Balance Impairments in People with Early-Stage Multiple Sclerosis: Boosting the Integration of Instrumented Assessment in Clinical Practice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Clinical Assessment
- Romberg test. As described by Gill-Body et al. [36], participants were required to stand for 30 s with feet together, arms crossed over their chest, and eyes closed [20,21]. The test was considered abnormal if the person was not able to maintain the position for 30 s or if postural sway larger than that expected by healthy subjects was observed by the clinician. The test was performed with eyes closed only, following previous studies [9,20,21].
- Tandem gait test. As described by Margolesky and Singer [23], participants were instructed to take ten consecutive heel-to-toe steps along a straight line with eyes open. The test was considered abnormal in case of larger than normal instability causing interruptions during the test.
- Fullerton advanced balance scale—short (FABs): This scale measures static and dynamic balance during six tasks rated on a five-point (0–4) ordinal scale (maximum score: 24) with higher scores indicating better performances. Scores lower than 23 are considered abnormal [35]. FABs included three items, i.e., Item 1 (“Turn 360° right and left”), Item 4 (“Standing on foam with eyes closed”, here called modified Romberg test, mRomberg), and Item 6 (“Walk with head turns”), which particularly challenge the vestibular system. For this reason, in the present study, these items were referred to as “Vestibular Tests”.
- A FABs-based balance assessment was used to identify individuals with and without clinically impaired balance: in particular, a person was identified as suffering from clinically impaired balance if at least one of the following conditions applied: (i) FABs total score < 23; (ii) FABs—Item 1 (“Turn 360° left and right”) score < 4; (iii) FABs—Item 6 (“Walk with head turns”) score < 4. Otherwise, the person was identified as having clinically normal balance. Conditions (ii) and (iii) were included to be more inclusive on the identification of persons with balance impairment since FABs—Item 1 and 6 demonstrated to be the most difficult tasks for early-stage PwMS [4].
- Timed up and go (TUG) test. This test measures mobility and dynamic balance. Subjects were instructed to stand up from a chair, walk 3 m, turn, walk back, and sit down. Time to complete TUG was recorded by the examiner using a stopwatch [37].
- Timed 25 foot walk test (T25FWT). The T25FWT measures the time taken by the subject to walk for 7.62 m “at their fastest but safest speed” [38]. The test was repeated twice, and the mean duration was computed.
- Twelve-item multiple sclerosis walking scale (MSWS-12). The MSWS-12 is a self-rated questionnaire on walking ability. The questions focused on the self-perceived impact of MS on 12 balance and locomotor activities of daily living in the last two weeks. In particular, question 4 asks the participant to evaluate how much MS makes it difficult to stand while performing an activity. The transformed total score ranges between 0 and 100 with higher scores indicating higher perceived walking difficulties [39,40].
- Romberg Test, tandem gait test, and FABs were administered to PwMS and HS while TUG, T25FWT, and MSWS-12 were administered to PwMS only. All tests were performed on a single day in random order.
2.4. Instrumented Assessment and Data Processing
2.5. Statistical Analysis
3. Results
3.1. Sample Description
3.2. Principal Component Analysis (PCA)
3.3. Sway Complexity and Sway Intensity: HS versus PwMS
3.4. Sway Complexity and Sway Intensity: Clinically Normal Balance versus Clinically Abnormal Balance Group
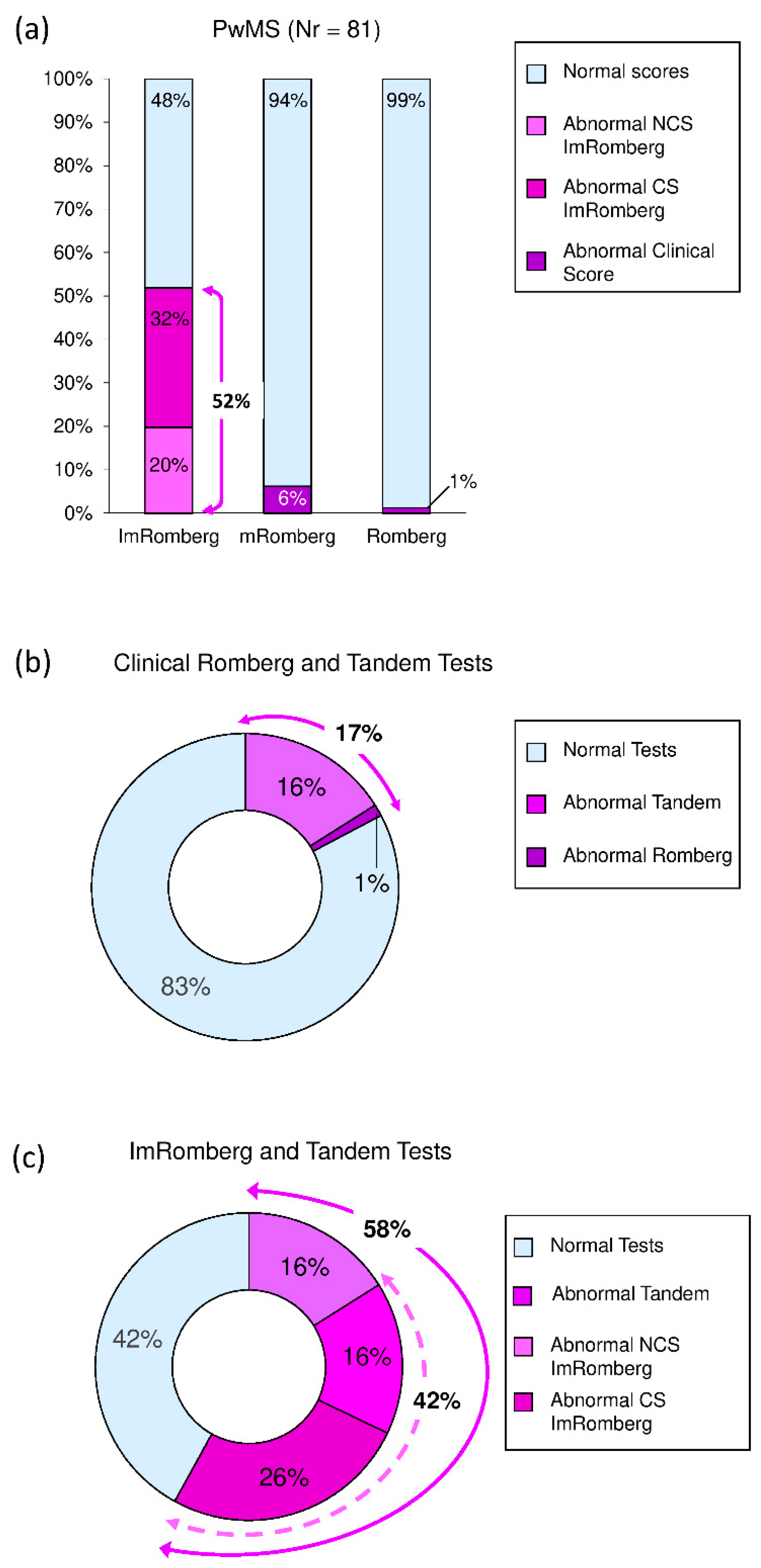
3.5. ImRomberg versus mRomberg versus Romberg Test
3.6. Sway Complexity and Sway Intensity: Correlations with Clinical Scales
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B
| Variable | A | B | μ | σ |
|---|---|---|---|---|
