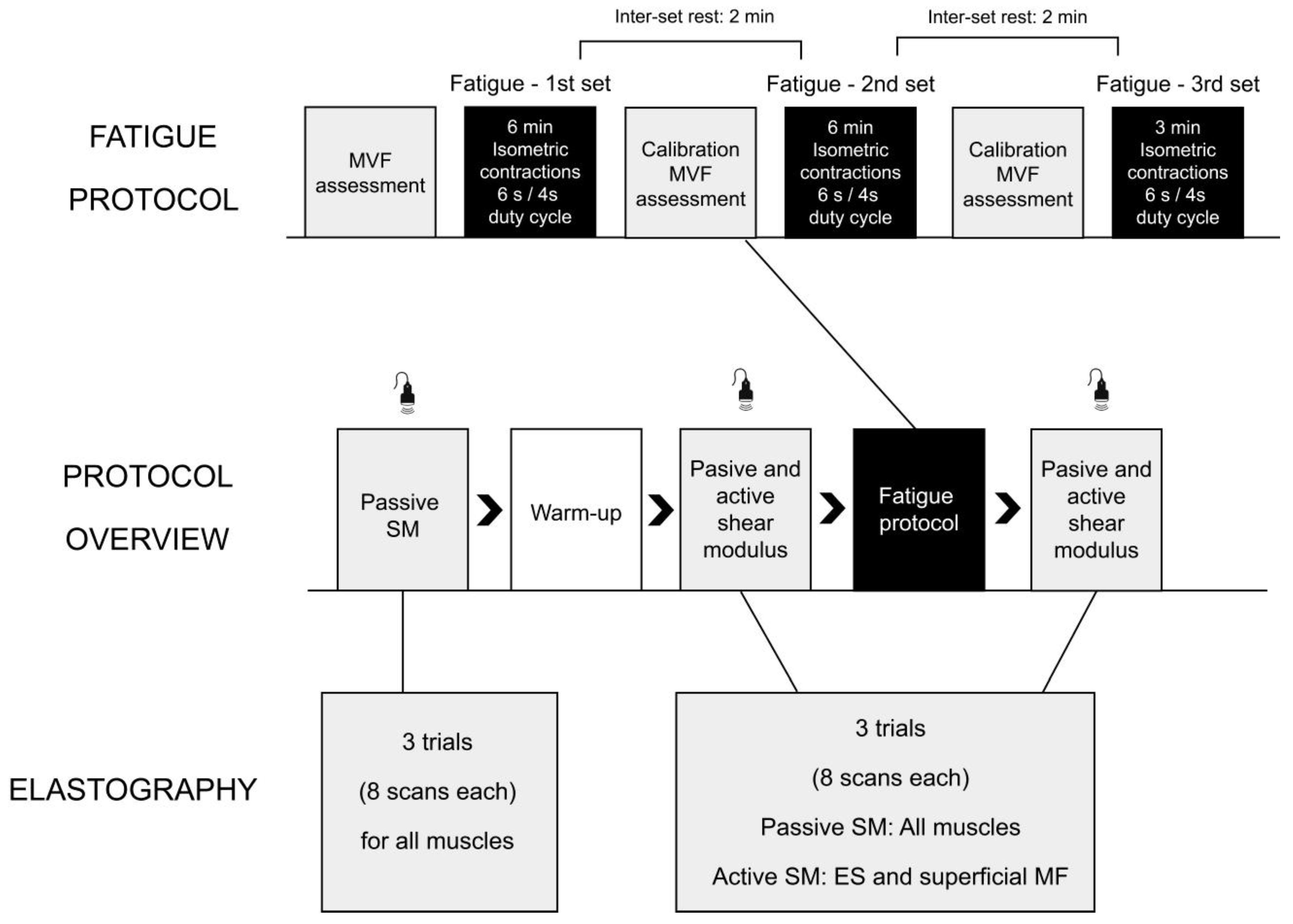
2.2. Study Protocol
2.3. Shear-Wave Elastography
2.4. Maximal Voluntary Force
2.5. Isometric Trunk Extension Protocol
2.6. Statistical Analysis
3. Results
3.1. The Effects of Warm-Up on Passive Shear Modulus
3.2. Changes in Passive Shear Modulus
3.3. Changes in Active Shear Modulus
3.4. Correlations between Changes in Shear Modulus and Maximal Voluntary Force
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A Systematic Review of the Global Prevalence of Low Back Pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What Low Back Pain Is and Why We Need to Pay Attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef] [Green Version]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-Specific Low Back Pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wettstein, M.; Eich, W.; Bieber, C.; Tesarz, J. Pain Intensity, Disability, and Quality of Life in Patients with Chronic Low Back Pain: Does Age Matter? Pain Med. 2019, 20, 464–475. [Google Scholar] [CrossRef] [Green Version]
- Husky, M.M.; Ferdous Farin, F.; Compagnone, P.; Fermanian, C.; Kovess-Masfety, V. Chronic Back Pain and Its Association with Quality of Life in a Large French Population Survey. Health Qual. Life Outcomes 2018, 16, 195. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.I.; Mirza, S.K.; Turner, J.A.; Comstock, B.A.; Sullivan, S.D.; Deyo, R.A.; Hollingworth, W. Expenditures and Health Status among Adults with Back and Neck Problems. JAMA 2008, 299, 656–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, K.E.; Florence, C.S.; Joski, P. Which Medical Conditions Account for the Rise in Health Care Spending? Health Aff. 2004, 23, W4-437. [Google Scholar] [CrossRef] [Green Version]
- Lacey, R.J.; Belcher, J.; Croft, P.R. Does Life Course Socio-Economic Position Influence Chronic Disabling Pain in Older Adults? A General Population Study. Eur. J. Public Health 2013, 23, 534–540. [Google Scholar] [CrossRef]
- Kent, P.M.; Keating, J.L. Can We Predict Poor Recovery from Recent-Onset Nonspecific Low Back Pain? A Systematic Review. Man. Ther. 2008, 13, 12–28. [Google Scholar] [CrossRef]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef]
- Creze, M.; Nordez, A.; Soubeyrand, M.; Rocher, L.; Maître, X.; Bellin, M.F. Shear Wave Sonoelastography of Skeletal Muscle: Basic Principles, Biomechanical Concepts, Clinical Applications, and Future Perspectives. Skeletal Radiol. 2018, 47, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Guo, J.-Y.; Cohen, J.H.; Parker, K.J. Relationship between Shear Elastic Modulus and Passive Muscle Force: An Ex-Vivo Study. J. Biomech. 2013, 46, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Blank, J.; Blomquist, M.; Arant, L.; Cone, S.; Roth, J. Characterizing Musculoskeletal Tissue Mechanics Based on Shear Wave Propagation—A 1 Systematic Review of Current Methods and Reported Measurements. Ann. Biomed. Eng. 2021, 50, 751–768. [Google Scholar] [CrossRef]