| AP Sway Amplitude | 0.050 | 0.287 | 0.21 | 0.19 |
| ML Sway Amplitude | 0.038 | 0.281 | 0.15 | 0.15 |
| AP Sway Velocity | 0.223 | 0.421 | 0.11 | 0.17 |
| ML Sway Velocity | 0.125 | 0.343 | 0.08 | 0.11 |
| AP Normalized Jerk | 0.363 | 0.171 | 3.64 | 0.44 |
| ML Normalized Jerk | 0.356 | 0.172 | 3.60 | 0.38 |
| AP Sample Entropy | 0.281 | 0.057 | 1.47 | 0.52 |
| ML Sample Entropy | 0.278 | 0.047 | 1.46 | 0.46 |
References
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising Prevalence of Multiple Sclerosis Worldwide: Insights from the Atlas of MS. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.L.; Phillips, B.A.; Kilpatrick, T.J.; Butzkueven, H.; Tubridy, N.; McDonald, E.; Galea, M.P. Gait and Balance Impairment in Early Multiple Sclerosis in the Absence of Clinical Disability. Mult. Scler. J. 2006, 12, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Thrue, C.; Riemenschneider, M.; Hvid, L.G.; Stenager, E.; Dalgas, U. Time Matters: Early-Phase Multiple Sclerosis Is Accompanied by Considerable Impairments across Multiple Domains. Mult. Scler. J. 2021, 27, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.; Gervasoni, E.; Anastasi, D.; Di Giovanni, R.; Brichetto, G.; Carpinella, I.; Cavalla, P.; Confalonieri, P.; Groppo, E.; Prosperini, L.; et al. Prevalence and Patterns of Subclinical Motor and Cognitive Impairments in Non-Disabled Individuals with Early Multiple Sclerosis: A Multicenter Cross-Sectional Study. Ann. Phys. Rehabil. Med. 2022, 65, 101491. [Google Scholar] [CrossRef]
- Kister, I.; Bacon, T.E.; Chamot, E.; Salter, A.R.; Cutter, G.R.; Kalina, J.T.; Herbert, J. Natural History of Multiple Sclerosis Symptoms. Int. J. MS Care 2013, 15, 146–158. [Google Scholar] [CrossRef]
- Dunn, J. Impact of Mobility Impairment on the Burden of Caregiving in Individuals with Multiple Sclerosis. Expert Rev. Pharmacoecon. Outcomes Res. 2010, 10, 433–440. [Google Scholar] [CrossRef]
- Spain, R.I.; St George, R.J.; Salarian, A.; Mancini, M.; Wagner, J.M.; Horak, F.B.; Bourdette, D. Body-Worn Motion Sensors Detect Balance and Gait Deficits in People with Multiple Sclerosis Who Have Normal Walking Speed. Gait Posture 2012, 35, 573–578. [Google Scholar] [CrossRef]
- Solomon, A.J.; Jacobs, J.V.; Lomond, K.V.; Henry, S.M. Detection of Postural Sway Abnormalities by Wireless Inertial Sensors in Minimally Disabled Patients with Multiple Sclerosis: A Case-Control Study. J. Neuroeng. Rehabil. 2015, 12, 74. [Google Scholar] [CrossRef]
- Melillo, F.; Di Sapio, A.; Martire, S.; Malentacchi, M.; Matta, M.; Bertolotto, A. Computerized Posturography Is More Sensitive than Clinical Romberg Test in Detecting Postural Control Impairment in Minimally Impaired Multiple Sclerosis Patients. Mult. Scler. Relat. Disord. 2017, 14, 51–55. [Google Scholar] [CrossRef]
- Brandstadter, R.; Ayeni, O.; Krieger, S.C.; Harel, N.Y.; Escalon, M.X.; Katz Sand, I.; Leavitt, V.M.; Fabian, M.T.; Buyukturkoglu, K.; Klineova, S.; et al. Detection of Subtle Gait Disturbance and Future Fall Risk in Early Multiple Sclerosis. Neurology 2020, 94, e1395–e1406. [Google Scholar] [CrossRef]
- Carpinella, I.; Gervasoni, E.; Anastasi, D.; Di Giovanni, R.; Tacchino, A.; Brichetto, G.; Confalonieri, P.; Solaro, C.; Rovaris, M.; Ferrarin, M.; et al. Walking With Horizontal Head Turns Is Impaired in Persons With Early-Stage Multiple Sclerosis Showing Normal Locomotion. Front. Neurol. 2022, 12, 821640. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Carpinella, I.; Gervasoni, E.; Anastasi, D.; Di Giovanni, R.; Tacchino, A.; Brichetto, G.; Confalonieri, P.; Rovaris, M.; Solaro, C.; Ferrarin, M.; et al. Instrumentally Assessed Gait Quality Is More Relevant than Gait Endurance and Velocity to Explain Patient-Reported Walking Ability in Early-Stage Multiple Sclerosis. Eur. J. Neurol. 2021, 28, 2259–2268. [Google Scholar] [CrossRef]
- Gunn, H.; Markevics, S.; Haas, B.; Marsden, J.; Freeman, J. Systematic Review: The Effectiveness of Interventions to Reduce Falls and Improve Balance in Adults With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2015, 96, 1898–1912. [Google Scholar] [CrossRef]