- Koppenhaver, S.; Kniss, J.; Lilley, D.; Oates, M.; Fernández-de-las-Peñas, C.; Maher, R.; Croy, T.; Shinohara, M. Reliability of Ultrasound Shear-Wave Elastography in Assessing Low Back Musculature Elasticity in Asymptomatic Individuals. J. Electromyogr. Kinesiol. 2018, 39, 49–57. [Google Scholar] [CrossRef]
- Masaki, M.; Aoyama, T.; Murakami, T.; Yanase, K.; Ji, X.; Tateuchi, H.; Ichihashi, N. Association of Low Back Pain with Muscle Stiffness and Muscle Mass of the Lumbar Back Muscles, and Sagittal Spinal Alignment in Young and Middle-Aged Medical Workers. Clin. Biomech. 2017, 49, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Murillo, C.; Falla, D.; Sanderson, A.; Rushton, A.; Heneghan, N.R. Shear Wave Elastography Investigation of Multifidus Stiffness in Individuals with Low Back Pain. J. Electromyogr. Kinesiol. 2019, 47, 19–24. [Google Scholar] [CrossRef]
- Koppenhaver, S.; Gaffney, E.; Oates, A.; Eberle, L.; Young, B.; Hebert, J.; Proulx, L.; Shinohara, M. Lumbar Muscle Stiffness Is Different in Individuals with Low Back Pain than Asymptomatic Controls and Is Associated with Pain and Disability, but Not Common Physical Examination Findings. Musculoskelet. Sci. Pract. 2020, 45, 102078. [Google Scholar] [CrossRef] [PubMed]
- Arippa, F.; Leban, B.; Fadda, P.; Fancello, G.; Pau, M. Trunk Sway Changes in Professional Bus Drivers during Actual Shifts on Long-Distance Routes. Ergonomics 2022, 65, 762–774. [Google Scholar] [CrossRef]
- Kett, A.R.; Milani, T.L.; Sichting, F. Sitting for Too Long, Moving Too Little: Regular Muscle Contractions Can Reduce Muscle Stiffness During Prolonged Periods of Chair-Sitting. Front. Sport. Act. Living 2021, 3, 760533. [Google Scholar] [CrossRef]
- Voglar, M.; Wamerdam, J.; Kingma, I.; Sarabon, N.; Van Dieën, J.H. Prolonged Intermittent Trunk Flexion Increases Trunk Muscles Reflex Gains and Trunk Stiffness. PLoS ONE 2016, 11, e0162703. [Google Scholar] [CrossRef]
- Hendershot, B.D.; Toosizadeh, N.; Muslim, K.; Madigan, M.L.; Nussbaum, M.A. Evidence for an Exposure-Response Relationship between Trunk Flexion and Impairments in Trunk Postural Control. J. Biomech. 2013, 46, 2554–2557. [Google Scholar] [CrossRef] [PubMed]
- Nourollahi-Darabad, M.; Mazloumi, A.; Saraji, G.N.; Afshari, D.; Foroushani, A.R. Full Shift Assessment of Back and Head Postures in Overhead Crane Operators with and without Symptoms. J. Occup. Health 2018, 60, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Hendershot, B.; Bazrgari, B.; Muslim, K.; Toosizadeh, N.; Nussbaum, M.A.; Madigan, M.L. Disturbance and Recovery of Trunk Stiffness and Reflexive Muscle Responses Following Prolonged Trunk Flexion: Influences of Flexion Angle and Duration. Clin. Biomech. 2011, 26, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Bazrgari, B.; Hendershot, B.; Muslim, K.; Toosizadeh, N.; Nussbaum, M.A.; Madigan, M.L. Disturbance and Recovery of Trunk Mechanical and Neuromuscular Behaviours Following Prolonged Trunk Flexion: Influences of Duration and External Load on Creep-Induced Effects. Ergonomics 2011, 54, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Kett, A.R.; Sichting, F. Sedentary Behaviour at Work Increases Muscle Stiffness of the Back: Why Roller Massage Has Potential as an Active Break Intervention. Appl. Ergon. 2020, 82, 102947. [Google Scholar] [CrossRef]