- Pavlikova, M.; Cattaneo, D.; Jonsdottir, J.; Gervasoni, E.; Stetkarova, I.; Angelova, G.; Markova, M.; Prochazkova, M.; Prokopiusova, T.; Hruskova, N.; et al. The Impact of Balance Specific Physiotherapy, Intensity of Therapy and Disability on Static and Dynamic Balance in People with Multiple Sclerosis: A Multi-Center Prospective Study. Mult. Scler. Relat. Disord. 2020, 40, 101974. [Google Scholar] [CrossRef]
- Donzé, C.; Massot, C. Rehabilitation in Multiple Sclerosis in 2021. Presse Med. 2021, 50, 104066. [Google Scholar] [CrossRef]
- Prosperini, L.; Fortuna, D.; Giannì, C.; Leonardi, L.; Marchetti, M.R.; Pozzilli, C. Home-Based Balance Training Using the Wii Balance Board: A Randomized, Crossover Pilot Study in Multiple Sclerosis. Neurorehabil. Neural Repair 2013, 27, 516–525. [Google Scholar] [CrossRef]
- Riemenschneider, M.; Hvid, L.G.; Stenager, E.; Dalgas, U. Is There an Overlooked “Window of Opportunity” in MS Exercise Therapy? Perspectives for Early MS Rehabilitation. Mult. Scler. J. 2018, 24, 886–894. [Google Scholar] [CrossRef]
- Dalgas, U.; Langeskov-Christensen, M.; Stenager, E.; Riemenschneider, M.; Hvid, L.G. Exercise as Medicine in Multiple Sclerosis—Time for a Paradigm Shift: Preventive, Symptomatic, and Disease-Modifying Aspects and Perspectives. Curr. Neurol. Neurosci. Rep. 2019, 19, 88. [Google Scholar] [CrossRef]
- Corporaal, S.H.A.; Gensicke, H.; Kuhle, J.; Kappos, L.; Allum, J.H.J.; Yaldizli, Ö. Balance Control in Multiple Sclerosis: Correlations of Trunk Sway during Stance and Gait Tests with Disease Severity. Gait Posture 2013, 37, 55–60. [Google Scholar] [CrossRef]
- Inojosa, H.; Schriefer, D.; Klöditz, A.; Trentzsch, K.; Ziemssen, T. Balance Testing in Multiple Sclerosis-Improving Neurological Assessment With Static Posturography? Front. Neurol. 2020, 11, 135. [Google Scholar] [CrossRef]
- Findling, O.; Rust, H.; Yaldizli, Ö.; Timmermans, D.P.H.; Scheltinga, A.; Allum, J.H.J. Balance Changes in Patients With Relapsing-Remitting Multiple Sclerosis: A Pilot Study Comparing the Dynamics of the Relapse and Remitting Phases. Front. Neurol. 2018, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Margolesky, J.; Singer, C. How Tandem Gait Stumbled into the Neurological Exam: A Review. Neurol. Sci. 2018, 39, 23–29. [Google Scholar] [CrossRef]
- Halmágyi, G.M.; Curthoys, I.S. Vestibular Contributions to the Romberg Test: Testing Semicircular Canal and Otolith Function. Eur. J. Neurol. 2021, 28, 3211–3219. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.; Carpinella, I.; Aprile, I.; Prosperini, L.; Montesano, A.; Jonsdottir, J. Comparison of Upright Balance in Stroke, Parkinson and Multiple Sclerosis. Acta Neurol. Scand. 2016, 133, 346–354. [Google Scholar] [CrossRef]
- Ghislieri, M.; Gastaldi, L.; Pastorelli, S.; Tadano, S.; Agostini, V. Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review. Sensors 2019, 19, 4075. [Google Scholar] [CrossRef]
- Zampogna, A.; Mileti, I.; Palermo, E.; Celletti, C.; Paoloni, M.; Manoni, A.; Mazzetta, I.; Costa, G.D.; Pérez-López, C.; Camerota, F.; et al. Fifteen Years of Wireless Sensors for Balance Assessment in Neurological Disorders. Sensors 2020, 20, 3247. [Google Scholar] [CrossRef] [PubMed]
- Brichetto, G.; Pedullà, L.; Podda, J.; Tacchino, A. Beyond Center-Based Testing: Understanding and Improving Functioning with Wearable Technology in MS. Mult. Scler. 2019, 25, 1402–1411. [Google Scholar] [CrossRef]
- Alexander, S.; Peryer, G.; Gray, E.; Barkhof, F.; Chataway, J. Wearable Technologies to Measure Clinical Outcomes in Multiple Sclerosis: A Scoping Review. Mult. Scler. 2021, 27, 1643–1656. [Google Scholar] [CrossRef]
- Papi, E.; Murtagh, G.M.; McGregor, A.H. Wearable Technologies in Osteoarthritis: A Qualitative Study of Clinicians’ Preferences. BMJ Open 2016, 6, e009544. [Google Scholar] [CrossRef]