- Chalchat, E.; Gennisson, J.L.; Peñailillo, L.; Oger, M.; Malgoyre, A.; Charlot, K.; Bourrilhon, C.; Siracusa, J.; Garcia-Vicencio, S. Changes in the Viscoelastic Properties of the Vastus Lateralis Muscle With Fatigue. Front. Physiol. 2020, 11, 307. [Google Scholar] [CrossRef]
- Siracusa, J.; Charlot, K.; Malgoyre, A.; Conort, S.; Tardo-Dino, P.E.; Bourrilhon, C.; Garcia-Vicencio, S. Resting Muscle Shear Modulus Measured with Ultrasound Shear-Wave Elastography as an Alternative Tool to Assess Muscle Fatigue in Humans. Front. Physiol. 2019, 10, 626. [Google Scholar] [CrossRef]
- Mendes, B.; Firmino, T.; Oliveira, R.; Neto, T.; Cruz-Montecinos, C.; Cerda, M.; Correia, J.P.; Vaz, J.R.; Freitas, S.R. Effects of Knee Flexor Submaximal Isometric Contraction until Exhaustion on Semitendinosus and Biceps Femoris Long Head Shear Modulus in Healthy Individuals. Sci. Rep. 2020, 10, 16433. [Google Scholar] [CrossRef]
- Kumamoto, T.; Seko, T.; Matsuda, R.; Miura, S. Repeated Standing Back Extension Exercise: Influence on Muscle Shear Modulus Change after Lumbodorsal Muscle Fatigue. Work 2021, 68, 1229–1237. [Google Scholar] [CrossRef]
- Botanlioglu, H.; Kantarci, F.; Kaynak, G.; Unal, Y.; Ertan, S.; Aydingoz, O.; Erginer, R.; Unlu, M.C.; Mihmanli, I.; Babacan, M. Shear Wave Elastography Properties of Vastus Lateralis and Vastus Medialis Obliquus Muscles in Normal Subjects and Female Patients with Patellofemoral Pain Syndrome. Skeletal Radiol. 2013, 42, 659–666. [Google Scholar] [CrossRef]
- Ando, R.; Suzuki, Y. Positive Relationship between Passive Muscle Stiffness and Rapid Force Production. Hum. Mov. Sci. 2019, 66, 285–291. [Google Scholar] [CrossRef]
- Yamazaki, K.; Inoue, K.; Miyamoto, N. Passive and Active Muscle Elasticity of Medial Gastrocnemius Is Related to Performance in Sprinters. Eur. J. Appl. Physiol. 2022, 122, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Ema, R. Association between Elastography-Assessed Muscle Mechanical Properties and High-Speed Dynamic Performance. Eur. J. Sport Sci. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ando, R.; Sato, S.; Hirata, N.; Tanimoto, H.; Imaizumi, N.; Suzuki, Y.; Hirata, K.; Akagi, R. Relationship between Resting Medial Gastrocnemius Stiffness and Drop Jump Performance. J. Electromyogr. Kinesiol. 2021, 58, 102549. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Zuriaga, D.; Adams, M.A.; Dolan, P. Is Activation of the Back Muscles Impaired by Creep or Muscle Fatigue? Spine 2010, 35, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Latimer, J.; Maher, C.G.; Refshauge, K.; Colaco, I. The Reliability and Validity of the Biering-Sorensen Test in Asymptomatic Subjects and Subjects Reporting Current or Previous Nonspecific Low Back Pain. Spine 1999, 24, 2085–2090. [Google Scholar] [CrossRef]