- Keogh, A.; Taraldsen, K.; Caulfield, B.; Vereijken, B. It’s Not about the Capture, It’s about What We Can Learn: A Qualitative Study of Experts’ Opinions and Experiences Regarding the Use of Wearable Sensors to Measure Gait and Physical Activity. J. Neuroeng. Rehabil. 2021, 18, 78. [Google Scholar] [CrossRef]
- Angelini, L.; Buckley, E.; Bonci, T.; Radford, A.; Sharrack, B.; Paling, D.; Nair, K.P.S.; Mazza, C. A Multifactorial Model of Multiple Sclerosis Gait and Its Changes Across Different Disability Levels. IEEE Trans. Biomed. Eng. 2021, 68, 3196–3204. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F.; Black, C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson Prentice Hall: New York, NY, USA, 2010. [Google Scholar]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic Criteria for Multiple Sclerosis: 2010 Revisions to the McDonald Criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Mestanza Mattos, F.G.; Gervasoni, E.; Anastasi, D.; Giovanni, R.D.; Tacchino, A.; Brichetto, G.; Carpinella, I.; Confalonieri, P.; Vercellino, M.; Solaro, C.; et al. Assessing Balance in Non-Disabled Subjects with Multiple Sclerosis: Validation of the Fullerton Advanced Balance Scale. Mult. Scler. Relat. Disord. 2020, 42, 102085. [Google Scholar] [CrossRef] [PubMed]
- Gill-body, K.M.; Beninato, M.; Krebs, D.E. Relationship among Balance Impairments, Functional Performance, and Disability in People with Peripheral Vestibular Hypofunction. Phys. Ther. 2000, 80, 748–758. [Google Scholar] [CrossRef]
- Cattaneo, D.; Regola, A.; Meotti, M. Validity of Six Balance Disorders Scales in Persons with Multiple Sclerosis. Disabil. Rehabil. 2006, 28, 789–795. [Google Scholar] [CrossRef]
- Phan-Ba, R.; Pace, A.; Calay, P.; Grodent, P.; Douchamps, F.; Hyde, R.; Hotermans, C.; Delvaux, V.; Hansen, I.; Moonen, G.; et al. Comparison of the Timed 25-Foot and the 100-Meter Walk as Performance Measures in Multiple Sclerosis. Neurorehabil. Neural Repair 2011, 25, 672–679. [Google Scholar] [CrossRef]
- Hobart, J.C.; Riazi, A.; Lamping, D.L.; Fitzpatrick, R.; Thompson, A.J. Measuring the Impact of MS on Walking Ability: The 12-Item MS Walking Scale (MSWS-12). Neurology 2003, 60, 31–36. [Google Scholar] [CrossRef]
- Solaro, C.; Trabucco, E.; Signori, A.; Cella, M.; Messmer Uccelli, M.; Brichetto, G.; Cavalla, P.; Gironi, M.; Patti, F.; Prosperini, L. Italian Validation of the 12-Item Multiple Sclerosis Walking Scale. Mult. Scler. Int. 2015, 2015, 540828. [Google Scholar] [CrossRef]
- Rose, D.J.; Lucchese, N.; Wiersma, L.D. Development of a Multidimensional Balance Scale for Use with Functionally Independent Older Adults. Arch. Phys. Med. Rehabil. 2006, 87, 1478–1485. [Google Scholar] [CrossRef]
- Hsieh, K.L.; Roach, K.L.; Wajda, D.A.; Sosnoff, J.J. Smartphone Technology Can Measure Postural Stability and Discriminate Fall Risk in Older Adults. Gait Posture 2019, 67, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Phan, D.; Pathirana, P.N.; Horne, M.; Power, L.; Szmulewicz, D. Quantification of Axial Abnormality Due to Cerebellar Ataxia with Inertial Measurements. Sensors 2018, 18, 2791. [Google Scholar] [CrossRef]
- Moe-Nilssen, R. A New Method for Evaluating Motor Control in Gait under Real-Life Environmental Conditions. Part 1: The Instrument. Clin. Biomech. 1998, 13, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Comber, L.; Sosnoff, J.J.; Galvin, R.; Coote, S. Postural Control Deficits in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Gait Posture 2018, 61, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.J.; Bruetsch, A.P.; Lynch, S.G.; Horak, F.B.; Huisinga, J.M. Instrumented Balance and Walking Assessments in Persons with Multiple Sclerosis Show Strong Test-Retest Reliability. J. Neuroeng. Rehabil. 2017, 14, 43. [Google Scholar] [CrossRef]
- Busa, M.A.; Jones, S.L.; Hamill, J.; van Emmerik, R.E.A. Multiscale Entropy Identifies Differences in Complexity in Postural Control in Women with Multiple Sclerosis. Gait Posture 2016, 45, 7–11. [Google Scholar] [CrossRef]