- Morel, B.; Hug, F.; Nordez, A.; Pournot, H.; Besson, T.; Mathevon, L.; Lapole, T. Reduced Active Muscle Stiffness after Intermittent Submaximal Isometric Contractions. Med. Sci. Sports Exerc. 2019, 51, 2603–2609. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, W.G. Measures of Reliability in Sports Medicine and Science. Sport. Med. 2000, 30, 375–381. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Eby, S.F.; Song, P.; Chen, S.; Chen, Q.; Greenleaf, J.F.; An, K.N. Validation of Shear Wave Elastography in Skeletal Muscle. J. Biomech. 2013, 46, 2381–2387. [Google Scholar] [CrossRef]
- Bernabei, M.; Lee, S.S.M.; Perreault, E.J.; Sandercock, T.G. Shear Wave Velocity Is Sensitive to Changes in Muscle Stiffness That Occur Independently from Changes in Force. J. Appl. Physiol. 2020, 128, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.; Firmino, T.; Oliveira, R.; Neto, T.; Infante, J.; Vaz, J.R.; Freitas, S.R. Hamstring Stiffness Pattern during Contraction in Healthy Individuals: Analysis by Ultrasound-Based Shear Wave Elastography. Eur. J. Appl. Physiol. 2018, 118, 2403–2415. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.K.F.; Richardson, C.A.; Jull, G.A. Electromyographic Amplitude and Frequency Changes in the Iliocostalis Lumborum and Multifidus Muscles during a Trunk Holding Test. Phys. Ther. 1997, 77, 954–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, S.J.; Burgess, K.; Onambele, G.N.L. Creep and the in Vivo Assessment of Human Patellar Tendon Mechanical Properties. Clin. Biomech. 2007, 22, 712–717. [Google Scholar] [CrossRef]
- Koo, T.K.; Hug, F. Factors That Influence Muscle Shear Modulus during Passive Stretch. J. Biomech. 2015, 48, 3539–3542. [Google Scholar] [CrossRef] [Green Version]
- Goubel, F.; Marini, J.F. Fibre Type Transition and Stiffness Modification of Soleus Muscle of Trained Rats. Pflügers Arch. Eur. J. Physiol. 1987, 410, 321–325. [Google Scholar] [CrossRef]
- Kovanen, V.; Suominen, H.; Heikkinen, E. Mechanical Properties of Fast and Slow Skeletal Muscle with Special Reference to Collagen and Endurance Training. J. Biomech. 1984, 17, 725–735. [Google Scholar] [CrossRef]
- Nocella, M.; Cecchi, G.; Bagni, M.A.; Colombini, B. Effect of Temperature on Crossbridge Force Changes during Fatigue and Recovery in Intact Mouse Muscle Fibers. PLoS ONE 2013, 8, e78918. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.S.; Humphrey, J.D. Heat-Induced Changes in the Mechanics of a Collagenous Tissue: Pseudoelastic Behavior at 37 °C. J. Biomech. 1997, 31, 211–216. [Google Scholar] [CrossRef]
- Tornberg, E. Effects of Heat on Meat Proteins—Implications on Structure and Quality of Meat Products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef]