- Pau, M.; Porta, M.; Coghe, G.; Corona, F.; Pilloni, G.; Lorefice, L.; Marrosu, M.G.; Cocco, E. Are Static and Functional Balance Abilities Related in Individuals with Multiple Sclerosis? Mult. Scler. Relat. Disord. 2017, 15, 1–6. [Google Scholar] [CrossRef]
- Huisinga, J.M.; Yentes, J.M.; Filipi, M.L.; Stergiou, N. Postural Control Strategy during Standing Is Altered in Patients with Multiple Sclerosis. Neurosci. Lett. 2012, 524, 124–128. [Google Scholar] [CrossRef]
- Sun, R.; Hsieh, K.L.; Sosnoff, J.J. Fall Risk Prediction in Multiple Sclerosis Using Postural Sway Measures: A Machine Learning Approach. Sci. Rep. 2019, 9, 16154. [Google Scholar] [CrossRef]
- Schubert, P.; Kirchner, M. Ellipse Area Calculations and Their Applicability in Posturography. Gait Posture 2014, 39, 518–522. [Google Scholar] [CrossRef]
- Mellone, S.; Palmerini, L.; Cappello, A.; Chiari, L. Hilbert-Huang-Based Tremor Removal to Assess Postural Properties from Accelerometers. IEEE Trans. Biomed. Eng. 2011, 58, 1752–1761. [Google Scholar] [CrossRef]
- Quijoux, F.; Nicolaï, A.; Chairi, I.; Bargiotas, I.; Ricard, D.; Yelnik, A.; Oudre, L.; Bertin-Hugault, F.; Vidal, P.P.; Vayatis, N.; et al. A Review of Center of Pressure (COP) Variables to Quantify Standing Balance in Elderly People: Algorithms and Open-Access Code. Physiol. Rep. 2021, 9, e15067. [Google Scholar] [CrossRef] [PubMed]
- Caby, B.; Kieffer, S.; de Saint Hubert, M.; Cremer, G.; Macq, B. Feature Extraction and Selection for Objective Gait Analysis and Fall Risk Assessment by Accelerometry. Biomed. Eng. Online 2011, 10, 1. [Google Scholar] [CrossRef]
- Mancini, M.; Salarian, A.; Carlson-Kuhta, P.; Zampieri, C.; King, L.; Chiari, L.; Horak, F.B. ISway: A Sensitive, Valid and Reliable Measure of Postural Control. J. Neuroeng. Rehabil. 2012, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological Time-Series Analysis Using Approximate Entropy and Sample Entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Peng, C.K.; Goldberger, A.L.; Hausdorff, J.M. Multiscale Entropy Analysis of Human Gait Dynamics. Physica A 2003, 330, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Carpinella, I.; Gervasoni, E.; Anastasi, D.; Di Giovanni, R.; Tacchino, A.; Brichetto, G.; Confalonieri, P.; Solaro, C.; Ferrarin, M.; Rovaris, M.; et al. A Single Wearable Inertial Sensor Detects Subtle Balance Impairments in Early-Stage, Normally Walking People with Multiple Sclerosis. RIMS Digit. Conf. Mult. Scler. 2020, 26, 24. [Google Scholar] [CrossRef]
- McDonald, J.H. Controlling the False Discovery Rate: Benjamini–Hochberg Procedure. In Handbook of Biological Statistics; Sparky House Publishing: Baltimore, MD, USA, 2009; pp. 258–259. [Google Scholar]
- Beavers, A.S.; Lounsbury, J.W.; Richards, J.K.; Huck, S.W.; Skolits, G.J.; Esquivel, S.L. Practical Considerations for Using Exploratory Factor Analysis in Educational Research. Pract. Assess. Res. Eval. 2013, 18, 1–13. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics; Pearson Education: Boston, MA, USA, 2013. [Google Scholar]
- Yong, A.G.; Pearce, S. A Beginner’s Guide to Factor Analysis: Focusing on Exploratory Factor Analysis. Tutor. Quant. Methods Psychol. 2013, 9, 79–94. [Google Scholar] [CrossRef]
- Campbell, M.J.; Swinscow, T.D.V. Statistics at Square One; Thomas, D.V., Ed.; Wiley-Blackwell/BMJ Books: Hoboken, NJ, USA, 2009. [Google Scholar]
- MacCallum, R.C.; Widaman, K.F.; Zhang, S.; Hong, S. Sample Size in Factor Analysis. Psychol. Methods 1999, 4, 84–99. [Google Scholar] [CrossRef]
- Mundfrom, D.J.; Shaw, D.G.; Ke, T.L. Minimum Sample Size Recommendations for Conducting Factor Analyses. Int. J. Test. 2005, 5, 159–168. [Google Scholar] [CrossRef]
- Yang, S.; Berdine, G. The Receiver Operating Characteristic (ROC) Curve. Southwest Respir. Crit. Care Chron. 2017, 5, 34. [Google Scholar] [CrossRef]
- Fjeldstad, C.; Pardo, G.; Bemben, D.; Bemben, M. Decreased Postural Balance in Multiple Sclerosis Patients with Low Disability. Int. J. Rehabil. Res. 2011, 34, 53–58. [Google Scholar] [CrossRef]
- Fanchamps, M.H.J.; Gensicke, H.; Kuhle, J.; Kappos, L.; Allum, J.H.J.; Yaldizli, Ö. Screening for Balance Disorders in Mildly Affected Multiple Sclerosis Patients. J. Neurol. 2012, 259, 1413–1419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barbado Murillo, D.; Sabido Solana, R.; Vera-Garcia, F.J.; Gusi Fuertes, N.; Moreno, F.J. Effect of Increasing Difficulty in Standing Balance Tasks with Visual Feedback on Postural Sway and EMG: Complexity and Performance. Hum. Mov. Sci. 2012, 31, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Manor, B.; Costa, M.D.; Kun, H.; Newton, E.; Starobinets, O.; Hyun, G.K.; Peng, C.K.; Novak, V.; Lipsitz, L.A. Physiological Complexity and System Adaptability: Evidence from Postural Control Dynamics of Older Adults. J. Appl. Physiol. 2010, 109, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Newell, K.M.; Vaillancourt, D.E.; Sosnoff, J.J. Aging, Complexity, and Motor Performance. In The Psychology of Aging; Birren, J.E., Schai, K.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 163–182. [Google Scholar]
- Stergiou, N.; Harbourne, R.T.; Cavanaugh, J.T. Optimal Movement Variability: A New Theoretical Perspective for Neurologic Physical Therapy. J. Neurol. Phys. Ther. 2006, 30, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.; Kerr, G.; Newell, K.M.; Silburn, P.A. Differential Time- and Frequency-Dependent Structure of Postural Sway and Finger Tremor in Parkinson’s Disease. Neurosci. Lett. 2008, 443, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Roerdink, M.; Hlavackova, P.; Vuillerme, N. Center-of-Pressure Regularity as a Marker for Attentional Investment in Postural Control: A Comparison between Sitting and Standing Postures. Hum. Mov. Sci. 2011, 30, 203–212. [Google Scholar] [CrossRef]
- Donker, S.F.; Roerdink, M.; Greven, A.J.; Beek, P.J. Regularity of Center-of-Pressure Trajectories Depends on the Amount of Attention Invested in Postural Control. Exp. Brain Res. 2007, 181, 1–11. [Google Scholar] [CrossRef]
- Roeing, K.L.; Wajda, D.A.; Sosnoff, J.J. Time Dependent Structure of Postural Sway in Individuals with Multiple Sclerosis. Gait Posture 2016, 48, 19–23. [Google Scholar] [CrossRef]
- Prosperini, L.; Sbardella, E.; Raz, E.; Cercignani, M.; Tona, F.; Bozzali, M.; Petsas, N.; Pozzilli, C.; Pantano, P. Multiple Sclerosis: White and Gray Matter Damage Associated with Balance Deficit Detected at Static Posturography. Radiology 2013, 268, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.P.; Roland, P.S.; Yellin, W. Vestibular Evaluation in Patients with Early Multiple Sclerosis. Am. J. Otol. 1997, 18, 93–100. [Google Scholar] [PubMed]
- Gabelić, T.; Krbot Skorić, M.; Adamec, I.; Barun, B.; Zadro, I.; Habek, M. The Vestibular Evoked Myogenic Potentials (VEMP) Score: A Promising Tool for Evaluation of Brainstem Involvement in Multiple Sclerosis. Eur. J. Neurol. 2015, 22, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, L.; Fortuna, D.; Giannì, C.; Leonardi, L.; Pozzilli, C. The Diagnostic Accuracy of Static Posturography in Predicting Accidental Falls in People with Multiple Sclerosis. Neurorehabil. Neural Repair 2013, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, D.; Carpinella, I.; Gervasoni, E.; Matsuda, P.N.; Bovi, G.; Ferrarin, M.; Cattaneo, D. Instrumented Version of the Modified Dynamic Gait Index in Patients With Neurologic Disorders. PM R 2019, 11, 1312–13198. [Google Scholar] [CrossRef]
- Carpinella, I.; Gervasoni, E.; Anastasi, D.; Lencioni, T.; Cattaneo, D.; Ferrarin, M. Instrumental Assessment of Stair Ascent in People with Multiple Sclerosis, Stroke, and Parkinson’s Disease: A Wearable-Sensor-Based Approach. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Fritz, N.E.; Marasigan, R.E.R.; Calabresi, P.A.; Newsome, S.D.; Zackowski, K.M. The Impact of Dynamic Balance Measures on Walking Performance in Multiple Sclerosis. Neurorehabil. Neural Repair 2015, 29, 62–69. [Google Scholar] [CrossRef]