- Kenny, G.P.; Reardon, F.D.; Zaleski, W.; Reardon, M.L.; Haman, F.; Ducharme, M.B. Muscle Temperature Transients before, during, and after Exercise Measured Using an Intramuscular Multisensor Probe. J. Appl. Physiol. 2003, 94, 2350–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombini, B.; Nocella, M.; Bagni, M.A. Non-Crossbridge Stiffness in Active Muscle Fibres. J. Exp. Biol. 2016, 219, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nocella, M.; Colombini, B.; Bagni, M.A.; Bruton, J.; Cecchi, G. Non-Crossbridge Calcium-Dependent Stiffness in Slow and Fast Skeletal Fibres from Mouse Muscle. J. Muscle Res. Cell Motil. 2012, 32, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Pincivero, D.M.; Gandaio, C.B.; Ito, Y. Gender-Specific Knee Extensor Torque, Flexor Torque, and Muscle Fatigue Responses during Maximal Effort Contractions. Eur. J. Appl. Physiol. 2003, 89, 134–141. [Google Scholar] [CrossRef]
- Hunter, S.K. Sex Differences in Human Fatigability: Mechanisms and Insight to Physiological Responses. Acta Physiol. 2014, 210, 768–789. [Google Scholar] [CrossRef] [Green Version]
- Clark, B.C.; Manini, T.M.; Thé, D.J.; Doldo, N.A.; Ploutz-Snyder, L.L. Gender Differences in Skeletal Muscle Fatigability Are Related to Contraction Type and EMG Spectral Compression. J. Appl. Physiol. 2003, 94, 2263–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haizlip, K.M.; Harrison, B.C.; Leinwand, L.A. Sex-Based Differences in Skeletal Muscle Kinetics and Fiber-Type Composition. Physiology 2015, 30, 30–39. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Wenner, M.M.; Stachenfeld, N.S. Sex Differences in Endothelial Function Important to Vascular Health and Overall Cardiovascular Disease Risk across the Lifespan. Am. J. Physiol. Circ. Physiol. 2018, 315, H1569–H1588. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Iemitsu, M.; Otsuki, T.; Maeda, S.; Ajisaka, R. Gender Differences in Brachial Blood Flow during Fatiguing Intermittent Handgrip. Med. Sci. Sports Exerc. 2008, 40, 684–690. [Google Scholar] [CrossRef]
- García-Vaquero, M.P.; Barbado, D.; Juan-Recio, C.; López-Valenciano, A.; Vera-Garcia, F.J. Isokinetic Trunk Flexion–Extension Protocol to Assess Trunk Muscle Strength and Endurance: Reliability, Learning Effect, and Sex Differences. J. Sport Health Sci. 2020, 9, 692–701. [Google Scholar] [CrossRef]
- Anders, C.; Wagner, H.; Puta, C.; Grassme, R.; Scholle, H.C. Healthy Humans Use Sex-Specific Co-Ordination Patterns of Trunk Muscles during Gait. Eur. J. Appl. Physiol. 2009, 105, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Larivière, C.; Shahvarpour, A.; Gravel, C.; Gauvin, M.; Jean, A.M.; Viau, A.; Mecheri, H. Revisiting the Effect of Manipulating Lumbar Stability with Load Magnitudes and Positions: The Effect of Sex on Trunk Muscle Activation. J. Electromyogr. Kinesiol. 2019, 46, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Taniguchi, M.; Tateuchi, H.; Hirono, T.; Yamagata, M.; Umehara, J.; Nojiri, S.; Kobayashi, M.; Ichihashi, N. Relationship between Individual Forces of Each Quadriceps Head during Low-Load Knee Extension and Cartilage Thickness and Knee Pain in Women with Knee Osteoarthritis. Clin. Biomech. 2022, 91, 105546. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.T.; Fung, P.K.; Ng, N.Y.; Ngan, T.L.; Chong, M.Y.; Tang, C.N.; He, J.F.; Zheng, Y.P. Dynamic Changes of Elasticity, Cross-Sectional Area, and Fat Infiltration of Multifidus at Different Postures in Men with Chronic Low Back Pain. Spine J. 2012, 12, 381–388. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Gao, J.; Hu, Y.; Liu, Y.; Li, W.; Chen, S.; Liu, F. Assessment of Ultrasound Shear Wave Elastography within Muscles Using Different Region of Interest Sizes, Manufacturers, Probes and Acquisition Angles: An Ex Vivo Study. Quant. Imaging Med. Surg. 2022, 12, 3227–3237. [Google Scholar] [CrossRef]