- Kalron, A.; Nitzani, D.; Achiron, A. Static Posturography across the EDSS Scale in People with Multiple Sclerosis: A Cross Sectional Study. BMC Neurol. 2016, 16, 70. [Google Scholar] [CrossRef]
- Sebastião, E.; Sandroff, B.M.; Learmonth, Y.C.; Motl, R.W. Validity of the Timed Up and Go Test as a Measure of Functional Mobility in Persons With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2016, 97, 1072–1077. [Google Scholar] [CrossRef]
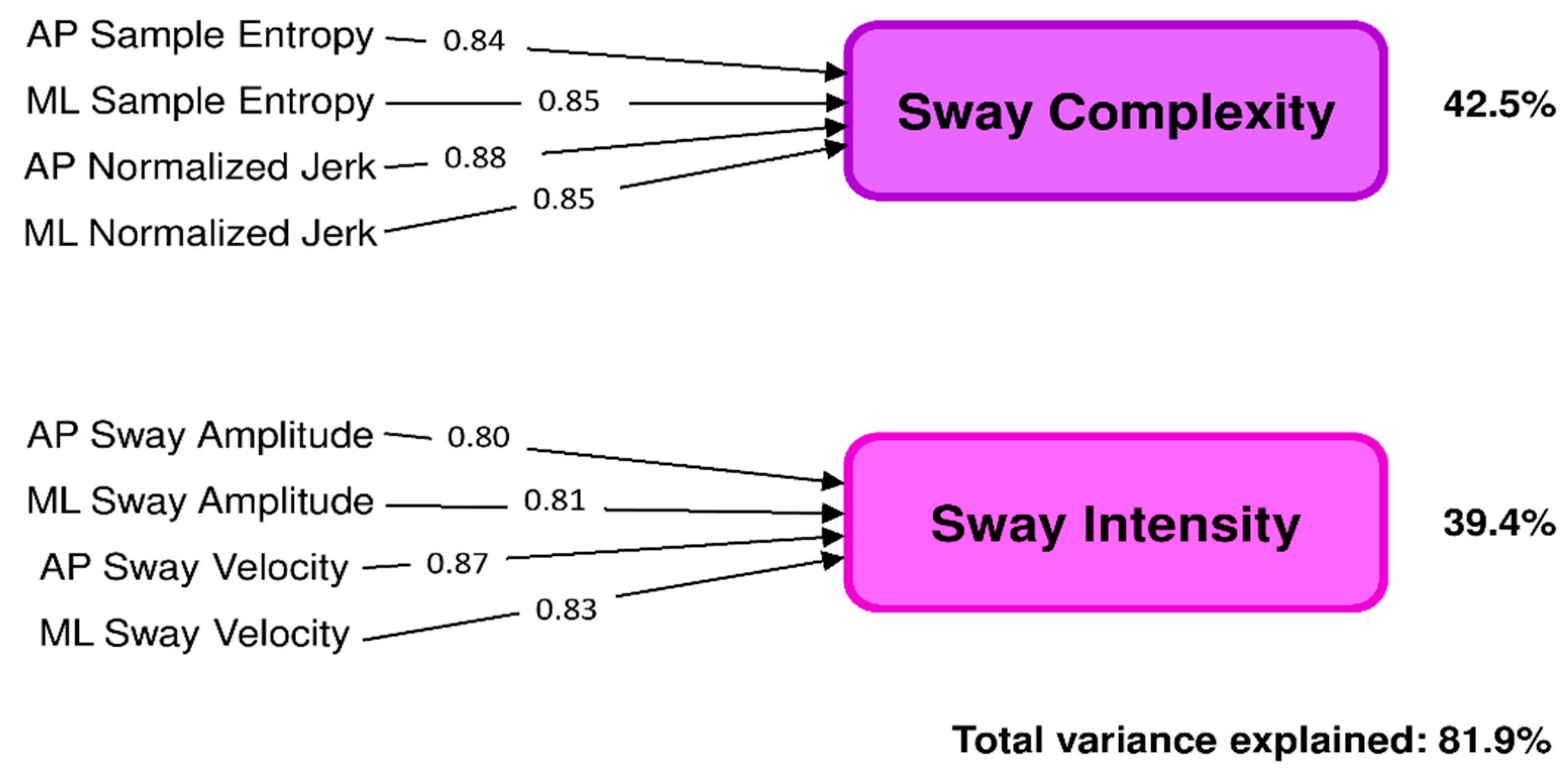


| Variable | Description |
|---|---|
| AP (ML) Sway Amplitude (SwAmp) [m/s2] | Root mean square of AP (ML) acceleration signal [7]. |
| AP (ML) Sway Range (SwRange) [m/s2] | Range (maximum−minimum value) of AP (ML) acceleration signal [46]. |
| 95% Confidence Ellipse Area (SwArea) [m2/s4] | Area of the ellipse containing 95% of ML/AP acceleration data points computed following Schubert and Kirchner [51] |
| AP (ML) Sway Velocity (SwVel) [m/s] | Mean of the absolute value of the AP (ML) velocity signal obtained by integrating the AP (ML) acceleration. Before integration, the accelerations were high-pass filtered at 0.15 Hz with a zero-lag, fourth order Butterworth filter to limit the drift effect [52]. |
| AP (ML) Sway Path (SwPath) [m] | Mean length of the AP (ML) trajectory traveled by the trunk and calculated as the product between AP (ML) sway velocity and the duration of the test [53]. |
| AP (ML) Normalized Jerk (nJerk) [-] | Logarithm of the normalized AP (ML) jerk (i.e., first time derivative of the acceleration) computed as described by Caby et al. [54]. In particular, AP (ML) jerk was normalized with respect to the range of AP (ML) acceleration and the test duration (see [7]). Decreasing values of nJerk indicate smoother trunk sway. |
| AP (ML) Total Spectral Power (Pwr) [m2/s4] | Integrated area of the power spectral density of AP (ML) acceleration computed using the Welch method with a Hanning window of 5 s and 50% overlap [52]. Increasing values of Pwr indicate higher energy expenditure [53] |
| AP (ML) 95% Power Frequency (F95) [Hz] | Frequency below which 95% of the AP (ML) acceleration power is contained [55]. Higher values of F95 indicate a higher frequency of trunk sway. |
| AP (ML) Centroidal Frequency (CF) [Hz] | Frequency at which the spectral mass of AP (ML) acceleration is concentrated [7,53] |
| AP (ML) Frequency Dispersion (FD) [-] | Measure of the variability of frequency content of the power spectral density (zero for pure sinusoid, increases with spectral bandwidth to one) [46,53] |
| AP (ML) Sample Entropy (SaEn) [-] | SaEn was computed on the standardized AP (ML) acceleration obtained by subtracting the mean and dividing by the standard deviation of the signal. SaEn is related to the conditional probability that two sequences of m consecutive data points similar to each other (i.e., distance between data points lower than a tolerance r) will remain similar when one more consecutive point is included [56,57]. Values of m and r were set equal to 2 and 0.15, respectively, following Busa et al. [47]. SaEn is a measure of the regularity and predictability of AP (ML) trunk accelerations, thus providing information on the complexity of the signal [49,57]. The higher SaEn, the more complex (less regular) the trunk acceleration. |
| Variable | HS (N = 38) | PwMS (N = 81) | p-Value |
|---|---|---|---|
| Age [years] | 34 (24; 58) | 39 (25; 56) | 0.360 |
| Female [N (%)] | 22 (58%) | 53 (65%) | 0.427 |
| Disease Duration [years] | - | 2 (0; 5) | - |
| EDSS Score [0–10] | - | 1.5 (0; 2.5) | - |
| MS Type [N (%)] | |||
| Relapsing-Remitting | - | 80 (99%) | - |
| Primary Progressive | - | 1 (1%) | - |
| Secondary Progressive | - | 0 (0%) | - |
| Abnormal Romberg Test [N (%)] | 0 (0%) | 1 (1.2%) | 0.555 |
| Abnormal Tandem Test [N (%)] | 0 (0%) | 13 (16%) | 0.024 |
| Abnormal mRomberg Test [N (%)] | 0 (0%) | 5 (6%) | 0.150 |
| FABs [0–24] | 24 (24; 24) | 23 (18; 24) | <0.001 |
| TUG [s] | - | 7.3 (5.2; 9.7) | - |
| T25FWT [s] | - | 4.0 (3.2; 5.7) | - |
| MSWS-12 score [0–100] | - | 4.2 (0; 43.8) | - |