- Heales, L.J.; Badya, R.; Ziegenfuss, B.; Hug, F.; Coombes, J.S.; van den Hoorn, W.; Tucker, K.; Coombes, B.K. Shear-Wave Velocity of the Patellar Tendon and Quadriceps Muscle Is Increased Immediately after Maximal Eccentric Exercise. Eur. J. Appl. Physiol. 2018, 118, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Lacourpaille, L.; Nordez, A.; Hug, F.; Couturier, A.; Dibie, C.; Guilhem, G. Time-Course Effect of Exercise-Induced Muscle Damage on Localized Muscle Mechanical Properties Assessed Using Elastography. Acta Physiol. 2014, 211, 135–146. [Google Scholar] [CrossRef]
- Alfuraih, A.M.; O’Connor, P.; Hensor, E.; Tan, A.L.; Emery, P.; Wakefield, R.J. The Effect of Unit, Depth, and Probe Load on the Reliability of Muscle Shear Wave Elastography: Variables Affecting Reliability of SWE. J. Clin. Ultrasound 2018, 46, 108–115. [Google Scholar] [CrossRef]
- van Dieën, J.H.; Westebring-van der Putten, E.P.; Kingma, I.; de Looze, M.P. Low-Level Activity of the Trunk Extensor Muscles Causes Electromyographic Manifestations of Fatigue in Absence of Decreased Oxygenation. J. Electromyogr. Kinesiol. 2009, 19, 398–406. [Google Scholar] [CrossRef]




| Muscle | Condition | Before Fatigue | After Fatigue | Mean Difference | t-Test | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Raw (kPa) | Relative (%) | t-Value | p | d | ||
| Erector spinae | Passive | 14.54 | 2.18 | 14.47 | 2.19 | −0.07 | −0.45 | 0.17 | 0.867 | 0.03 |
| LC | 20.73 | 4.66 | 20.53 | 5.03 | −0.20 | −0.99 | 0.39 | 0.701 | 0.04 | |
| HC | 23.47 | 4.61 | 21.94 | 4.26 | −1.53 | −6.74 | 2.80 | 0.011 | 0.34 | |
| Superficial MF | Passive | 11.10 | 3.09 | 9.88 | 3.18 | −1.22 | −11.62 | 2.48 | 0.022 | 0.39 |
| LC | 24.50 | 8.69 | 24.24 | 7.75 | −0.25 | −1.04 | 0.39 | 0.697 | 0.03 | |
| HC | 27.23 | 5.89 | 25.44 | 6.38 | −1.78 | −6.77 | 2.79 | 0.011 | 0.29 | |
| Deep MF | Passive | 13.15 | 2.56 | 11.97 | 2.92 | −1.18 | −9.36 | 3.07 | 0.006 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vatovec, R.; Kozinc, Ž.; Voglar, M. The Effects of Isometric Fatigue on Trunk Muscle Stiffness: Implications for Shear-Wave Elastography Measurements. Sensors 2022, 22, 9476. https://doi.org/10.3390/s22239476
Vatovec R, Kozinc Ž, Voglar M. The Effects of Isometric Fatigue on Trunk Muscle Stiffness: Implications for Shear-Wave Elastography Measurements. Sensors. 2022; 22(23):9476. https://doi.org/10.3390/s22239476
Chicago/Turabian StyleVatovec, Rok, Žiga Kozinc, and Matej Voglar. 2022. "The Effects of Isometric Fatigue on Trunk Muscle Stiffness: Implications for Shear-Wave Elastography Measurements" Sensors 22, no. 23: 9476. https://doi.org/10.3390/s22239476