| MSWS-12 Item 4 [1–5] | - | 1 (1; 3) | - |
| Variable | HS (N = 38) | PwMS (N = 81) | p-Value | ∆% |
|---|---|---|---|---|
| AP Sway Amplitude [m/s2] | 0.12 (0.08; 0.18) | 0.17 (0.09; 0.58) | <0.001 | +42% |
| ML Sway Amplitude [m/s2] | 0.07 (0.04; 0.13) | 0.10 (0.05; 0.57) | <0.001 | +43% |
| AP Sway Range [m/s2] | 0.78 (0.51; 1.20) | 1.03 (0.54; 5.54) | <0.001 | +32% |
| ML Sway Range [m/s2] | 0.43 (0.25; 0.87) | 0.63 (0.32; 4.98) | <0.001 | +47% |
| 95% Conf. Ellipse Area [m2/s4] | 0.15 (0.08; 0.35) | 0.24 (0.10; 6.19) | <0.001 | +60% |
| AP Sway Velocity [m/s] | 0.05 (0.02; 0.09) | 0.08 (0.03; 0.30) | <0.001 | +60% |
| ML Sway Velocity [m/s] | 0.02 (0.01; 0.08) | 0.05 (0.02; 0.33) | <0.001 | +150% |
| AP Sway Path [m] | 1.07 (0.42; 1.74) | 1.64 (0.58; 582) | <0.001 | +53% |
| ML Sway Path [m] | 0.49 (0.22; 1.56) | 1.16 (0.31; 6.58) | <0.001 | +137% |
| AP Normalized Jerk [-] | 3.74 (3.27; 4.45) | 3.53 (2.89; 4.32) | 0.007 | −6% |
| ML Normalized Jerk [-] | 3.75 (3.19; 4.28) | 3.55 (2.92; 4.10) | 0.002 | −5% |
| AP Total Spectral Power [m2/s4] | 0.08 (0.04; 0.16) | 0.11 (0.04; 1.79) | 0.002 | +38% |
| ML Total Spectral Power [m2/s4] | 0.03 (0.01; 0.11) | 0.06 (0.01; 1.25) | <0.001 | +100% |
| AP 95% power frequency [Hz] | 1.61 (1.03; 2.34) | 1.76 (0.88; 2.78) | 0.281 | +9% |
| ML 95% power frequency [Hz] | 1.83 (1.17; 2.93) | 1.90 (1.03; 2.93) | 0.620 | +4% |
| AP Centroidal Frequency [Hz] | 0.76 (0.56; 1.02) | 0.81 (0.53; 1.23) | 0.243 | +7% |
| ML Centroidal Frequency [Hz] | 0.91 (0.62; 1.16) | 0.89 (0.58; 1.37) | 0.750 | −2% |
| AP Frequency Dispersion [-] | 0.68 (0.58; 0.76) | 0.66 (0.54; 0.75) | 0.153 | −9% |
| ML Frequency Dispersion [-] | 0.63 (0.52; 0.72) | 0.62 (0.50; 0.72) | 0.869 | −2% |
| AP Sample Entropy [-] | 1.73 (1.12; 2.13) | 1.34 (0.53; 2.21) | 0.010 | −23% |
| ML Sample Entropy [-] | 1.77 (1.16; 2.23) | 1.33 (0.46; 2.09) | <0.001 | −25% |
| Variable | HS (N = 38) | PwMS (N = 81) | p-Value | ∆% |
|---|---|---|---|---|
| Sway Complexity [-] | 0.29 (−0.82; 1.59) | −0.37 (−1.77; 1.61) | 0.003 | −228% |
| Sway Intensity [-] | −0.41 (−0.69; 0.11) | −0.15 (−0.72; 1.61) | <0.001 | +63% |
| Variable | Clinically Normal Balance Group (N = 63) | Clinically Abnormal Balance Group (N = 56) | p-Value | ∆% |
|---|---|---|---|---|
| Sway Complexity [-] | 0.27 (−1.01; 1.59) | −0.48 (−1.18; 1.74) | 0.002 | −278% |
| Sway Intensity [-] | −0.31 (−0.63; 0.59) | −0.15 (−0.83; 2.69) | 0.041 | +52% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpinella, I.; Anastasi, D.; Gervasoni, E.; Di Giovanni, R.; Tacchino, A.; Brichetto, G.; Confalonieri, P.; Rovaris, M.; Solaro, C.; Ferrarin, M.; et al. Balance Impairments in People with Early-Stage Multiple Sclerosis: Boosting the Integration of Instrumented Assessment in Clinical Practice. Sensors 2022, 22, 9558. https://doi.org/10.3390/s22239558
Carpinella I, Anastasi D, Gervasoni E, Di Giovanni R, Tacchino A, Brichetto G, Confalonieri P, Rovaris M, Solaro C, Ferrarin M, et al. Balance Impairments in People with Early-Stage Multiple Sclerosis: Boosting the Integration of Instrumented Assessment in Clinical Practice. Sensors. 2022; 22(23):9558. https://doi.org/10.3390/s22239558
Chicago/Turabian StyleCarpinella, Ilaria, Denise Anastasi, Elisa Gervasoni, Rachele Di Giovanni, Andrea Tacchino, Giampaolo Brichetto, Paolo Confalonieri, Marco Rovaris, Claudio Solaro, Maurizio Ferrarin, and et al. 2022. "Balance Impairments in People with Early-Stage Multiple Sclerosis: Boosting the Integration of Instrumented Assessment in Clinical Practice" Sensors 22, no. 23: 9558. https://doi.org/10.3390/s22239558
APA StyleCarpinella, I., Anastasi, D., Gervasoni, E., Di Giovanni, R., Tacchino, A., Brichetto, G., Confalonieri, P., Rovaris, M., Solaro, C., Ferrarin, M., & Cattaneo, D. (2022). Balance Impairments in People with Early-Stage Multiple Sclerosis: Boosting the Integration of Instrumented Assessment in Clinical Practice. Sensors, 22(23), 9558. https://doi.org/10.3390/s22239558









